Abstract
In preoperative planning, fMRI and repetitive navigated transcranial magnetic stimulation (rTMS) repeatedly revealed differences in the detected language sites, which can be attributed to tumor-induced oxygenation changes impairing the accuracy of fMRI. We therefore compared the accordance of those techniques in healthy subjects using exactly the same tasks in both investigations. 19 healthy right-handed subjects performed object naming, pseudoword reading, verb generation, and action naming during fMRI at 3 T and rTMS. For rTMS language mapping, we stimulated 46 cortical spots over the left hemisphere; each site was stimulated for three times. Language positive points during rTMS for one, two, or three errors out the three stimulations per spot (1/3, 2/3, 3/3) were exported via DICOM, and compared to the positive fMRI clusters. As a result of this comparison, the best correlation was observed between 3/3 errors and fMRI for pseudoword reading and verb generation with t-values of pu < 0.001, uncorrected for multiple comparisons, on average across the whole rTMS-spot map. We found a close spatial agreement between several rTMS-spots (2/3 and 3/3 errors) and fMRI clusters accentuated in the frontal lobe, followed by the parietal lobe and less in the temporal lobe. Compared to the fMRI clusters, there was a higher congruence for 2/3 and 3/3 errors than for 1/3 errors. Overall, results of language mapping in healthy subjects by fMRI and rTMS correspond well yet depending on the used language task.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A variety of noninvasive methods is used in neuroscience to investigate cortical language function in the human brain, such as functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and electroencephalography (EEG) (Salmelin et al. 2000). Additionally, transcranial magnetic stimulation (TMS) was introduced for stimulating the human cortex by Barker et al. (1985). Pascual-Leone et al. (1991, 1999) introduced the term “virtual lesion” and was able to induce speech arrests and counting errors by the use of rapid-rate TMS (Pascual-Leone et al. (1991, 1999)). Recently, the combination of TMS with optically tracked stereotactic navigation systems was established making it possible to visualize the stimulation sites via MRI-based 3D reconstructions of the patients’ brain, called navigated TMS (nTMS) (Paus 1999; Neggers et al. 2004; Ilmoniemi et al. 1999; Ruohonen and Ilmoniemi 1999; Ruohonen and Karhu 2010).
With the development of nTMS, TMS gains increasing importance for neuroscientific language research by combining it with an object naming task and by using repetitive nTMS (rTMS) (Sollmann et al. 2013b; Lioumis et al. 2012). Moreover, the correlation with intraoperative direct cortical stimulation (DCS) was investigated repeatedly showing a high sensitivity for language-involved brain areas (Picht et al. 2013; Tarapore et al. 2013; Krieg et al. 2014b). As a consequence of these studies, in some institutions, rTMS is already used routinely in addition to fMRI for non-invasive language mapping (Rosler et al. 2014; Krieg et al. 2014a; Ille et al. 2015b; d). Moreover, there are studies showing that fMRI is impaired by intraparenchymal lesions due to impaired oxygenation levels in the proximity of intracerebral lesions and therefore seems not to be appropriate for preoperative mapping of cortical language function (Giussani et al. 2010; Ille et al. 2015b). Nonetheless, fMRI and rTMS are two highly capable techniques for mapping of cortical language function and are therefore highly relevant for neuroscientists per se. Thus, the present study is one of the first to examine the results of cortical mapping of language function obtained by rTMS and fMRI within one cohort of healthy volunteers (Kononen et al. 2015). The study therefore investigates the correlation between a lesion-based technique (rTMS) and neuro-vascular coupling as the base of fMRI in the same volunteers and by the same four language tasks mirroring different language sub-functions and networks.
Our hypothesis is that neuronal activation as measured by changed blood oxygenation levels and a modality using virtual lesions to identify functionally relevant cortical tissue correlate well in their location with reduced correlation in the precentral gyrus due to the absence of the motoric component during the fMRI examination.
Methods
Participants
We enrolled 20 subjects (10 male, 10 female) without neurological disorders in this prospective study. One male participant could not tolerate the TMS stimulation, so we had to stop his investigation and we excluded him from further analysis. The remaining subjects had a mean age of 24.6 ± 1.7 years (range 22–29 years). Their right-handedness was tested by the Edinburgh handedness inventory (Oldfield 1971) and for all subjects German was the sole mother tongue. Prerequisite inclusion criteria were a written informed consent and an age of at least 18 years. General TMS and fMRI exclusion criteria like pacemaker, cochlear implant, or deep brain stimulation (Rossi et al. 2009), as well as previous seizures, second mother tongue, developmental language impairment, bilateral or left handedness, aberrant medical history, or any neurological deficits or pathologies on cranial MRI led to an exclusion of the study.
Ethics
The experimental procedures were approved by the local ethical committee in accordance with the Declaration of Helsinki (registration number: 2793/10). All participants gave their written informed consent prior to the first investigation.
Language tasks
rTMS and fMRI experiments used 4 paradigms with identical stimulus material in both methods. We utilized four language tasks consisting of a set of 100 items each: object naming, pseudoword reading, verb generation, and action naming. All tasks were visual tasks and had to be performed in German. During the rTMS investigation, the participants had to name or read aloud the word, whereas in the fMRI investigation, the volunteers were advised only to think the word.
For the object naming task, we used a colored picture set of common objects comparable to the picture set of Snodgrass and Vanderwart (1980) (Picht et al. 2013; Tarapore et al. 2013), which had to be named (or thought about in fMRI) without article.
The pseudoword reading task consisted of 50 pseudowords randomly mixed with 50 real words (as control) from a German word list by Felty et al. (2007). Felty’s list contained disyllabic nouns, verbs, and adjectives in the CVCCVC structure (C=Consonant, V=Vowel) and pseudowords that were derived from those real words. The subjects were asked to read aloud (or calm in fMRI) the presented words.
For the verb generation task, common objects were visually presented on a screen. The instruction for the volunteers was to build verbs out of those objects.
During the action naming task, the participants were asked to name aloud (or calm in fMRI) daily activities that were displayed by pictures on a screen (e.g., dancing, sleeping).
rTMS language mapping
Experimental setup
One investigator performed all rTMS language investigations by using the Nexstim eXimia NBS system version 4.3 and a NexSpeech® module (Nexstim Plc, Helsinki, Finland). In each participant, language mapping was conducted for the left hemisphere. Thereby, we followed the same mapping protocol as published earlier, with a picture to trigger interval of 0 ms (Krieg et al. 2014b; Picht et al. 2013). In brief, we firstly had to determine the Resting Motor Threshold (RMT) by motor mapping of the cortical representation of the left-sided hand area (right-sided abductor pollicis brevis muscle or abductor digiti minimi muscle, if no satisfying answer of the abductor pollicis brevis muscle is observed) as described in detail by Picht et al. (Picht et al. 2009). Stimulus intensity for language mapping was 100% RMT in all mapping sessions. We applied ten bursts per rTMS train with a repetition rate of 5 Hz (stimulation duration of approximately 2 s).
rTMS language mapping procedure
We presented the pictures or words in a random order on a screen 60 cm in front of the participant. Since we wanted to make sure that items, which were shown during stimulation, had been perfectly recognized without stimulation, baseline testing of each task was performed prior to the stimulation session. Thus, inter-individual differences in the vocabulary were considered. During baseline testing, pictures or words had to be named or read accurately and quickly. Misnamed or misread items were rejected from the stimulus sequence, correctly identified items were documented, counted and were presented in the following stimulation session. During the rTMS investigation, the display time of items of the object naming, action naming, and verb generation task was 700 ms. Words of the pseudoword reading task were displayed for 1.0 s. The inter picture interval (Shepherd et al. 2013) was 3.0 s for all tasks. We applied magnetic pulses simultaneously with the item presentation.
Altogether, one investigation required 100–120 min per subject, including 4 different tasks. Baseline performance and rTMS language mapping were video recorded for objective and detailed post-hoc language analysis (Lioumis et al. 2012).
Stimulated points
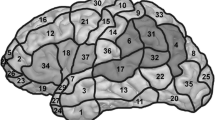
We determined 46 cortical spots on the left hemisphere, which were tagged on the 3D MRI prior to each mapping session (Fig. 1). The spots were easy to reproduce in the cortical 3D reconstruction of healthy volunteers and spared areas in which stimulation would cause unacceptable pain (orbital part of the inferior frontal gyrus (orIFG), polar superior and polar middle temporal gyrus (polSTG, polMTG), anterior middle temporal gyrus (aMTG), and polar superior (polSFG), polar middle (polMFG) and polar inferior frontal gyrus (polIFG). Because of the increasing distance between skin and brain in the inferior temporal gyrus (ITG) and the consecutive decreasing stimulation intensity below 50 V/m, this region was also not mapped (Krieg et al. 2013b). Each of the 46 spots was stimulated three times per language task, thus we applied 138 stimulations per task. Thereby, the coil was placed in a strictly anterior-posterior field orientation tangentially to the skull (Epstein et al. 1996; Lioumis et al. 2012; Wassermann et al. 1999). We accepted minimum field strength of 55 V/m at the region of interest; across subjects, the field strength raged from 55 to 80 V/m.
The localization of language-positive points was described by Corina et al.’s cortical parcellation system (CPS) (Corina et al. 2005).
Data analysis
Video analysis of all mapping sessions was conducted as described previously (Lioumis et al. 2012; Picht et al. 2013; Sollmann et al. 2013b). The analysis was blinded to subject, as well as to stimulated cortical spots and previous results. We directly compared any language impairment to the baseline. To describe language impairment as precise as possible, we divided the evoked errors into different error categories: No response errors, performance errors, hesitations, neologisms, semantic paraphasias, phonological paraphasias (for detailed description see (Corina et al. 2010; Sollmann et al. 2013a)), and nominalizations. We documented nominalization errors during the verb generation and action naming task, when volunteers were not able to find the appropriate verb, but the noun. The error category “all errors” included all error types. We considered a spot as language positive, if at least one out of the three stimulations per spot evoked any type of error. Thereby, we excluded errors attributed to pain or muscle stimulation. Errors were categorized in “1/3 error” (at least one induced language error out of three stimulations), “2/3 error” (at least two induced language error out of three stimulations), and “3/3 error” (all three stimulations induced a language error). For comparison of tasks and the correlation of rTMS with fMRI-positive brain volume, we calculated error rates. The error rate resulted from the number of elicited language errors per number of stimulations and was expressed as a percentage value.
Creation of 3D maps of responsive rTMS spots
Each stimulation spot was represented by a cascade of three spots in increasing cortical depth from 0 mm to 10 mm below cortical surface, corresponding to variable possible stimulation depth. For each task three maps in subject space representing spots with one, two, and three errors (out of three applications) elicited by rTMS were created. For single subject analysis (see 2.6.2), images were smoothed in order to compensate for discrete positions of rTMS-stimulation sites which are commonly about (14 ± 5) mm apart. A 10 mm Gaussian kernel was sufficient to create overlap between representations of the spots. Spot images were in register with T1-weighted images used for rTMS stimulation, which were co-registered to the T1-weighted image acquired with the fMRI data for each subject with SPM 8.0 (http://www.fil.ion.ucl.ac.uk/spm/) on Matlab 2007b (http://www.mathworks.de/).
fMRI language mapping
Paradigm
Before scanning started, the subject received a detailed introduction and had enough time to practice, until he/she was able to perform the task correctly. Within the scanner, tasks were communicated to the subject as follows: a screen was fixed at the end of the gantry, which the subject was able to see via a mirror attached to the head coil. The paradigm was shown using a beamer, which was placed in the adjoining room. The paradigm was programmed in “Presentation” (http://www.neurobs.com).
The paradigm for the language activation comprised one fMRI run per paradigm. Activation period lasted 16 dynamics (32 s) with an intermittent resting period of 14 dynamics with an empty display. On the whole, each task type was presented 8 times with two additional scans before the first task. A monitoring of the subjects’ compliance was not possible, but intrinsic motivation was high.
Image acquisition
All subjects received four runs of task-fMRI, a T1 weighted 3-dimensional sequence at a 3 T Philips Achieva MRI scanner (Philips Medical Systems, the Netherlands) with a sense-head-8 coil. To acquire the fMRI data, an EPI gradient echo sequence was utilized. Imaging parameters were the following: TR/TE = 2000/30 ms, 90° flip angle, 64 × 64 matrix, 192 mm FOV, 37 slices, 3 mm slice thickness with 10% inter-slice gap, consisting in 242 dynamics. The 3-dimensional gradient echo T1 weighted sequence was conducted to assess the underlying anatomy. Imaging parameters were the following: TR/TE = 9/4 ms, flip angle 8°, FOV 240 mm, 170 slices, 1 mm isovoxel without gap.
Preprocessing and statistical analysis
The imaging datasets acquired were preprocessed using SPM 8.0 (http://www.fil.ion.ucl.ac.uk/spm/) on matlab 2007b (http://www.mathworks.de/). Discarding of three initial images was automatically performed by the scanner. The EPI data of the task fMRI were realigned and unwarped, to account for residual head motion of the subjects in the scanner and the 3D T1 images were co-registered to the EPI series (register with rTMS spot maps was maintained). Subsequently EPI volumes were smoothed with an 8 mm kernel (Friston et al. 1996; Jenkinson et al. 2002).
The statistical analysis was performed for each task separately. The onsets and duration of the condition were modeled in a General Linear Model (GLM) as blocks. Contrast images and t-maps for task versus baseline were calculated for each subject and task.
Fusion of rTMS and fMRI derived maps
Creation of group maps
In the next step, all images, including the statistical images from the GLM analysis and the rTMS-spot images, were normalized to standard MNI space (Montreal Neurological Institute, see SPM software) via the subjects’ anatomical images.
At the second level for each task, normalized contrast images were entered into a one-sample t-test within SPM.
Unsmoothed, but normalized rTMS spot images were averaged per error-rate they represent in order to create group images, which then were smoothed with a 16 mm Gaussian kernel according to the matched sampling theorem.
The overall procedure resulted in complete accordance of statistical functional, anatomical and rTMS-spot images in subject space, and of second level statistical images and normalized averaged rTMS spot images in standard space for group analysis.
Group-level analysis of distance between rTMS spot peaks and fMRI peaks
The coordinates of the significant fMRI peaks (p < 0.05, family-wise-error corrected for multiple comparisons) of the group analysis for each task as well as for the averaged rTMS spots in MNI space were analyzed in Euclidean space. After visualizing the fMRI clusters and the rTMS spots on a standard brain in the SPM viewer, one radiologist verified visually, if the rTMS spot was located within a significant fMRI cluster separately for each of the four tasks. The congruent rTMS spots for a specific task were matched to the fMRI clusters of the four differing tasks (task 1–4).
For the rTMS spots, which were located within the fMRI clusters, the Euclidean distance was calculated:
Thereby,‘x’ corresponds to the respective coordinate of the fMRI peaks and “y “to the respective coordinate of the rTMS peaks. The rTMS peaks, which showed an optical congruity with the fMRI clusters, had a Euclidean distance of <18 mm.
Quantitative dependence of fMRI activation significance on rate of regionally rTMS-elicited errors
Binarized maps for each task and each error rate (one to three errors elicited by rTMS) were used to mask the t-maps resulting from fMRI analysis for the respective task on a single subject basis. Average t-values were calculated across the masked t-maps. Mean and standard error of mean (SEM) across subjects were subsequently calculated. Additionally, the percentage of at least marginally activated voxels at a level of pu < 0.001, uncorrected for multiple comparisons, within the rTMS masks was calculated per error rate, task, and subject, and subsequently mean and SEM of this measure were calculated.
Creation of overlay maps for visual comparison
Overlay maps were created for each task separately for the frontal, the parietal and the temporal lobes of the left hemisphere. Thereby, frontal areas included the inferior frontal, the middle frontal, the superior frontal, and the precentral gyrus. Parietal areas consisted of the postcentral gyrus, the anterior and the posterior supramarginal gyrus, the superior parietal lobe, and the angular gyrus. Temporal areas involved the superior temporal and the middle temporal gyrus. Binary masks were created within the WFUpickatlas toolbox for SPM (http://fmri.wfubmc.edu/software/pickatlas). Group statistical maps are displayed within the respective lobe, along with the smoothed rTMS-spot maps of spots eliciting two and three errors, respectively. For visualization of the degree of congruence the top 20% of activations are shown, thereby focus is laid on peak positions.
Creation of fMRI and rTMS volumes
Group images per task of the t-maps from the random effects analysis, as well as those of two and three rTMS-elicited errors were masked with binary left-sided masks (International Consortium for Brain Mapping, ICBM) of the frontal, the parietal and the temporal lobes derived from the WFU-pickatlas toolbox (http://fmri.wfubmc.edu/software/pickatlas). Volumes of the fMRI activations were created by summing up the number of voxels surviving a threshold of pu < 0.001, uncorrected for multiple comparisons for the t-maps and multiplying by the voxel volume of 2 × 2 × 2 mm3. Volumes of the rTMS responsive areas were created by summing up the voxels surviving a constant arbitrary threshold serving to eliminate low rim values for the rTMS error maps, and also multiplying by the voxel size of 2 × 2 × 2 mm3. This was performed per task, per lobe and per map. The resulting volume data were subsequently available for trend calculations in terms of Pearson correlations towards each other and overall error rates.
Statistics
Representative correct baseline pictures, pain by visual analogue scale (VAS), and RMT were presented as mean ± standard deviation (SD). We used Friedman’s test for nonparametric matched groups for testing differences between the numbers of correctly named baseline pictures in each language task. Additionally, correlation between the baseline error rate of each task and the error rate during stimulation of that task was tested by nonparametric Spearman correlation with a two-tailed p value and 95% confidence interval. Differences among distribution of error rates per stimulation point in different tasks were tested using Friedman’s test followed by Dunn’s post hoc test. A value of p < 0.05 was considered significant (GraphPad Prism 6.0, La Jolla, CA, USA).
Results
General TMS results
Toleration of stimulation was well in 19 out of the 20 involved subjects. However, one participant suffered from intensive discomfort, nausea and perspiration during RMT determination via non-repetitive TMS. Because of those symptoms we interrupted the examination and renounced further investigations in this case.
Concerning rTMS language mapping, the highest error rate was detected during object naming. Verb generation and action naming generated fewer errors. rTMS combined with pseudoword reading barely evoked errors.
All error types evoked by rTMS for each language task are described in (Hauck et al. 2015b). Table 1 shows the percentage of participants in which stimulation induced an error in the respective stimulation point and demonstrates data for 1/3, 2/3, and 3/3 errors.
Correlation of fMRI and rTMS according to the 1/3, 2/3 and 3/3 rule
In order to assess the regional overlap of fMRI-visible task-specific activation with sensitivity to rTMS interference, we analyzed t-values of fMRI-activation at brain regions, which were weak or strongly affected by rTMS on a single subject basis. Table 2 shows that, as a general trend, areas in which rTMS reproducibly led to errors showed higher average t-values derived from fMRI. There is a correlation for pseudoword reading and verb generation tasks with t-values for 3/3 errors corresponding to pu < 0.001, uncorrected for multiple comparisons, on average across the whole smoothed rTMS spot map. In object naming averaged t-values for 3/3 errors clearly exceed corresponding values 1/3 and 2/3 errors. On the other hand, in action naming the trend of average t-values is clearly decreasing from 1/3 to 3/3 errors.
When analyzing the percentage overlap of area activated at pu < 0.001, uncorrected for multiple comparisons, in fMRI on top of the area sensitive to rTMS at the three levels the positive association of fMRI activation with increasing rTMS error rate is stronger. Trend in all tasks is positive, except again for action naming, in which it is mildly negative.
fMRI volume vs. rTMS error rate
Table 3 and Fig. 2 show that increased proneness of a task to rTMS interference is accompanied by a larger volume of significant fMRI activation in the left frontal, parietal as well as temporal lobes. Action naming takes a special role in that fMRI shows a large number of activated regions, although comparatively few errors can be elicited by rTMS.
This figure shows that increased proneness of a task to rTMS interference is accompanied by a larger volume of significant fMRI activation in the left frontal (red), parietal (blue) as well as temporal (green) lobes. Action naming takes a special role in that fMRI shows a large number of activated regions, although comparatively few errors can be elicited by rTMS. R values: 0.6834, −0.1061, 0.1331, 0.4553; p-values: 0.3166, 0.8939, 0.8669, 0.5447
rTMS volume vs. fMRI volume
Table 4 and Fig. 3 show that in sum across the whole left hemisphere the rTMS volume increases well in correlation with the fMRI volume across tasks when investigating 3/3 errors. The dependence is less clear for 2/3 errors. When looking at the three lobes under consideration separately, the similar pattern shows for the parietal and temporal lobes. In the frontal lobe, though rTMS volumes are comparable to the other two lobes, fMRI volume is in general much larger and linear dependence for 3/3 errors is not present.
This figure shows that in sum across the whole left hemisphere (black) the rTMS volume increases well in correlation with the fMRI volume across tasks when investigating 2/3 (a) and 3/3 (b) errors. The dependence is less clear for 2/3 errors. When looking at the three lobes under consideration separately, the similar pattern shows for the parietal (blue) and temporal (green) lobes. In the frontal lobe (red), though rTMS volumes are comparable to the other two lobes, fMRI volume is in general much larger and linear dependence for 3/3 errors is not present. R values: −0.1649, 0.9892, 0.8447, 0.9184; p-values: 0.8351, 0.0108, 0.1553, 0.0816
Peak distances
The Euclidean distances were calculated between the coordinates (x,y,z) of the fMRI clusters’ peaks and the rTMS-spots (2/3 and 3/3 errors), averaged for all subjects as described in 2.6.1. After visualization of the rTMS spots of 1/3 errors and the fMRI clusters, overlaid to a normalized brain, we desisted from analyzing the distances due to a visually wide spreading of the rTMS spots.
Analyses confirmed the visual impression of a close location between several rTMS-spots (2/3 and 3/3 errors) and fMRI clusters in the 3-dimensional room accentuated in the frontal lobe, followed by the parietal lobe and less in the temporal lobe. A total of 40 fMRI clusters (p ≤ 0.05) were compared to 61 rTMS spots. Out of these, 27 rTMS spots had an Euclidean distance of ≤18 mm compared to 16 fMRI clusters. There was a significant difference between the rTMS spots of 1/3 errors and the ones of 2/3 and 3/3 errors together, in comparison to the fMRI clusters in terms of a higher congruence for 2/3 and 3/3 errors. No significant difference could be detected between the subgroups of 2/3 errors compared to the fMRI clusters and the ones of 3/3 errors. While conferring the rTMS spots separately for each task, some coordinates of the rTMS spots stood out to be highly congruent to the fMRI clusters of any of the tasks. Analogously some fMRI clusters showed a stable recurrence for each task (Table 5).
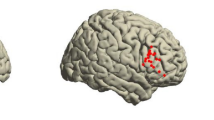
Object naming
Analyzing the task ‚object naming’, seven fMRI clusters (p ≤ 0.05) were compared to 17 rTMS spots (Fig. 4). Out of these, seven rTMS spots were closely located to four fMRI clusters (Euclidean distance ≤18 mm). Most conformity appeared to be in the frontal lobe, followed by the parietal and the temporal lobe (Table 6).
Pseudoword reading
Analyzing the task ‚pseudoword reading’, nine fMRI clusters (p ≤ 0.05) were compared to eight rTMS spots (Fig. 5). Out of these, four rTMS spots were closely located to three fMRI clusters (Euclidean distance ≤18 mm). Most conformity appeared to be in the frontal lobe, followed by the parietal lobe. There were no conformities in the temporal lobe. (Table 7).
Verb generation
Analyzing the task ‚verb generation’, 12 fMRI clusters (p ≤ 0.05) were compared to 14 rTMS spots (Fig. 6). Out of these, eight rTMS spots were closely located to six fMRI clusters (Euclidean distance ≤18 mm). Most conformity appeared to be in the frontal lobe, followed by the parietal lobe. There were no conformities in the temporal lobe (Table 8).
Action naming
Analyzing the task ‚action naming’, twelve fMRI clusters (p ≤ 0.05) were compared to 22 rTMS spots (Fig. 7). Out of these, eleven rTMS spots were closely located to seven fMRI clusters (Euclidean distance ≤18 mm). Most conformity appeared to be in the frontal lobe, followed by the parietal and the temporal lobe (Table 9).
Error categories
In the first step, which was just described in detail above, rTMS and fMRI clusters had been examined as a composite of all different language task types. Further analysis comprised the congruency of rTMS and fMRI clusters for each task type separately. Table 10 specifies analysis of spatial congruency of rTMS spots and fMRI clusters grouped into different language tasks. Results showed a moderate concordance in object naming, good concordance in pseudoword reading and moderate to good concordance in verb generation and action naming. No response answers had repeatable congruencies in the frontal areas for all language tasks, due to the inhibition of the precentral cortex. However, hesitation, as it was the most common type of error showed good to moderate congruencies in all types of language tasks. Discrepant results could be found especially in temporal regions. Neologism, phonological and semantic errors show less to none spatial congruencies in object naming and pseudoword reading.
Discussion
General results
This is the first study to compare the two most broadly used methods of non-invasive language mapping in a considerably large cohort. With neurovascular coupling and direct neuronal inhibition in terms of a ‘virtual lesion’, both methods utilize two totally different approaches to reveal language-related cortex.
As a result of this comparison, the best correlation was observed between 3/3 errors and fMRI for pseudoword reading and verb generation with t-values of pu < 0.001, uncorrected for multiple comparisons, on average across the whole rTMS-spot map. There is a close spatial agreement between several rTMS-spots (2/3 and 3/3 errors) and fMRI clusters accentuated in the frontal lobe, followed by the parietal lobe and less in the temporal lobe. There was a higher congruence for 2/3 and 3/3 errors than for 1/3 errors compared to the fMRI clusters.
Table 10 shows the analysis of the spatial congruency of rTMS spots and fMRI clusters grouped into different language tasks. According to the results mentioned above higher concordance could be found in frontal and parietal lobes and less concordance in temporal lobes. Concerning different language tasks there was moderate concordance in object naming, good concordance in pseudoword reading and moderate to good concordance in verb generation and action naming. No response answers showed repeatable congruencies in the frontal areas for all language tasks, due to the inhibition of the precentral cortex. However, hesitation, as it was the most common type of error showed good to moderate congruencies in all types of language tasks. Discrepant results could be found especially in temporal regions. Neologism, phonological and semantic errors show less to none spatial congruencies in object naming and pseudoword reading.
fMRI
FMRI is absolutely non-invasive in patients matching the requirements for MRI scanning. Mapping of language function is more challenging than e.g. motor function as it is more variable across individuals and of higher complexity (Bookheimer 2007). Nevertheless, it has been shown that language function can be localized with high reliability of over 79% in terms of presence of activation across patients (Van Westen et al. 2005). Activations elicited by object naming, verb generation, and action naming tasks were repeatedly detected in left frontal areas in agreement with our results (Roux et al. 2003; Giussani et al. 2010; Price et al. 1996; Murtha et al. 1999; Kiyosawa et al. 1996; FitzGerald et al. 1997).
Several studies already compared fMRI activation patterns of language tasks with direct cortical stimulation with varying results (Bookheimer 2007; Giussani et al. 2010). Of course, these studies were carried out in patient collectives. Especially the aspect of sensitivity of detecting language relevant areas takes an important role when using fMRI in pre-surgical diagnostics for location of language function reliably (Bookheimer 2007). Compared to DCS results sensitivity values of fMRI investigations were above 80% with the exception of 59% in 3 cases of meningioma. However, the authors of a larger meta-analysis state that due to the extreme range in sensitivity and specificity between the different trials, fMRI cannot be recommended for pre-surgical mapping of language function in brain tumor patients (Giussani et al. 2010).
Navigated transcranial magnetic stimulation
TMS directly inhibits cortical function and does not use surrogate markers like fMRI. Combining TMS with a navigation system, its precision und usability for neuroscientific and neurosurgical applications gained tremendously. Thus, it now allows precise mapping of cortical function. Most importantly, there are major differences between TMS and other well-known functional brain imaging modalities such as fMRI and MEG. By inhibiting cortical areas TMS evokes an assessable response. Consequently, brain areas, which cause a response while being inhibited, are mandatory for the observed reaction. In contrast, fMRI and MEG identify all brain areas, which actually participate at a given specific task but do not distinguish essential from non-essential areas.
For motor mapping there is a high accuracy between nTMS and the DCS when calculating the distances between the respective hot spots or centers of gravity of a target muscle. But although nTMS and DCS both have severe methodological imprecision including navigational errors or brain shift, the distances between both methods was reported to be around 5 to 6 mm for the adductor pollicis brevis muscle (Tarapore et al. 2012; Picht et al. 2012; Krieg et al. 2013a). While nTMS motor cortical mapping is well established, rTMS language mapping is still developing. First studies on the correlation of preoperative rTMS language mapping and DCS during awake surgery showed a good overall correlation especially in true negatively mapped brain regions and thus a high negative predictive value (Picht et al. 2013; Krieg et al. 2014b).
Differences between fMRI and rTMS
The study does not state which of the two used modalities are the ground truth. We just investigated the correlation between both. Both methods are defined as equivalent. DCS as the current gold standard obviously cannot be used in healthy volunteers.
The subjects were actively performing the task during rTMS, while this was not the case for fMRI. During rTMS, we had the participants to speak out loud the asked term (overt speech). In fMRI investigations the subjects only had to think of the word (internal/covert speech) because of potential movement artifacts in the scanner during articulation. Previous studies have shown involvement of Broca’s area and the motor cortex both in overt and in covert speech (Aziz-Zadeh et al. 2005; Bookheimer 2002). Nevertheless, this fundamental difference in task performance might lead to inconsistencies between both techniques in certain brain areas (Riecker et al. 2000; Pei et al. 2011). Furthermore, although the subjects’ motivation was high, any lack of the subjects’ activity while lying in the scanner during fMRI could not be monitored.
Moreover, the different methodological approaches of both modalities have to be considered. BOLD contrast mechanism in fMRI and the lesion-based approach in rTMS are two fundamental different principles in the mapping of language function. BOLD contrast imaging relies on different magnetic properties of the oxygenated and deoxygenated forms of hemoglobin and thus is able to indicate task-specific brain activity (Ogawa et al. 1990). fMRI measures cerebrovascular effects related to local metabolic demand. This means that crucial neuronal activity might not be detected if not surpassing a required threshold for increased demand. As BOLD signal increase mainly relates to neuronal input rather than axonal output, it is biased towards areas receiving top-down influence and might indicate regions which are potentially involved in language but not essential for it (Binder et al. 2009; Viswanathan and Freeman 2007; Logothetis et al. 2001). rTMS, in contrast, inhibits single brain areas but is therefore unable to identify functional networks. These basically distinct methodological approaches imply a crucial disadvantage of fMRI compared to rTMS: in case of patients suffering from intracerebral lesions, the changing of metabolism, vessel reactivity and anatomy hamper the accuracy of fMRI (Giussani et al. 2010; Sollmann et al. 2013b; Ille et al. 2015a; Kuchcinski et al. 2015).
Another difference between rTMS and fMRI exists also regarding the ability of detecting language sub-function. fMRI studies task dependent cortical networks and does not provide any information about language sub-functions within one task. Activation in one area can only tell the investigator if this region is involved in language processing of the respective task or not. However, it provides no knowledge about the effect on language performance in case of damage of that region. Thus, in order to obtain information on language sub-function, several language paradigms are required (Gaillard et al. 2004).
Also in rTMS, as we can see in this study and in recently published results, distinct language tasks identify different regions involved in language performance (Hauck et al. 2015b). However, the examiner is furthermore able to make a statement about language sub-functions within one task by categorizing language disturbances into different error categories, which have already been established in previous invasive and non-invasive mapping procedures (Corina et al. 2005; Sollmann et al. 2013b).
On the other hand, one of the important advantages of fMRI is its ability to include the entire brain in its functional brain mapping. Against this, rTMS is unable to reach brain regions buried deep within sulci. Furthermore, stimulation has to be restricted due to induced pain in lower parts of the temporal lobe.
fMRI shows good spatial resolution which depends on the inherent smoothness of the data. This depends on intrinsic factors like signal-to-noise ratio and intrinsic hemodynamic response, but also on preprocessing strategies including spatial smoothing, in practice resulting in a resolution of (10 mm)3. The most prominent BOLD effect is shifted from the site of neuronal activity towards draining veins at the top of the cortical layers which introduces spatial uncertainty also in the range of centimeters (Kim and Ogawa 2012). Regarding the spatial resolution of rTMS, there exists still a lack of knowledge. Obviously, the distance of stimulation sites in our study dominates it. We accounted for spatial resolution of the methods by choice of smoothing kernels. The above-mentioned aspects highlight the rather good spatial agreement of both methods within our sample.
Because of the elemental differences in methods of both techniques, fMRI and rTMS will probably never come to the same results. We therefore suggest combining both modalities to obtain the most accurate information on language organization.
Differences between tasks in language location
Object naming task was shown in many reports on different mapping modalities to be reliable by involving various cortical and subcortical sub-functions of language (Petrovich Brennan et al. 2007; Indefrey 2011). Thus, object naming is the predominating task in awake surgery and has also been the mostly used task for rTMS studies (Lioumis et al. 2012; Picht et al. 2013; Krieg et al. 2013b; Tarapore et al. 2013; Hervey-Jumper et al. 2015). Moreover, according to recent studies on rTMS language mapping compared to awake surgery, limitations of rTMS seem to be predominantly in posterior perisylvian brain regions (Picht et al. 2013; Krieg et al. 2014b; Sollmann et al. 2015b). Keeping these aspects in mind, the other used language tasks of this study might be more suitable for posterior brain regions by requiring more semantic processing; at least for rTMS (Hauck et al. 2015b).
In fMRI the different tasks were compared to the rest state as a baseline condition. Thus, probably only a partial subtraction of language-related activation was achieved. Meaning that next to language-related areas, additional activations can be found in regions involved indirectly, like e.g. the visual cortices due to reading and areas of the verbal working memory system (Stark and Squire 2001).
Object naming
Object naming procedure starts with lemma retrieval for the visually recognized object. Therefore, access to semantic and phonological store is evident. The phonological information must then be transformed into suitable articulatory codes. Subsequently, motor command is initiated. Simplified, involved brain areas within object naming processing are MTG, which is associated with semantic and phonological representations, opIFG for syllabification, and vPrG for motor command initiation (Indefrey 2011).
Both the performed fMRI and the rTMS investigations consistently detected temporal areas, opIFG, pMFG, the postcentral gyrus, and SPL to be involved in object naming. pMFG as a part of the supplementary motor area is thereby attributed a role in planning and execution of motor commands for speech production (Alario et al. 2006). The postcentral gyrus is related to somato-sensory feedback (Peschke et al. 2009).
Areas significantly activated during fMRI examination were additionally areas that cannot be stimulated during rTMS with the current mapping protocol: the fusiform gyrus and the cerebellum. Within temporal sites, fMRI activation was mainly found in the temporal pole, which was shown to contribute to semantic processing (Tsapkini et al. 2011; de Zubicaray et al. 2006). Activation in SPL and in the fusiform gyrus during object naming was observed in earlier studies and was suggested to reflect lexical decisions and semantic access (Perani et al. 1999; Moore and Price 1999). Further, the cerebellum was also reported to be involved object naming, albeit less frequently than in verb naming (Saccuman et al. 2006).
Regarding language disruption during object naming in rTMS, additionally to the above named regions we found errors mainly in trIFG, mSFG, vPrG, vPoG, and mMTG within temporal regions. Errors in mMTG can be ascribed to lemma retrieval and selection, which is assumed to be located in this area (Indefrey and Levelt 2004). Recent studies suggested that information that is stored in temporal regions is then selected in SFG, so that SFG plays a role in semantic processing (Binder et al. 2009; Binder and Desai 2011). TrIFG as part of the inferior frontal gyrus participates in phonological and semantic decisions (Gold and Buckner 2002).
Pseudoword reading
Reading makes no overt demands on semantic systems, but may elicite an automatic semantic processing, depending on how meaningful the stimulus is (Binder et al. 1999). Generally, the pseudoword reading task seemed to be more vulnerable to fMRI than to rTMS. Hence, we presume that rTMS combined with a task relying on less automatic mechanisms is generally more suitable to detect language areas.
Concerning word and pseudoword reading, involved regions within the left hemisphere are the fusiform gyrus, the angular gyrus, and the middle temporal gyrus for lexical and semantic processing. Further, spelling-sound conversion is located in the inferior parietal gyrus. Phonological output is then initiated by the inferior frontal gyrus (Taylor et al. 2013).
Consistent areas detected by fMRI and rTMS during pseudoword reading are the left inferior frontal, the precentral, and the postcentral gyrus.
Regarding fMRI, further activation was found in the inferior occipital gyrus. Besides other left-hemispheric posterior regions, this area has repeatedly been observed to be evident for proper reading abilities (Shaywitz et al. 2002; Shaywitz and Shaywitz 2005). In addition, significant activation was revealed within the inferior parietal gyrus.
In contrast, rTMS evoked only few errors the inferior parietal gyrus, which disagrees with recent lesion study observations (Sanai and Berger 2008), and ventro-occipital areas like the inferior occipital gyrus could not be reached with sufficient stimulation intensity via rTMS. Areas, in which stimulation caused remarkable language disruption were (beside the above named) temporal regions like mMTG and pSTG, and the inferior frontal gyrus. pSTG was attributed a role as phonological storage in reading (Simos et al. 2002). Highest error rates were observed in Broca’s area, where phonological information is transformed into articulatory codes (Poldrack et al. 1999).
Verb generation
Verb generation is a task requiring semantic categorization and thus, making great demands on semantic processing. The subjects were asked to construct verbs out of visually demonstrated objects so that involved areas match widely with those in object naming (Ojemann et al. 2002). Generally, a majority of previous non-lesion based imaging investigations detected activation mainly in anterior regions during verb generation. Despite fMRI and rTMS revealed remarkable involvement of anterior regions, both techniques also found association of verb processing with posterior areas.
Consistent participating sites in fMRI and rTMS were determined within the IFG, mainly trIFG, and in the precentral gyrus. Also previous investigations attached great importance to IFG when producing a semantically suitable verb to the presented object (Bak et al. 2001; Buckner et al. 1995; Crescentini et al. 2010).
Verb generation during fMRI resulted further in substantial activation of the SMG within the parietal lobe, whereas rTMS did not evoke a considerable high error rate in this region. Parietal sites were detected as verb generation sites in several patients in an earlier study (Ojemann et al. 2002), and were suggested to be part of the lexico-semantic network of language production (Demonet et al. 1992). In return, rTMS induced frequent verb generation disruption in mMTG and MFG (mMFG and pMFG). MTG was attributed a role in semantic associations when naming tools or generating verbs (Martin et al. 1996; Allendorfer et al. 2012). MFG might be relevant for semantically driven word retrieval (Buckner et al. 1995). Additionally, as supplementary motor area it plays a role in initiation of language (Wise et al. 1991; Crosson et al. 2001).
Action naming
Action naming occupies an outstanding role within the applied tasks when comparing fMRI and rTMS. While other language tasks showed best accordance between both techniques when applying the 2/3 or 3/3 rule for rTMS, action naming revealed better correlation when using the 1/3 rule. Furthermore, in general, fMRI revealed wide distributed activation, whereas rTMS induced fewer errors during action naming than during object naming and verb generation. Also, an earlier electrostimulation study in brain tumor patients found naming interference more often in object than in action naming (Lubrano et al. 2014). An explanation for these observations could be that action naming requires different and more demands on the language processing network than other tasks like object naming (Matzig et al. 2009). Thus, fMRI measures activity within several brain regions and consequently shows high activity. Stimulation induced by rTMS, however, is applied focally and therefore might have less influence on action naming.
The underlying reasons for differences between localization of object and action naming have been widely investigated. Thereby, grammatical class effects as well as semantic differences have been discussed (Moseley and Pulvermuller 2014; Siri et al. 2008). As a limitation of this study, we are not capable to negate semantic correlations between lexical classes of noun and verbs.
Regions identified by both techniques are IFG, precentral gyrus, SFG, SMG, and SPL. Previous findings demonstrated that SPL was significantly more active when processing verbs compared to nouns (Shapiro et al. 2006; Perani et al. 1999). However, in the current study rTMS induced a higher overall error rate in SPL during object naming.
In fMRI action naming showed activation also in the fusiform gyrus. This region was reported to be involved in action naming, albeit it showed greater activation during object naming (Shapiro et al. 2006). rTMS, moreover, revealed higher error rates in temporal areas (mSTG, mMTG) and in the prefrontal cortex (mainly pMFG), which is in accordance with greater activity during verb production within those regions in earlier findings (Shapiro et al. 2006).
Stimulation protocol and task selection
Previous rTMS studies have shown that both the underlying language mapping protocol as well as the utilized language tasks are of great significance. Thus, different mapping setups and tasks might result in various mapping outcomes (Sollmann et al. 2015a; Hauck et al. 2015a, b).
We chose the above-described mapping protocol which has been used repeatedly in previous investigations and which appeared to be consistent and safe over the last years (Tarapore et al. 2013; Krieg et al. 2014b). As we achieved language errors in each subject while applying a frequency of 5 Hz, we decided to use constantly 5 Hz repetition rate in all cases.
Comparing rTMS with DCS, one of the main disadvantages of rTMS is the high number of false positive points (Krieg et al. 2014b). Therefore, the current study might be useful for future rTMS mapping protocols concerning the cut-off error rate for language positive sites. We obtained only few correlations between fMRI and rTMS when considering one out of three errors as language positive in rTMS. As a consequence, we rather suggest regarding a site as language positive when at least two out of three stimulations evoked any kind of error.
Mirroring different language sub-functions, we performed four language tasks. Essentially for this study, for better comparability of the two different mapping techniques, we used exactly the same language tasks for both methods. Besides the probably most frequently applied object naming task we selected tests that are commonly used in language assessment and easily to perform both during fMRI and rTMS. Furthermore, they all previously showed wide distributed cluster of activation in the left hemisphere (Vigliocco et al. 2011; Price 2012; Hagoort et al. 1999).
Limitations
Some limitations of the current study should be kept in mind. As far as we currently know from comparison studies of fMRI and intraoperative DCS, the positive predictive value of fMRI language mapping is considerably low (Giussani et al. 2010). Moreover, this corresponds to what we know about rTMS when compared to DCS (Picht et al. 2013; Ille et al. 2015a, c; Krieg et al. 2014b). Regarding the current study, when applying the 1/3 rule, language positive sites cover almost the entire lateral surface of the hemisphere. This fact renders the technique less informative for language mapping and plausibly contributes to the high number of false positive points compared to DCS, which has been observed in earlier studies. On the other hand, with the 3/3 rule there remains an insufficient number of language positive sites. Thus, the 2/3 rule seems to be the most suitable one for informative results. It furthermore delivered best accordance with fMRI results.
A further limitation of rTMS language mapping is that rTMS has limitations in terms of reachable brain regions, such as the lower parts of the temporal lobe since rTMS in these regions induces severe pain in most subjects. Therefore, these brain areas are not reachable for rTMS in most cases (Krieg et al. 2015; Sollmann et al. 2014). However, we were not able to find any more activation in these brain areas by fMRI in this study. Additionally, a further limitation of rTMS language mapping in general is that the analysis of language errors remains subjective to a certain degree (Sollmann et al. 2013a).
Clinical implications
During intraoperative DCS mapping, positive naming sites during object naming are defined as essential to language function so that their resection leads to any degree of aphasia (Haglund et al. 1994; Chang et al. 2011; Hervey-Jumper et al. 2015). While fMRI failed to show sufficient precision for preoperative mapping in brain tumor patients when compared to DCS, rTMS at least showed a high negative predictive value (Krieg et al. 2014b; Giussani et al. 2010). Another study revealed fMRI to be severely impaired in the proximity of intraparenchymal tumors which might explain these previous findings by the impairment of the BOLD signal (Ille et al. 2015a).
In combination with the results of this study, fMRI seems feasible to identify language-related brain areas in healthy brains and the results correlate well with rTMS. Yet, the presence of brain tumors still hampers the usefulness of BOLD-based detection of language sites and minor the clinical value of fMRI in brain tumor patient. Consequently, we need to investigate how the change BOLD data processing in order to reduce the tumor-induced bias and therefore increase its clinical usefulness.
Remarkable in the current study is the positive correlation of error susceptibility of one task during rTMS with fMRI volumes of that task–except for action naming. This indicates the general superior applicability of certain language paradigms compared to others. Nevertheless, language tasks should also be selected with respect to lesion location and language deficit in case of tumor patients.
A combination of appropriate tasks combined with fMRI and rTMS, which has also been suggested in previous patient and volunteer studies (Sack et al. 2009; Kononen et al. 2015) might very likely lead to most accurate language detection.
Conclusion
This study provides the first actually comparable data on the correlation of fMRI and nTMS and thus of BOLD signal and a lesion-based technique. Although some data already exists, this study used the same language tasks with the same picture sets in healthy volunteers without any impaired cortical function. Overall results of fMRI and rTMS correspond well although the degree of correlation depends on the used language task. Additionally, this study also shows the easy implementation of different tasks for language testing in an existing fMRI or rTMS setup.
Acknowlegdements
The first author gratefully acknowledges the support of the Graduate School’s Faculty Graduate Center of our university.
Abbreviations
- aMTG:
-
Anterior middle temporal gyrus
- CPS:
-
Cortical parcellation system
- DCS:
-
Direct cortical stimulation
- EEG:
-
Electroencephalography
- EPI:
-
Echo planar imaging
- fMRI:
-
Functional magnetic resonance imaging
- GLM:
-
General linear model
- ITG:
-
Inferior temporal gyrus
- MEG:
-
Magnetoencephalography
- NPV:
-
Negative predictive value
- nTMS:
-
Navigated transcranial magnetic stimulation
- orIFG:
-
Orbital part of the inferior frontal gyrus
- PET:
-
Positron emission tomography
- polIFG:
-
Polar inferior frontal gyrus
- polMFG:
-
Polar middle frontal gyrus
- polMTG:
-
Polar middle temporal gyrus
- polSFG:
-
Polar superior frontal gyrus
- polSTG:
-
Polar superior temporal gyrus
- PTI:
-
Picture to trigger interval
- RMT:
-
Resting Motor Threshold
- rTMS:
-
Repetitive navigated transcranial magnetic stimulation
- SEM:
-
Standard error of mean
- VAS:
-
Visual analogue scale
References
Alario, F. X., Chainay, H., Lehericy, S., & Cohen, L. (2006). The role of the supplementary motor area (SMA) in word production. Brain Research, 1076(1), 129–143. https://doi.org/10.1016/j.brainres.2005.11.104.
Allendorfer, J. B., Kissela, B. M., Holland, S. K., & Szaflarski, J. P. (2012). Different patterns of language activation in post-stroke aphasia are detected by overt and covert versions of the verb generation fMRI task. Medical Science Monitor, 18(3), CR135–CR137.
Aziz-Zadeh, L., Cattaneo, L., Rochat, M., & Rizzolatti, G. (2005). Covert speech arrest induced by rTMS over both motor and nonmotor left hemisphere frontal sites. Journal of Cognitive Neuroscience, 17(6), 928–938. https://doi.org/10.1162/0898929054021157.
Bak, T. H., O'Donovan, D. G., Xuereb, J. H., Boniface, S., & Hodges, J. R. (2001). Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain, 124(Pt 1), 103–120.
Barker, A. T., Jalinous, R., & Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. Lancet, 1(8437), 1106–1107.
Binder, J. R., & Desai, R. H. (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536, https://doi.org/10.1016/j.tics.2011.7001.
Binder, J. R., Frost, J. A., Hammeke, T. A., Bellgowan, P. S., Rao, S. M., & Cox, R. W. (1999). Conceptual processing during the conscious resting state. A functional MRI study. Journal of Cognitive Neuroscience, 11(1), 80–95.
Binder, J. R., Desai, R. H., Graves, W. W., & Conant, L. L. (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. https://doi.org/10.1093/cercor/bhp055.
Bookheimer, S. (2002). Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience, 25, 151–188. https://doi.org/10.1146/annurev.neuro.25.112701.142946.
Bookheimer, S. (2007). Pre-surgical language mapping with functional magnetic resonance imaging. Neuropsychology Review, 17(2), 145–155. https://doi.org/10.1007/s11065-007-9026-x.
Buckner, R. L., Raichle, M. E., & Petersen, S. E. (1995). Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. Journal of Neurophysiology, 74(5), 2163–2173.
Chang, E. F., Clark, A., Smith, J. S., Polley, M. Y., Chang, S. M., Barbaro, N. M., et al. (2011). Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: Improvement of long-term survival. Clinical article. Journal of Neurosurgery, 114(3), 566–573. https://doi.org/10.3171/2010.6.JNS091246.
Corina, D. P., Gibson, E. K., Martin, R., Poliakov, A., Brinkley, J., & Ojemann, G. A. (2005). Dissociation of action and object naming: evidence from cortical stimulation mapping. Human Brain Mapping, 24(1), 1–10. https://doi.org/10.1002/hbm.20063.
Corina, D. P., Loudermilk, B. C., Detwiler, L., Martin, R. F., Brinkley, J. F., & Ojemann, G. (2010). Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain and Language, 115(2), 101–112. https://doi.org/10.1016/j.bandl.2010.04.001.
Crescentini, C., Shallice, T., & Macaluso, E. (2010). Item retrieval and competition in noun and verb generation: an FMRI study. Journal of Cognitive Neuroscience, 22(6), 1140–1157. https://doi.org/10.1162/jocn.2009.21255.
Crosson, B., Sadek, J. R., Maron, L., Gokcay, D., Mohr, C. M., Auerbach, E. J., et al. (2001). Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. Journal of Cognitive Neuroscience, 13(2), 272–283.
de Zubicaray, G., McMahon, K., Eastburn, M., Pringle, A., & Lorenz, L. (2006). Classic identity negative priming involves accessing semantic representations in the left anterior temporal cortex. Neuroimage, 33(1), 383–390. https://doi.org/10.1016/j.neuroimage.2006.06.024.
Demonet, J. F., Chollet, F., Ramsay, S., Cardebat, D., Nespoulous, J. L., Wise, R., et al. (1992). The anatomy of phonological and semantic processing in normal subjects. Brain, 115(Pt 6), 1753–1768.
Epstein, C. M., Lah, J. J., Meador, K., Weissman, J. D., Gaitan, L. E., & Dihenia, B. (1996). Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology, 47(6), 1590–1593.
Felty, R. A. (2007). Context effects in spoken word recognition of English and German by native and non-native listeners. East Lansing: Michigan State University.
FitzGerald, D. B., Cosgrove, G. R., Ronner, S., Jiang, H., Buchbinder, B. R., Belliveau, J. W., et al. (1997). Location of language in the cortex: A comparison between functional MR imaging and electrocortical stimulation. AJNR. American Journal of Neuroradiology, 18(8), 1529–1539.
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., & Turner, R. (1996). Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355.
Gaillard, W. D., Balsamo, L., Xu, B., McKinney, C., Papero, P. H., Weinstein, S., et al. (2004). fMRI language task panel improves determination of language dominance. Neurology, 63(8), 1403–1408.
Giussani, C., Roux, F. E., Ojemann, J., Sganzerla, E. P., Pirillo, D., & Papagno, C. (2010). Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery, 66(1), 113–120. https://doi.org/10.1227/01.NEU.0000360392.15450.C9.
Gold, B. T., & Buckner, R. L. (2002). Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron, 35(4), 803–812.
Haglund, M. M., Berger, M. S., Shamseldin, M., Lettich, E., & Ojemann, G. A. (1994). Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery, 34(4), 567–576 discussion 576.
Hagoort, P., Indefrey, P., Brown, C., Herzog, H., Steinmetz, H., & Seitz, R. J. (1999). The neural circuitry involved in the reading of German words and pseudowords: a PET study. Journal of Cognitive Neuroscience, 11(4), 383–398.
Hauck, T., Tanigawa, N., Probst, M., Wohlschlaeger, A., Ille, S., Sollmann, N., et al. (2015a). Stimulation frequency determines the distribution of language positive cortical regions during navigated transcranial magnetic brain stimulation. BMC Neuroscience, 16, 5. https://doi.org/10.1186/s12868-015-0143-9.
Hauck, T., Tanigawa, N., Probst, M., Wohlschlaeger, A., Ille, S., Sollmann, N., et al. (2015b). Task type affects location of language-positive cortical regions by repetitive navigated transcranial magnetic stimulation mapping. PLoS One, 10(4), e0125298. https://doi.org/10.1371/journal.pone.0125298.
Hervey-Jumper, S. L., Li, J., Lau, D., Molinaro, A. M., Perry, D. W., Meng, L., et al. (2015). Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. Journal of Neurosurgery, 123(2), 325–339. https://doi.org/10.3171/2014.10.JNS141520.
Ille, S., Sollmann, N., Hauck, T., Maurer, S., Tanigawa, N., Obermueller, T., et al. (2015a). Impairment of preoperative language mapping by lesion location: a functional magnetic resonance imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation study. Journal of Neurosurgery, 123(2), 314–324. https://doi.org/10.3171/2014.10.JNS141582.
Ille, S., Sollmann, N., Hauck, T., Maurer, S., Tanigawa, N., Obermueller, T., et al. (2015b). Impairment of preoperative language mapping by lesion location: a functional magnetic resonance imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation study. J Neurosurg, 1–11, https://doi.org/10.3171/2014.10.JNS141582.
Ille, S., Sollmann, N., Hauck, T., Maurer, S., Tanigawa, N., Obermueller, T., et al. (2015c). Combined noninvasive language mapping by navigated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. [Comparative Study. Research Support, Non-U.S. Gov't]. Journal of Neurosurgery, 123(1), 212–225. https://doi.org/10.3171/2014.9.JNS14929.
Ille, S., Sollmann, N., Hauck, T., Maurer, S., Tanigawa, N., Obermueller, T., et al. (2015d). Combined noninvasive language mapping by navigated transcranial magnetic stimulation and functional MRI and its comparison with direct cortical stimulation. Journal of Neurosurgery, 1–14, https://doi.org/10.3171/2014.9.JNS14929.
Ilmoniemi, R. J., Ruohonen, J., & Karhu, J. (1999). Transcranial magnetic stimulation--a new tool for functional imaging of the brain. Critical Reviews in Biomedical Engineering, 27(3–5), 241–284.
Indefrey, P. (2011). The spatial and temporal signatures of word production components: A critical update. Frontiers in Psychology, 2, 255. https://doi.org/10.3389/fpsyg.2011.00255.
Indefrey, P., & Levelt, W. J. (2004). The spatial and temporal signatures of word production components. Cognition, 92(1–2), 101–144. https://doi.org/10.1016/j.cognition.2002.06.001.
Jenkinson, M., Bannister, P., Brady, M., & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), 825–841.
Kim, S. G., & Ogawa, S. (2012). Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. Journal of Cerebral Blood Flow and Metabolism, 32(7), 1188–1206. https://doi.org/10.1038/jcbfm.2012.23.
Kiyosawa, M., Inoue, C., Kawasaki, T., Tokoro, T., Ishii, K., Ohyama, M., et al. (1996). Functional neuroanatomy of visual object naming: a PET study. Graefe's Archive for Clinical and Experimental Ophthalmology, 234(2), 110–115.
Kononen, M., Tamsi, N., Saisanen, L., Kemppainen, S., Maatta, S., Julkunen, P., et al. (2015). Non-invasive mapping of bilateral motor speech areas using navigated transcranial magnetic stimulation and functional magnetic resonance imaging. Journal of Neuroscience Methods, 248, 32–40. https://doi.org/10.1016/j.jneumeth.2015.03.030.
Krieg, S. M., Shiban, E., Buchmann, N., Meyer, B., & Ringel, F. (2013a). Presurgical navigated transcranial magnetic brain stimulation for recurrent gliomas in motor eloquent areas. Clinical Neurophysiology, 124(3), 522–527. https://doi.org/10.1016/j.clinph.2012.08.011.
Krieg, S. M., Sollmann, N., Hauck, T., Ille, S., Foerschler, A., Meyer, B., et al. (2013b). Functional language shift to the right hemisphere in patients with language-eloquent brain tumors. PLoS One, 8(9), e75403. https://doi.org/10.1371/journal.pone.0075403.
Krieg, S. M., Sollmann, N., Hauck, T., Ille, S., Meyer, B., & Ringel, F. (2014a). Repeated mapping of cortical language sites by preoperative navigated transcranial magnetic stimulation compared to repeated intraoperative DCS mapping in awake craniotomy. BMC Neuroscience, 15, 20. https://doi.org/10.1186/1471-2202-15-20.
Krieg, S. M., Tarapore, P. E., Picht, T., Tanigawa, N., Houde, J., Sollmann, N., et al. (2014b). Optimal timing of pulse onset for language mapping with navigated repetitive transcranial magnetic stimulation. Neuroimage, 100C, 219–236. https://doi.org/10.1016/j.neuroimage.2014.06.016.
Krieg, S. M., Sollmann, N., Tanigawa, N., Foerschler, A., Meyer, B., & Ringel, F. (2015). Cortical distribution of speech and language errors investigated by visual object naming and navigated transcranial magnetic stimulation. Brain Structure and Function. https://doi.org/10.1007/s00429-015-1042-7.
Kuchcinski, G., Mellerio, C., Pallud, J., Dezamis, E., Turc, G., Rigaux-Viode, O., et al. (2015). Three-tesla functional MR language mapping: Comparison with direct cortical stimulation in gliomas. Neurology, 84(6), 560–568. https://doi.org/10.1212/WNL.0000000000001226.
Lioumis, P., Zhdanov, A., Makela, N., Lehtinen, H., Wilenius, J., Neuvonen, T., et al. (2012). A novel approach for documenting naming errors induced by navigated transcranial magnetic stimulation. Journal of Neuroscience Methods, 204(2), 349–354. https://doi.org/10.1016/j.jneumeth.2011.11.003.
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., & Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature, 412(6843), 150–157. https://doi.org/10.1038/35084005.
Lubrano, V., Filleron, T., Demonet, J. F., & Roux, F. E. (2014). Anatomical correlates for category-specific naming of objects and actions: a brain stimulation mapping study. Human Brain Mapping, 35(2), 429–443. https://doi.org/10.1002/hbm.22189.
Martin, A., Wiggs, C. L., Ungerleider, L. G., & Haxby, J. V. (1996). Neural correlates of category-specific knowledge. Nature, 379(6566), 649–652. https://doi.org/10.1038/379649a0.
Matzig, S., Druks, J., Masterson, J., & Vigliocco, G. (2009). Noun and verb differences in picture naming: past studies and new evidence. Cortex, 45(6), 738–758. https://doi.org/10.1016/j.cortex.2008.10.003.
Moore, C. J., & Price, C. J. (1999). Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage, 10(2), 181–192. https://doi.org/10.1006/nimg.1999.0450.
Moseley, R. L., & Pulvermuller, F. (2014). Nouns, verbs, objects, actions, and abstractions: local fMRI activity indexes semantics, not lexical categories. Brain and Language, 132, 28–42. https://doi.org/10.1016/j.bandl.2014.03.001.
Murtha, S., Chertkow, H., Beauregard, M., & Evans, A. (1999). The neural substrate of picture naming. Journal of Cognitive Neuroscience, 11(4), 399–423.
Neggers, S. F., Langerak, T. R., Schutter, D. J., Mandl, R. C., Ramsey, N. F., Lemmens, P. J., et al. (2004). A stereotactic method for image-guided transcranial magnetic stimulation validated with fMRI and motor-evoked potentials. Neuroimage, 21(4), 1805–1817. https://doi.org/10.1016/j.neuroimage.2003.12.006.
Ogawa, S., Lee, T. M., Kay, A. R., & Tank, D. W. (1990). Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proceedings of the National Academy of Sciences of the United States of America, 87(24), 9868–9872.
Ojemann, J. G., Ojemann, G. A., & Lettich, E. (2002). Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming. Journal of Neurosurgery, 97(1), 33–38. https://doi.org/10.3171/jns.2002.97.1.0033.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113.
Pascual-Leone, A., Gates, J. R., & Dhuna, A. (1991). Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology, 41(5), 697–702.
Pascual-Leone, A., Bartres-Faz, D., & Keenan, J. P. (1999). Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of 'virtual lesions'. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 354(1387), 1229–1238. https://doi.org/10.1098/rstb.1999.0476.
Paus, T. (1999). Imaging the brain before, during, and after transcranial magnetic stimulation. Neuropsychologia, 37(2), 219–224.
Pei, X., Leuthardt, E. C., Gaona, C. M., Brunner, P., Wolpaw, J. R., & Schalk, G. (2011). Spatiotemporal dynamics of electrocorticographic high gamma activity during overt and covert word repetition. Neuroimage, 54(4), 2960–2972. https://doi.org/10.1016/j.neuroimage.2010.10.029.
Perani, D., Cappa, S. F., Schnur, T., Tettamanti, M., Collina, S., Rosa, M. M., et al. (1999). The neural correlates of verb and noun processing. A PET Study, 122(12), 2337–2344. https://doi.org/10.1093/brain/122.12.2337.
Peschke, C., Ziegler, W., Kappes, J., & Baumgaertner, A. (2009). Auditory-motor integration during fast repetition: the neuronal correlates of shadowing. Neuroimage, 47(1), 392–402. https://doi.org/10.1016/j.neuroimage.2009.03.061.
Petrovich Brennan, N. M., Whalen, S., de Morales Branco, D., O'Shea, J. P., Norton, I. H., & Golby, A. J. (2007). Object naming is a more sensitive measure of speech localization than number counting: converging evidence from direct cortical stimulation and fMRI. Neuroimage, 37(Suppl 1), S100–S108. https://doi.org/10.1016/j.neuroimage.2007.04.052.
Picht, T., Mularski, S., Kuehn, B., Vajkoczy, P., Kombos, T., & Suess, O. (2009). Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery, 65(6 Suppl), 93–98.
Picht, T., Schulz, J., Hanna, M., Schmidt, S., Suess, O., & Vajkoczy, P. (2012). Assessment of the influence of navigated transcranial magnetic stimulation on surgical planning for tumors in or near the motor cortex. Neurosurgery, 70(5), 1248–1256; discussion 1256-1247. https://doi.org/10.1227/NEU.0b013e318243881e.
Picht, T., Krieg, S. M., Sollmann, N., Rosler, J., Niraula, B., Neuvonen, T., et al. (2013). A comparison of language mapping by preoperative navigated transcranial magnetic stimulation and direct cortical stimulation during awake surgery. Neurosurgery, 72(5), 808–819. https://doi.org/10.1227/NEU.0b013e3182889e01.
Poldrack, R. A., Wagner, A. D., Prull, M. W., Desmond, J. E., Glover, G. H., & Gabrieli, J. D. (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage, 10(1), 15–35. https://doi.org/10.1006/nimg.1999.0441.
Price, C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage, 62(2), 816–847. https://doi.org/10.1016/j.neuroimage.2012.04.062.
Price, C. J., Wise, R. J., Warburton, E. A., Moore, C. J., Howard, D., Patterson, K., et al. (1996). Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain, 119(Pt 3), 919–931.
Riecker, A., Ackermann, H., Wildgruber, D., Dogil, G., & Grodd, W. (2000). Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport, 11(9), 1997–2000.
Rosler, J., Niraula, B., Strack, V., Zdunczyk, A., Schilt, S., Savolainen, P., et al. (2014). Language mapping in healthy volunteers and brain tumor patients with a novel navigated TMS system: evidence of tumor-induced plasticity. Clinical Neurophysiology, 125(3), 526–536. https://doi.org/10.1016/j.clinph.2013.08.015.
Rossi, S., Hallett, M., Rossini, P. M., & Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. https://doi.org/10.1016/j.clinph.2009.08.016.
Roux, F. E., Boulanouar, K., Lotterie, J. A., Mejdoubi, M., LeSage, J. P., & Berry, I. (2003). Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery, 52(6), 1335–1345 discussion 1345-1337.
Ruohonen, J., & Ilmoniemi, R. J. (1999). Modeling of the stimulating field generation in TMS. Electroencephalography and Clinical Neurophysiology. Supplement, 51, 30–40.
Ruohonen, J., & Karhu, J. (2010). Navigated transcranial magnetic stimulation. Neurophysiologie Clinique, 40(1), 7–17.
Saccuman, M. C., Cappa, S. F., Bates, E. A., Arevalo, A., Della Rosa, P., Danna, M., et al. (2006). The impact of semantic reference on word class: an fMRI study of action and object naming. Neuroimage, 32(4), 1865–1878. https://doi.org/10.1016/j.neuroimage.2006.04.179.
Sack, A. T., Cohen Kadosh, R., Schuhmann, T., Moerel, M., Walsh, V., & Goebel, R. (2009). Optimizing functional accuracy of TMS in cognitive studies: A comparison of methods. Journal of Cognitive Neuroscience, 21(2), 207–221. https://doi.org/10.1162/jocn.2009.21126.
Salmelin, R., Helenius, P., & Service, E. (2000). Neurophysiology of fluent and impaired reading: a magnetoencephalographic approach. Journal of Clinical Neurophysiology, 17(2), 163–174.
Sanai, N., & Berger, M. S. (2008). Mapping the horizon: techniques to optimize tumor resection before and during surgery. Clinical Neurosurgery, 55, 14–19.
Shapiro, K. A., Moo, L. R., & Caramazza, A. (2006). Cortical signatures of noun and verb production. Proceedings of the National Academy of Sciences of the United States of America, 103(5), 1644–1649. https://doi.org/10.1073/pnas.0504142103.
Shaywitz, S. E., & Shaywitz, B. A. (2005). Dyslexia (specific reading disability). Biological Psychiatry, 57(11), 1301–1309. https://doi.org/10.1016/j.biopsych.2005.01.043.
Shaywitz, B. A., Shaywitz, S. E., Pugh, K. R., Mencl, W. E., Fulbright, R. K., Skudlarski, P., et al. (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry, 52(2), 101–110.
Shepherd, F. A., Domerg, C., Hainaut, P., Janne, P. A., Pignon, J. P., Graziano, S., et al. (2013). Pooled analysis of the prognostic and predictive effects of KRAS mutation status and KRAS mutation subtype in early-stage resected non-small-cell lung cancer in four trials of adjuvant chemotherapy. Journal of Clinical Oncology, 31(17), 2173–2181. https://doi.org/10.1200/JCO.2012.48.1390.
Simos, P. G., Breier, J. I., Fletcher, J. M., Foorman, B. R., Castillo, E. M., & Papanicolaou, A. C. (2002). Brain mechanisms for reading words and pseudowords: an integrated approach. Cerebral Cortex, 12(3), 297–305.
Siri, S., Tettamanti, M., Cappa, S. F., Della Rosa, P., Saccuman, C., Scifo, P., et al. (2008). The neural substrate of naming events: effects of processing demands but not of grammatical class. Cerebral Cortex, 18(1), 171–177. https://doi.org/10.1093/cercor/bhm043.
Snodgrass, J. G., & Vanderwart, M. (1980). A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 6(2), 174–215.
Sollmann, N., Hauck, T., Hapfelmeier, A., Meyer, B., Ringel, F., & Krieg, S. M. (2013a). Intra- and interobserver variability of language mapping by navigated transcranial magnetic brain stimulation. BMC Neuroscience, 14, 150. https://doi.org/10.1186/1471-2202-14-150.
Sollmann, N., Picht, T., Makela, J. P., Meyer, B., Ringel, F., & Krieg, S. M. (2013b). Navigated transcranial magnetic stimulation for preoperative language mapping in a patient with a left frontoopercular glioblastoma. Journal of Neurosurgery, 118(1), 175–179. https://doi.org/10.3171/2012.9.JNS121053.
Sollmann, N., Tanigawa, N., Ringel, F., Zimmer, C., Meyer, B., & Krieg, S. M. (2014). Language and its right-hemispheric distribution in healthy brains: An investigation by repetitive transcranial magnetic stimulation. Neuroimage, 102(Pt 2), 776–788. https://doi.org/10.1016/j.neuroimage.2014.09.002.
Sollmann, N., Ille, S., Obermueller, T., Negwer, C., Ringel, F., Meyer, B., et al. (2015a). The impact of repetitive navigated transcranial magnetic stimulation coil positioning and stimulation parameters on human language function. European Journal of Medical Research, 20, 47. https://doi.org/10.1186/s40001-015-0138-0.
Sollmann, N., Tanigawa, N., Tussis, L., Hauck, T., Ille, S., Maurer, S., et al. (2015b). Cortical regions involved in semantic processing investigated by repetitive navigated transcranial magnetic stimulation and object naming. Neuropsychologia, 70, 185–195. https://doi.org/10.1016/j.neuropsychologia.2015.02.035.
Stark, C. E., & Squire, L. R. (2001). When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America, 98(22), 12760–12766. https://doi.org/10.1073/pnas.221462998.
Tarapore, P. E., Tate, M. C., Findlay, A. M., Honma, S. M., Mizuiri, D., Berger, M. S., et al. (2012). Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. Journal of Neurosurgery, 117(2), 354–362. https://doi.org/10.3171/2012.5.JNS112124.
Tarapore, P. E., Findlay, A. M., Honma, S. M., Mizuiri, D., Houde, J. F., Berger, M. S., et al. (2013). Language mapping with navigated repetitive TMS: proof of technique and validation. Neuroimage, 82, 260–272. https://doi.org/10.1016/j.neuroimage.2013.05.018.
Taylor, J. S., Rastle, K., & Davis, M. H. (2013). Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin, 139(4), 766–791. https://doi.org/10.1037/a0030266.
Tsapkini, K., Frangakis, C. E., & Hillis, A. E. (2011). The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain, 134(Pt 10), 3094–3105. https://doi.org/10.1093/brain/awr050.
Van Westen, D., Skagerberg, G., Olsrud, J., Fransson, P., & Larsson, E. M. (2005). Functional magnetic resonance imaging at 3T as a clinical tool in patients with intracranial tumors. Acta Radiologica, 46(6), 599–609.
Vigliocco, G., Vinson, D. P., Druks, J., Barber, H., & Cappa, S. F. (2011). Nouns and verbs in the brain: a review of behavioural, electrophysiological, neuropsychological and imaging studies. Neuroscience and Biobehavioral Reviews, 35(3), 407–426. https://doi.org/10.1016/j.neubiorev.2010.04.007.
Viswanathan, A., & Freeman, R. D. (2007). Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nature Neuroscience, 10(10), 1308–1312. https://doi.org/10.1038/nn1977.
Wassermann, E. M., Blaxton, T. A., Hoffman, E. A., Berry, C. D., Oletsky, H., Pascual-Leone, A., et al. (1999). Repetitive transcranial magnetic stimulation of the dominant hemisphere can disrupt visual naming in temporal lobe epilepsy patients. Neuropsychologia, 37(5), 537–544.
Wise, R., Chollet, F., Hadar, U., Friston, K., Hoffner, E., & Frackowiak, R. (1991). Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain, 114(Pt 4), 1803–1817.
Funding
The study was financed by institutional grants from the Department of Neurosurgery and the Section of Neuroradiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SK, FR, and BM are consultants for Brainlab AG (Munich, Germany). SK is consultant for Nexstim Plc (Helsinki, Finland).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hauck, T., Probst, M., Zimmer, C. et al. Language function shows comparable cortical patterns by functional MRI and repetitive nTMS in healthy volunteers. Brain Imaging and Behavior 13, 1071–1092 (2019). https://doi.org/10.1007/s11682-018-9921-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-018-9921-1