Abstract
Fibromyalgia (FMS) is a complex clinical syndrome that includes many symptoms beyond chronic pain. The studies that have addressed brain morphometry in FMS have had very heterogeneous results. Thus, the question of which specific FMS symptoms and clinical features—pain, but also psychological distress, sleep-related problems, health status, and medication intake—impact on brain morphometry remains open. Here, we wanted to determine if brain changes in FMS are “symptom-related” more than “diagnostic-related”. We performed an observational study of 46 premenopausal women (23 FMS patients and 23 age-matched healthy participants). Magnetic resonance images were analyzed using voxel-based morphometry and subcortical segmentation. We used multiple regression models to assess the associations between total and local brain volumes and FMS clinical characteristics. Furthermore, we calculated associations between subcortical structures’ shapes and volumes and FMS clinical characteristics. Larger psychological distress, anxiety, and sleepiness, and higher analgesic consumption accounted for 38 % of FMS patients’ smaller total gray matter volume (GMV). For both groups, local decrements of GMV in the medial orbitofrontal cortex were associated to larger psychological distress. Local increases of GMV were positively related to pain scores (superior frontal gyrus), psychological distress (cerebellum), anxiety (medial orbitofrontal cortex), and sleepiness (frontal superior medial cortex). FMS clinical characteristics were also associated to deformations in subcortical structures and volumes changes. This study reveals that total and local GMV changes in FMS go beyond the traditional “pain matrix” alterations. We demonstrated that brain morphology is altered by pain, but also by clinical characteristics that define the FMS experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic pain is a major public health problem, due to its high prevalence and clinical challenges (Goren et al. 2014). Fibromyalgia syndrome (FMS) is one of the most common chronic pain conditions. Depending on the diagnostic criteria used, prevalence rates range from 1.1 to 6.4 % of the general population (Branco et al. 2010; Nakamura et al. 2014; Vincent et al. 2013), with middle-aged and older women having higher rates (Branco et al. 2010; Clauw 2014). FMS is primarily characterized by chronic widespread musculoskeletal pain and multiple tender points (Wolfe et al. 1990) (see Table 1). However, other non-specific symptoms, such as psychological distress and sleep disturbances, are also very common (Wolfe et al. 2011) and distressing (Shillam et al. 2011). Additionally, patients usually have multiple comorbidities (Rehm et al. 2010).
Importantly, the frequent symptoms of allodynia and hyperalgesia might express a central pain processing disturbance (Woolf 2011). Therefore, since the pathogenesis of FMS is unknown, many efforts have been made to understand the central pain processing in these patients, as well as the brain changes in pain processing areas. Firstly, functional magnetic resonance imaging (fMRI) studies have provided evidence that FMS patients process pain differently than healthy controls (Schweinhardt and Bushnell 2010; Vachon-Presseau et al. 2013). For example, they rate experimental pain stimuli as more painful and, moreover, enhanced pain-evoked neural responses are also elicited. Similarly, non-painful stimuli in healthy subjects are considered as painful by FMS patients, and they activate relevant pain-related brain areas when they are applied (Gracely et al. 2002). Secondly, voxel-based morphometry (VBM) studies have shown morphometric brain changes among FMS patients, either in total volume or in specific areas (May 2011). Although pioneering research by Kuchinad and colleagues (2007) and more recent work by Jensen and colleagues (2013) found that FMS patients had significantly lower volumes in total gray matter volume (GMV) than healthy controls, most similar studies have not found significant differences between FMS patients and healthy controls (Burgmer et al. 2009; Ceko et al. 2013; Fallon et al. 2013; Hsu et al. 2009; Robinson et al. 2011). Among VBM studies focusing on local GMV differences, decrements and increments of cortical GMV have been found among FMS patients when they are compared to healthy controls (see Table 2), with certain level of agreement regarding a significant decrement of GMV in the anterior cingulate cortex (Burgmer et al. 2009; Ceko et al. 2013; Jensen et al. 2013; Kuchinad et al. 2007; Robinson et al. 2011; Wood et al. 2009). These heterogeneous results in VBM studies might be due to the small sample sizes assessed and some methodological inconsistencies among studies involving, for example, a high variability between participants (demographic and clinical features), as well as differences in the methods used (data acquisition and statistical analyses). Another explanation for this heterogeneity might come from the intrinsic diversity that characterizes the FMS population. Since the clinical profiles of these patients are quite variable, FMS might not constitute a single clinical entity (Rehm et al. 2010). Finally, studies showing significant morphological alterations to subcortical structures (including the bilateral basal ganglia, thalamus, hippocampus, and brainstem) in FMS patients are scarce (Fallon et al. 2013), even when it is known that subcortical structures are involved in pain processing (Bingel et al. 2002).
Despite the heterogeneity in the results from VBM studies in FMS, both in the direction of the differences and in the localization of the affected areas, it is reasonable to conclude that FMS is associated with structural alterations of the brain. Some studies agrees on the presence of structural changes in areas such as the cingulate cortex or the insula—known to be involved in pain regulation (Catani et al. 2013; Gasquoine 2013). All the studies presented in Table 2 had a cross-sectional design and no statement can be made regarding the cause of this structural (re)organization. However, some longitudinal evidence showed that brain structural changes might be subsequent to pain as those changes returned to a normal state when the pain was resolved (Henry et al. 2011). Therefore, some authors suggest that this “experience-dependent” (Kolb and Gibb 2014) central nervous system (CNS) reorganization is likely to reflect a “maladaptive plasticity” (May 2008). Similar to the structural brain changes produced during sensory learning (May et al. 2007), it might be that the continuous nociceptive activation provides a similar recurring stimulus input (i.e., pain-induced plasticity). However, the FMS experience includes symptoms other than pain (Müller et al. 2007) and CNS changes might be importantly shaped by other symptoms as well (May 2011).
Insights into the CNS reorganization associated with FMS symptoms are still lacking. Among the symptoms experienced in FMS (see Table 1), psychological distress, poor sleep quality, and subsequent daytime sleepiness are highly frequent and incapacitating (Diaz-Piedra et al. 2015; Goesling et al. 2013). Pioneering research (Burgmer et al. 2009; Hsu et al. 2009; Schmidt-Wilcke et al. 2007), trying to disentangle the effect of pain and psychological distress on brain morphology in FMS patients, has shown a partial CNS reorganization which is due to depression and/or anxiety independent of pain. On the other hand, other studies have found certain brain structural changes that could not be explained by depression in FMS (Schmidt-Wilcke et al. 2007). Additionally, despite the importance of sleep quality in relation to other FMS symptoms (Diaz-Piedra et al. 2014), the effect of sleep-related variables in altering brain structure among FMS patients has been neglected.
To summarize, although the primary symptom of FMS is pain, additional symptoms are also involved. Structural brain changes might be stimulus-dependent (Draganski et al. 2004) and, therefore, frequent FMS symptoms might have an active role in the pathophysiology of the syndrome. It is not clear, however, whether structural brain changes occur in relationship with other symptoms, other than pain, commonly reported in FMS patients and, if so, what is the specific impact of these symptoms on structural brain changes. Even when it is important to know which—and where—specific FMS symptoms alter brain structure (May 2011; Smallwood et al. 2013), the question remains open. We investigated, for the first time, the role that FMS symptoms (including pain) and clinical characteristics play in explaining changes in brain structure. We hypothesized that symptom severity other than pain intensity, such as psychological distress, anxiety, depression, sleep quality, and daytime sleepiness, as well as clinical variables (length of diagnosis and medication intake) would be related to morphological cortical and subcortical brain changes regardless of the diagnosis.
Methods
Participants
We performed an observational study on 48 Caucasian premenopausal women (from 74 potential participants who were interviewed for their possible inclusion in the study). This sample size is typical of MRI studies that do simple group comparisons (Table 2). The study was conducted at the Mind, Brain, and Behavior Research Center, Granada, Spain. Twenty-four FMS patients were referred from the Service of Rheumatology of the University Hospital Virgen de las Nieves (Granada, Spain). Twenty-four healthy controls were recruited from the local community by advertisements and word-of-mouth. No significant differences were observed in age, body mass index, marital status, and employment status between groups (all p-values > .05). Inclusion criteria for patients were female gender (pain responses differ between sexes (Mogil 2012)), the diagnosis of FMS performed by an experienced rheumatologist (MAG), following the American College of Rheumatology criteria for FMS (Wolfe et al. 1990), and premenopausal status. Inclusion criteria for healthy controls were that they were premenopausal women, had no history of chronic pain, no sleep disorders, and were not taking CNS medications. Subjects were excluded from both groups if they had any of the following: severe physical impairment; coexisting physical injury; comorbid medical illnesses (morbid obesity, autoimmune diseases, cardiopulmonary diseases, uncontrolled endocrine disorders, uncontrolled allergic disorder/asthma, cancer, and/or medical history of significant head injury or neurological disorder); existing psychopathological disorder (history of psychosis, current suicide risk—or attempt within 2 years of the study–, history of substance abuse, major depressive disorder); pregnancy; use of recreational drugs; alcohol consumption of more than 40 g per day (established as harmful consumption by the World Health Organization (Anderson et al. 2008)). Patients were not asked to alter or stop their medication prior to the study. One patient was excluded after the pregnancy test. The scan of one healthy control was excluded because of catastrophic artifacts in the MRI data (orthodontic brackets). The final sample was composed of 23 FMS patients and 23 healthy controls. Table 3 displays the sociodemographic descriptive statistics of the participants.
Psychological assessment
Sociodemographic and clinical data were collected through a semi-structured interview and questionnaires. The assessment was conducted by an expert psychologist (CDP) in the evaluation of people suffering from chronic pain and/or sleep disorders. During the interview, we obtained data regarding 1) FMS and comorbid conditions: length of diagnosis, level of disability due to FMS, medication consumption (name and frequency of use of prescription medicines actually consumed with or without prescription, as well as over-the-counter medicines), current treatments other than medication (e.g., physiotherapy, alternative medicine treatments,…), pain experience (severity, localization, and quality), and other symptoms suffered; 2) lifestyle habits (e.g., tobacco use, alcohol consumption); 3) depressive disorders and other non-psychotic psychopathological disorders, assessed using an adaptation of the structured clinical interview for DSM-IV disorders (First et al. 1999), and 4) sleep disturbances, assessed using an adaptation of the Insomnia Interview Schedule (Morin and Espie 2003). Finally, to assess symptoms severity, participants were asked to complete the validated Spanish versions of several self-reports and questionnaires: A visual analogue scale to assess pain intensity (VAS), the Hospital Anxiety and Depression Scale to assess psychological distress (HADS; Herrero et al. 2003), the State-Trait Anxiety Inventory to assess anxiety (STAI; Spielberger et al. 2011), the Beck Depression Inventory to assess depression (BDI; Sanz et al. 2005), the Pittsburgh Sleep Quality Index to assess sleep quality (PSQI; Carpenter and Andrykowski 1998; Royuela and Macías 1997), the Epworth Sleepiness Scale to assess daytime sleepiness (ESS; Ferrer et al. 1999), and the Fibromyalgia Impact Questionnaire to assess health status (physical function, disease impact and symptoms) (FIQ; Rivera and Gonzalez 2004) (see Table 4).
MRI procedures
MRI was performed on a 3T Phillips Achieva whole body MRI system (Philips Medical Systems, Best, The Netherlands) operating with an eight channel phased-array head coil for reception. For each participant, a T1-weighted 3D volume was acquired using a MPRAGE sequence in sagittal orientation with 0.94 × 0.94 × 1.0 mm resolution (160 slides, FOV = 240 × 240mm2, matrix: 256 × 256, repetition time: 8 ms; echo time: 4 ms; flip angle: 8°; fat saturation band with 191 HZ/pixel). The sequence was designed to optimize the reduction of magnetic field inhomogeneities and susceptibility artifacts.
Image processing
Voxel-based morphometry
T1 MRI-images were manually checked for morphological abnormalities or artifacts and aligned to the anterior-posterior commissures line. For data processing and analysis, we used SPM 8 processing pipelines (http://www.fil.ion.ucl.ac.uk/spm/). For the voxel-based morphometry of structural images, we used the DARTEL algorithm (Ashburner 2007). After brain tissue segmentation (Ashburner and Friston 2005), maps of gray matter (GM) and white matter (WM), were fed to DARTEL. DARTEL has proved to improve anatomical precision of alternative standard spatial normalization methods (Klein et al. 2009). Through an iterative procedure, it created optimized templates for the whole sample and the deformation field for each participant, which were later used for warping segments onto the new reference space. The GM and WM segments were resampled to 1.5 mm isotropic voxels, using trilinear interpolation and remapped onto the Montreal Neurological Institute (MNI) space using affine transformations. These volumes were scaled by the Jacobian determinants of the deformation fields, to consider the local compression and stretching inherent to warping and affine transformation processes. GM and WM maps were then smoothed by an 8 mm FWHM Gaussian kernel.
Subcortical segmentation and volumetric analysis
Seven subcortical structures (hippocampus, dorsal striatum [i.e., caudate nucleus and putamen], ventral striatum [i.e., accumbens nucleus], amygdala, globus pallidus, thalamus, and brainstem + 4th ventricle) were segmented for each woman’s scan using a semi-automated, model-based subcortical tool (FMRIB’s Integrated Registration and Segmentation Tool; FIRST v1.2) (Nugent et al. 2013; Patenaude et al. 2011) in FMRIB’s Software Library (FSL) version 4.1.4 (http://www.fmrib.ox.ac.uk/fsl) (Smith et al. 2004). Based on manually segmented models of the Center for Morphometric Analysis, Massachusetts General Hospital (Boston, MA), FIRST computed a mesh composed by a set vertices defined by adjacent triangles. Structures can be compared across subjects as the number of vertices for each structure is fixed, and the pose (rotation and translation) is removed, as they are aligned to a common space in several stages: 1) a 12° of freedom registration to the MNI152, 2) a 12° of freedom registration in which a mask is used to exclude voxels not belonging to the subcortical structure, 3) minimization of the sum-of-squares differences between subject’s vertices and average vertices (Patenaude et al. 2007). The radial distances of each vertex to the medial line of the nucleus allow for the examination of the expansions and contractions of the surface. This approach has been used both in manually and automated subcortical segmentation (Beacher et al. 2009; Becker et al. 2006; Madsen et al. 2010). Segmentations were visually checked for errors. The volume of each participant’s subcortical structures was measured in mm3, and bilateral brain volume values were used in subsequent analyses.
Statistical analysis
Sociodemographic and clinical variables were compared between groups using chi-square and two sample t-tests for categorical (educational, marital, and work statuses) and quantitative variables (age and body mass index), respectively. Pearson’s correlation coefficients were computed for the association between total gray and white matter volumes and clinical variables, including length of diagnosis and medication intake (number or different medications for each category: antidepressants, anxiolytics, opioids, aniline analgesics, nonsteroidal anti-inflammatory analgesics, and anticonvulsants) in order to determine possible covariates for successive analyses. Only analgesic consumption was related to brain volumes, and served as covariate in the next analyses together with questionnaire measures. Analyses of brain volumes were done on three levels: global, voxel-by-voxel, and subcortical structures.
Total gray matter volume (T-GMV) and total white matter volume (T-WMV) from FMS and control groups were submitted to two separate between subjects ANCOVA in which analgesic consumption, pain intensity (VAS), psychological distress (HADS), anxiety (STAI-T), daytime sleepiness (ESE), and health status (FIQ) served as covariates. Multiple imputations were used for 7 missing values (information about aniline analgesics intake from one patient and HADS, STAI and FIQ scores from two controls). The significant results reported were similar for imputed and non-imputed data. All statistical analyses were done using IBM SPSS (20.0, IBM Corporation, New York, USA).
To determine whether the set of predictors of T-GMV and T-WMV were also related to voxel volumes, we analyzed smoothed volumes using the generalized linear model approach implemented in SPM 8. We compared FMS and control groups voxel-by-voxel, using analgesic consumption, pain intensity (VAS), psychological distress (HADS), anxiety (STAI-T), daytime sleepiness (ESE), and health status (FIQ) as covariates. Statistical analyses were carried out with a corrected p-value on the cluster level (p < .05, computed from an uncorrected voxel level p < .001, with a cluster size of 420 contiguous voxels), as estimated by AlphaSim (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim) (Ward 2000) after a Monte Carlo simulation (5000 runs), in which the brain mask included cortical, subcortical, and cerebellum gray matter.
To evaluate the association between the shape of subcortical structures and pain intensity (VAS), psychological distress (HADS), anxiety (STAI-T), daytime sleepiness (ESE), and health status (FIQ), we calculated the partial correlation between those measures from each participant and each radial distance (distances from the medial line of the structure to each surface vertex) while controlling for age, marital status, work status, and total intracranial volume (TIV). Cluster-based permutation tests were performed (10,000 permutations) using the r p-max to obtain the empirical distribution of the correlation coefficients. This test was used to correct for multiple comparisons. Hierarchical forward stepwise multivariate regressions were also carried out to predict clinical measures from subcortical structures volumes. Psychological distress (HADS), anxiety (STAI-T), sleepiness (ESE), sleep quality (PSQI), pain intensity (VAS), and health status (FIQ) were used as dependent variables in those analyses. Confounders (block 1) were age, educational, marital, and work statuses, and TIV. Variables of interest (block 2) were subcortical volumes.
Results
Clinical data
FMS patients reported greater pain intensity, psychological distress, anxiety, depression, and somnolence than healthy controls, as well as poorer sleep quality and health status (all p-values < .05). Sample means and standard deviations for symptoms are shown in Supplementary Table 1.
Neuroimaging data: total volumes
T-GMV was larger for controls than for FMS patients (see Table 5), F(1,37) = 5.69, p < .03, adjusted R2 p = .13. We observed significant effects of psychological distress, F(1,37) = 12.10, p < .01, anxiety, F(1,37) = 10.62, p < .01, daytime sleepiness, F(1,37) = 6.14, p = .02, and analgesic consumption, F(1,37) = 11.96, p < .01 on T-GMV. Larger psychological distress, anxiety, daytime sleepiness, and higher consumption were associated to lower T-GMV. The whole model accounted for a significant amount of total variance in T-GMV (adjusted R2 p = .38). Analysis of residuals indicated they were normally distributed (minimum p-value for the Shapiro-Wilk estimates = .38), and showed equal variance for the control and FMS groups (0.99 vs 1.08, respectively), and no outliers were detected.
We observed no significant differences in T-WMV between the FMS and control groups (see Table 5), F(1,37) = 2.63, p = .11, adjusted R2 p = .07. However, there was a significant effect of psychological distress F(1,37) = 9.61, p < .01, anxiety F(1,37) = p < .01, and analgesic consumption, F(1,37) = 8.45, p < .01 on T-WMV. Larger psychological distress, anxiety, and higher consumption were associated to lower T-WMV. Analysis of residuals indicated they were normally distributed (minimum Shapiro-Wilk p = .10) and showed homogeneity of variances for the control and FMS groups (1.08 vs 1.01, respectively).
No variables were significantly associated to cerebrospinal fluid volume (all p-values > .05), which indicated that the above effects were specific for parenchyma volumes.
Neuroimaging data: local volumes
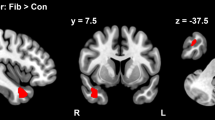
Higher pain intensity was associated with an increase of the left and right superior frontal gyrus (including parts of BA9 and BA10). Higher psychological distress was significantly associated to a decrease of the right and left medial orbitofrontal cortex (BA10, BA11) and an increase of the left and right cerebellum. Higher daytime sleepiness was associated to an increase of the right frontal superior medial cortex (BA9 and BA10) and a decrement of the right cerebellum. Anxiety was also associated to increased volumes in the left medial orbitofrontal cortex (BA10, BA11). None of the variables were related to changes in local WMV (all p-values > .05). Table 6 and Fig. 1 displays the main results observed at the voxel level in relation to symptoms. The comparison of local GMV between FMS patients and healthy controls can be found in the supplementary material.
Coronal (left column), sagittal (central column), and axial (right column) brain slices showing the areas exhibiting significant local gray matter volume (GMV) increments (red areas) and decrements (blue areas) associated to a pain scores, b and c distress, d anxiety, e and f sleepiness, and g analgesics consumption (all corrected p-values < .05). GMV changes are superimposed on a high-resolution T1-weighted template (generated by averaging all scans, N = 46). Table 7 shows the specific coordinates of the respective peaks
Neuroimaging data: subcortical structures shape and volume
Vertex analysis was performed to evaluate the relationship of the shape of the seven subcortical structures and clinical measurements. Subcortical analyses revealed surface contractions and enlargements of several subregions of those subcortical structures related to clinical measurements. Table 7 shows the specific locations and directions of those shape changes. The Video 1 shows in detail surface contractions and enlargements depending on symptoms.
Multiple regression models were performed to establish the relevance of subcortical volumes explaining clinical variables. Adjusted R2 are provided. The volumes of the left caudate nucleus and the right putamen accounted for 22.2 % of the variance of pain intensity; β = −.521, β = .335, respectively, F(2,43) = 7.43, p = .002. The volume of the right caudate nucleus accounted for 8.1 % of the variance of sleepiness (ESE); β = −.318, F(1,44) = 4.94, p = .031. The volume of the left caudate nucleus accounted for 22.8 % of the variance of sleep quality (PSQI); β = −.495, F(1,44) = 14.26, p < .001. Anxiety scores (STAI-T) were predicted by educational status (16.7 %) and the volume of the left hippocampus (10.1 %) (whole adjusted R2 = .268); β1 = −.380, β2 = −.344, F(2,43) = 9.25, p < .001. FIQ scores were predicted by educational status (10.1 %) and the volume of the left caudate nucleus (7.6 %) and the right putamen (6.6 %) (whole adjusted R2 = .243); β1 = −.153, β2 = −.468, β3 = .313, F(3,42) = 5.81, p = .002.
Discussion
Many human brain alterations are stimulus-dependent (Draganski et al. 2004), thus it is plausible to assume that FMS experience, beyond pain, might alter brain structure in patients. In the present study, for the first time, we used VBM to examine the specific effects of FMS clinical features on brain volumes. Our results show that psychological distress, anxiety, daytime sleepiness, and analgesic consumption account for structural brain differences in T-GMV and T-WMV between FMS patients and healthy controls. At a local level, for both groups, decrements of GMV in the medial orbitofrontal cortex are associated to higher psychological distress. Additionally, local increases of GMV in the superior frontal gyrus, the cerebellum, the medial orbitofrontal cortex, and the frontal superior medial cortex are positively related to pain intensity, psychological distress, anxiety, and sleepiness, respectively. Shape analyses also revealed surface contractions and enlargements related to FMS symptoms in subcortical structures.
Even though an increasing number of studies have examined brain morphology in FMS (see Table 2), the interpretation of past results is limited, the significance of brain structure changes in FMS patients being still under debate. It has been suggested that brain matter volumes reflect trait rather than state characteristics of the brain (Hsu et al. 2009). However, previous studies (e.g., May 2008) have provided evidence of causal paths between FMS and brain alterations, and suggested that brain structure changes in FMS reflects “experience-dependent” plasticity. Consequently, alterations in the “pain matrix” might follow the chronicity of pain (May 2011) and could be reversible (Rodriguez-Raecke et al. 2009). Furthermore, pain processing is based on complex neural networks which involve the sensory, cognitive, and emotional dimensions of pain (Melzack 1999). Thus, in the case of chronic pain syndromes, such as FMS, understanding the pathophysiology is a challenge. Moreover, the integration of experimental findings across studies is hard to undertake. Previous studies (see Table 2) have reported changes in brain volumes in FMS patients in a number of regions known to be critically involved in the modulation of pain experiences, but with disparate findings across studies. The very few consistencies appear in the cingulate (Burgmer et al. 2009; Ceko et al. 2013; Kuchinad et al. 2007; Robinson et al. 2011; Wood et al. 2009) and insular (Hsu et al. 2009; Kuchinad et al. 2007; Robinson et al. 2011) cortices, both of which being core “pain matrix” areas. One reason for these inconsistencies might arise at the statistical level. Many of the studies mentioned above (e.g., Burgmer et al. 2009; Hsu et al. 2009; Robinson et al. 2011; Wood et al. 2009) have selected a priori hypothesized regions. This statistical approach is useful for exploring MRI data, but it increases the probability of a type I error (Kriegeskorte et al. 2010). Therefore, we dismissed this approach. Furthermore, we controlled for menopausal status, a well-known factor that influences brain volumes (Takahashi et al. 2011) that is usually neglected.
FMS pathophysiology: the putative role of clinical symptoms in brain structure changes
Our findings are relevant to understand the possible mechanisms underlying chronic pain, with complex relationships between the chronic pain experience and structural brain changes. Thus, brain changes related to chronic pain might not be just the result of a continuous nociceptive stimulation (“acute pain that it is lasting too long”), but the consequence of the chronic pain experience. In line with Schmidt-Wilcke (2015), such brain reorganization might reflect the preeminent role of various CNS levels maintaining pain (even in the absence of the original nociceptive stimulation), rather than persistent peripheral nociceptive inputs leading to a chronic stimulation of the central pain system. Therefore, central mechanisms, including cortical processing of pain and other symptoms, might play a crucial role in pain chronification. This plausible working hypothesis had already been proposed—although not proven—by a recent meta-analysis about brain alterations in a diversity of chronic pain syndromes (Smallwood et al. 2013). Our results suggest that, actually, there are relevant structural brain alterations in FMS which are “symptom-related”. Not only pain intensity, but also FMS symptoms such as psychological distress, anxiety, and sleepiness are associated with changes in GMV.
Pain intensity
FMS patients experience persistent pain, as well as altered pain processing (Gracely et al. 2002; Wolfe et al. 1990). Moreover, chronic pain patients exhibit prefrontal hyperactivity to painful stimuli (Apkarian et al. 2001). Therefore, the association between a larger frontal pole area (BA 9/10) and pain intensity, as found in the present study, might be intrinsically related to the chronic pain experience. The prefrontal area is related to the sensory dimension of pain and receives nociceptive information from the cingulate gyrus (Treede and Apkarian 2010). Furthermore, the prefrontal area has been related to endogenous pain control (Schmahl et al. 2006), providing a decision making on stimulus value and the expected outcome (Marchand 2010). Besides, in the present study, we found an increment of GMV in parietal association areas (BA40), which is coherent with their involvement in functions related to the cognitive dimension of pain (Treede and Apkarian 2010)—pain-related memory and stimulus evaluation.
We also found subcortical contractions and enlargements related to pain intensity in multiple subcortical structures. fMRI studies support a relevant role for the basal ganglia in several aspects of pain processing in chronic pain (e.g., in FMS, Gracely et al. 2002, 2004). These aspects might include the integration of motor, emotional/affective, autonomic, and cognitive responses to pain (for a review, see Borsook and Becerra 2007; Borsook et al. 2010). Schmidt-Wilcke and colleagues (2007) already reported an increase in GMV in the striatum in FMS patients. We also found greater putamen volumes related to pain scores. These results might suggest some underlying functional alterations as a result of experiencing pain chronically. However, increments and decrements in other basal ganglia nuclei volumes, as well as their shapes, were also related to other symptoms in FMS.
Psychological distress
FMS patients suffer from repeated exposure to uncontrollable psychological distress, which is inherent to chronic pain (Weir et al. 2006). The affective dimension of pain goes beyond its immediate unpleasantness. High psychological distress usually involves an inability to effectively cope with the syndrome (Ridner 2004). This ineffective coping includes social isolation, reduced daily living and leisure activities, and the avoidance of physical activity. This absence of behavioral activation might underlie the decrements of GMV in frontal and prefrontal areas (BA10/11) described here. Additionally, although the cerebellum is not usually though as a chronic pain-related area, our results agree with those of previous studies relating the cerebellum to the affective domain of pain processing in FMS patients (Kim et al. 2015).
Anxiety
Pain-related anxiety and fear of pain has a modulating effect on FMS severity (Wolfe et al. 1990). In the present study, the medial orbitofrontal cortex (BA 10/11) had higher volume with greater anxiety levels, which seems coherent with its associated functions. The orbitofrontal cortex is implicated in fear extinction, specifically in expressing extinction memory and therefore reducing conditioned fear to a previously conditioned stimulus (Milad and Rauch 2007). Hence, increased activation in the orbitofrontal regions of anxiety disorder patients has been reported in symptom provocation studies (Adler et al. 2000). Moreover, recent evidence suggests a relationship between dopamine receptor availability in the orbitofrontal cortex and spontaneous pain in FMS patients (Albrecht et al. 2015).
Hippocampus volume and shape were related to anxiety scores. Previous studies already connected anterior hippocampus with anxiety, fear, and stress responses, as it receives extensive connections from the amygdala (whose shape changes are also related to anxiety scores) and hypothalamus (Fournier and Duman 2013).
Sleepiness
FMS patients show more sleep disturbances, which impair daytime performance (Diaz-Piedra et al. 2014). This implies that patients might have to improve their alertness, overcoming sleepiness, to exhibit better performance. The ability to overcome sleepiness in task execution has been previously related to the hyperactivation of the frontal cortex, specifically in the right prefrontal cortex (Honma et al. 2010). In line with this, in the present study, greater daytime sleepiness was related to GMV increments in right frontal areas (BA 9/10).
Daytime sleepiness and poor sleep quality were also related to changes in caudate nucleus shape and volume. This nucleus might be involved in the regulation of arousal and sleep. Specifically, the dorsal striatum might enhance wakefulness (Lazarus et al. 2012). In fact, a recent fMRI study found that patients with primary insomnia presented an impaired recruitment of the head of the left caudate nucleus during an executive functioning task (Stoffers et al. 2014).
Limitations
Although the current study sheds further light on the role played by clinical characteristics altering brain morphology in the FMS pathophysiology, there are some potential limitations to this work that should be considered. Firstly, because it is a cross-sectional study, it does not directly establish a causal relationship between FMS clinical characteristics and the brain volume changes reported. It is not clear how these changes develop over time, and whether they are reversible. Secondly, although our sample was relatively homogeneous and the matching with controls was carefully made, the generalizability of our results might be partially limited. Because our clinical sample was referred for FMS from the same rheumatologist who thoroughly checked the diagnosis criteria, our results cannot be generalized to non-referred samples (e.g., patients who are diagnosed and treated by primary care physicians). Further longitudinal work with larger samples is needed to answer these questions.
Conclusions
This study deepens our understanding of the brain changes associated with the chronic pain experience in FMS, and shows that those changes in total and local GMV changes are associated with pain scores, but also with distress, anxiety, sleepiness, and analgesic consumption. Therefore, many of the brain changes do not occur in the “pain matrix” areas, in which previous research has mostly focused on. This implies that altered brain morphology is associated to other frequent symptoms and clinical characteristics, and not exclusively to alterations of the ‘pain matrix’. From our results, we conclude that “maladaptive plasticity” is definitively related to continuous nociceptive activation, and as well as to continuous psychological distress and attempts to overcome daytime sleepiness. Future studies may focus on the specific therapies that may change the brain in a manner that corresponds with therapeutic effects.
References
Adler, C. M., McDonough-Ryan, P., Sax, K. W., Holland, S. K., Arndt, S., & Strakowski, S. M. (2000). fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. Journal of Psychiatric Research, 34(4–5), 317–324.
Albrecht, D. S., MacKie, P. J., Kareken, D. A., Hutchins, G. D., Chumin, E. J., Christian, B. T., & Yoder, K. K. (2015). Differential dopamine function in fibromyalgia. Brain Imaging and Behavior, 1–11. doi:10.1007/s11682-015-9459-4.
Anderson, P., Gual, A., & Colon, J. (2008). Alcohol y atención primaria de la salud. Informaciones clínicas básicas para la identificación y el manejo de riesgos y problemas. Washington DC: Pan American Health Organization.
Apkarian, A. V., Thomas, P. S., Krauss, B. R., & Szeverenyi, N. M. (2001). Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neuroscience Letters, 311(3), 193–197. doi:10.1016/S0304-3940(01)02122-X.
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. doi:10.1016/j.neuroimage.2007.07.007.
Ashburner, J., & Friston, K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. doi:10.1016/j.neuroimage.2005.02.018.
Beacher, F., Daly, E., Simmons, A., Prasher, V., Morris, R., Robinson, C., Lovestone, S., Murphy, K., & Murphy, D. G. M. (2009). Alzheimer’s disease and Down’s syndrome: an in vivo MRI study. Psychological Medicine, 39(04), 675–684. doi:10.1017/S0033291708004054.
Becker, J. T., Davis, S. W., Hayashi, K. M., Meltzer, C. C., Toga, A. W., Lopez, O. L., & Thompson, P. M. (2006). Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Archives of Neurology, 63(1), 97–101. doi:10.1001/archneur.63.1.97.
Bingel, U., Quante, M., Knab, R., Bromm, B., Weiller, C., & Büchel, C. (2002). Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain, 99(1–2), 313–321. doi:10.1016/S0304-3959(02)00157-4.
Borsook, D., & Becerra, L. (2007). Phenotyping central nervous system circuitry in chronic pain using functional MRI: considerations and potential implications in the clinic. Current Pain and Headache Reports, 11(3), 201–207. doi:10.1007/s11916-007-0191-7.
Borsook, D., Upadhyay, J., Chudler, E. H., & Becerra, L. (2010). A key role of the basal ganglia in pain and analgesia - insights gained through human functional imaging. Molecular Pain, 6(1), 27. doi:10.1186/1744-8069-6-27.
Branco, J. C., Bannwarth, B., Failde, I., Abello Carbonell, J., Blotman, F., Spaeth, M., Saraiva, F., Nacci, F., Thomas, E., Caubère, J. P., Le Lay, K., Taieb, C., & Matucci-Cerinic, M. (2010). Prevalence of fibromyalgia: a survey in five European countries. Seminars in Arthritis and Rheumatism, 39, 448–453.
Burgmer, M., Gaubitz, M., Konrad, C., Wrenger, M., Hilgart, S., Heuft, G., & Pfleiderer, B. (2009). Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosomatic Medicine, 71(5), 566–573. doi:10.1097/PSY.0b013e3181a32da0.
Carpenter, J. S., & Andrykowski, M. A. (1998). Psychometric evaluation of the Pittsburgh sleep quality index. Journal of Psychosomatic Research, 45, 5–13.
Catani, M., Dell’Acqua, F., & Thiebaut de Schotten, M. (2013). A revised limbic system model for memory, emotion and behaviour. Neuroscience & Biobehavioral Reviews, 37, 1724–1737. doi:10.1016/j.neubiorev.2013.07.001.
Ceko, M., Bushnell, M. C., Fitzcharles, M.-A., & Schweinhardt, P. (2013). Fibromyalgia interacts with age to change the brain. NeuroImage: Clinical, 3, 249–260. doi:10.1016/j.nicl.2013.08.015.
Clauw, D. J. (2014). Fibromyalgia. A clinical review. JAMA, 311(15), 1547–1555. doi:10.1001/jama.2014.3266.
Diaz-Piedra, C., Catena, A., Miro, E., Martinez, M. P., Sanchez, A. I., & Buela-Casal, G. (2014). The impact of pain on anxiety and depression is mediated by objective and subjective sleep characteristics in fibromyalgia patients. The Clinical Journal of Pain, 30(10), 852–859. doi:10.1097/AJP.0000000000000040.
Diaz-Piedra, C., Di Stasi, L. L., Baldwin, C. M., Buela-Casal, G., & Catena, A. (2015). Sleep disturbances of adult women suffering from fibromyalgia: a systematic review of observational studies. Sleep Medicine Reviews, 21, 86–99. doi:10.1016/j.smrv.2014.09.001.
Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., & May, A. (2004). Neuroplasticity: changes in grey matter induced by training. Nature, 427(6972), 311–312. doi:10.1038/427311a.
Fallon, N., Alghamdi, J., Chiu, Y., Sluming, V., Nurmikko, T., & Stancak, A. (2013). Structural alterations in brainstem of fibromyalgia syndrome patients correlate with sensitivity to mechanical pressure. NeuroImage: Clinical, 3, 163–170. doi:10.1016/j.nicl.2013.07.011.
Ferrer, M., Vilagut, G., Monasterio, C., Montserrat, J. M., Mayos, M., & Alonso, J. (1999). Measurement of the perceived impact of sleep problems: the Spanish version of the functional outcomes sleep questionnaire and the Epworth sleepiness scale. Medicina Clínica, 113(7), 250–255.
First, M., Spitzer, R., Gibbon, M., & Williams, J. (1999). Entrevista clínica estructurada para los trastornos del Eje I del DSM-IV (SCID-I) (versión clínica). Barcelona: Masson S. A.
Fournier, N. M., & Duman, R. S. (2013). Illuminating hippocampal control of fear memory and anxiety. Neuron, 77(5), 803–806. doi:10.1016/j.neuron.2013.02.017.
Gasquoine, P. G. (2013). Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neuroscience & Biobehavioral Reviews, 37, 340–348. doi:10.1016/j.neubiorev.2013.01.002.
Goesling, J., Clauw, D. J., & Hassett, A. L. (2013). Pain and depression: an integrative review of neurobiological and psychological factors. Current Psychiatry Reports, 15, 421. doi:10.1007/s11920-013-0421-0.
Goren, A., Mould-Quevedo, J., & daCosta DiBonaventura, M. (2014). Prevalence of pain reporting and associated health outcomes across emerging markets and developed countries. Pain Medicine. doi:10.1111/pme.12542.
Gracely, R. H., Petzke, F., Wolf, J. M., & Clauw, D. J. (2002). Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis & Rheumatism, 46(5), 1333–1343. doi:10.1002/art.10225.
Gracely, R. H., Geisser, M. E., Giesecke, T., Grant, M. A. B., Petzke, F., Williams, D. A., & Clauw, D. J. (2004). Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain: A Journal of Neurology, 127(Pt 4), 835–843. doi:10.1093/brain/awh098.
Henry, D. E., Chiodo, A. E., & Yang, W. (2011). Central nervous system reorganization in a variety of chronic pain states: a review. PM&R, 3, 1116–1125. doi:10.1016/j.pmrj.2011.05.018.
Herrero, M. J., Blanch, J., Peri, J. M., De Pablo, J., Pintor, L., & Bulbena, A. (2003). A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. General Hospital Psychiatry, 25(4), 277–283. doi:10.1016/S0163-8343(03)00043-4.
Honma, M., Soshi, T., Kim, Y., & Kuriyama, K. (2010). Right prefrontal activity reflects the ability to overcome sleepiness during working memory tasks: a functional near-infrared spectroscopy study. PLoS One, 5(9), e12923. doi:10.1371/journal.pone.0012923.
Hsu, M. C., Harris, R. E., Sundgren, P. C., Welsh, R. C., Fernandes, C. R., Clauw, D. J., & Williams, D. A. (2009). No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain, 143(3), 262–267. doi:10.1016/j.pain.2009.03.017.
Jensen, K. B., Srinivasan, P., Spaeth, R., Tan, Y., Kosek, E., Petzke, F., Carville, S., Fransson, P., Marcus, H., Williams, S. C., Choy, E., Vitton, O., Gracely, R., Ingvar, M., & Kong, J. (2013). Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis & Rheumatism, 65(12), 3293–3303.
Kim, H., Kim, J., Loggia, M. L., Cahalan, C., Garcia, R. G., Vangel, M. G., Wasan, A. D., Edwards, R. R., & Napadow, V. (2015). Fibromyalgia is characterized by altered frontal and cerebellar structural covariance brain networks. NeuroImage: Clinical, 7, 667–677. doi:10.1016/j.nicl.2015.02.022.
Klein, A., Andersson, J., Ardekani, B. A., Ashburner, J., Avants, B., Chiang, M.-C., Christensen, G. E., Collins, D. L., Gee, J., Hellier, P., Song, J. H., Jenkinson, M., Lepage, C., Rueckert, D., Thompson, P., Vercauteren, T., Woods, R. P., Mann, J. J., & Parsey, R. V. (2009). Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage, 46(3), 786–802. doi:10.1016/j.neuroimage.2008.12.037.
Kolb, B., & Gibb, R. (2014). Searching for the principles of brain plasticity and behavior. Cortex, 58, 251–260. doi:10.1016/j.cortex.2013.11.012.
Kriegeskorte, N., Lindquist, M. A., Nichols, T. E., Poldrack, R. A., & Vul, E. (2010). Everything you never wanted to know about circular analysis, but were afraid to ask. Journal of Cerebral Blood Flow & Metabolism, 30(9), 1551–1557. doi:10.1038/jcbfm.2010.86.
Kuchinad, A., Schweinhardt, P., Seminowicz, D. A., Wood, P. B., Chizh, B. A., & Bushnell, M. C. (2007). Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? The Journal of Neuroscience, 27(15), 4004–4007. doi:10.1523/JNEUROSCI.0098-07.2007.
Lazarus, M., Huang, Z.-L., Lu, J., Urade, Y., & Chen, J.-F. (2012). How do the basal ganglia regulate sleep–wake behavior? Trends in Neurosciences, 35(12), 723–732. doi:10.1016/j.tins.2012.07.001.
Lutz, J., Jäger, L., de Quervain, D., Krauseneck, T., Padberg, F., Wichnalek, M., Beyer, A., Stahl, R., Zirngibl, B., Morhard, D., Reiser, M., Schelling, G. (2008). White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis & Rheumatism, 58(12), 3960–3969. doi:10.1002/art.24070.
Madsen, S. K., Ho, A. J., Hua, X., Saharan, P. S., Toga, A. W., Jack, C. R., Jr., Weiner, M. W., & Thompson, P. M. (2010). 3D maps localize caudate nucleus atrophy in 400 Alzheimer’s disease, mild cognitive impairment, and healthy elderly subjects. Neurobiology of Aging, 31(8), 1312–1325. doi:10.1016/j.neurobiolaging.2010.05.002.
Marchand, W. R. (2010). Cortico-basal ganglia circuitry: a review of key research and implications for functional connectivity studies of mood and anxiety disorders. Brain Structure & Function, 215(2), 73–96. doi:10.1007/s00429-010-0280-y.
May, A. (2008). Chronic pain may change the structure of the brain. Pain, 137(1), 7–15. doi:10.1016/j.pain.2008.02.034.
May, A. (2011). Structural brain imaging: a window into chronic pain. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry, 17(2), 209–220. doi:10.1177/1073858410396220.
May, A., Hajak, G., Gänßbauer, S., Steffens, T., Langguth, B., Kleinjung, T., & Eichhammer, P. (2007). Structural brain alterations following five days of intervention: dynamic aspects of neuroplasticity. Cerebral Cortex, 17(1), 205–210. doi:10.1093/cercor/bhj138.
Melzack, R. (1999). From the gate to the neuromatrix. Pain, 82(Supplement 1), S121–S126. doi:10.1016/S0304-3959(99)00145-1.
Milad, M. R., & Rauch, S. L. (2007). The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences, 1121(1), 546–561. doi:10.1196/annals.1401.006.
Mogil, J. S. (2012). Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience, 13(12), 859–866. doi:10.1038/nrn3360.
Morin, C., & Espie, C. (2003). Insomnia - A clinical guide to assessment and treatment. New York: Springer.
Müller, W., Schneider, E. M., & Stratz, T. (2007). The classification of fibromyalgia syndrome. Rheumatology International, 27(11), 1005–1010. doi:10.1007/s00296-007-0403-9.
Nakamura, I., Nishioka, K., Usui, C., Osada, K., Ichibayashi, H., Ishida, M., Turk, D. C., Matsumoto, Y., & Nishioka, K. (2014). An epidemiologic internet survey of fibromyalgia and chronic pain in Japan. Arthritis Care & Research, 66(7), 1093–1101. doi:10.1002/acr.22277.
Nugent, A. C., Luckenbaugh, D. A., Wood, S. E., Bogers, W., Zarate, C. A., & Drevets, W. C. (2013). Automated subcortical segmentation using FIRST: test-retest reliability, interscanner reliability, and comparison to manual segmentation. Human Brain Mapping, 34(9), 2313–2329. doi:10.1002/hbm.22068.
Patenaude, B., Smith, S., Kennedy, D., & Jenkinson, M. (2007). Bayesian shape and appearance models. FMRIB technical report TR07BP1. Retrieved from http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.298.909&rep=rep1&type=pdf.
Patenaude, B., Smith, S. M., Kennedy, D. N., & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. doi:10.1016/j.neuroimage.2011.02.046.
Rehm, S. E., Koroschetz, J., Gockel, U., Brosz, M., Freynhagen, R., Tölle, T. R., & Baron, R. (2010). A cross-sectional survey of 3035 patients with fibromyalgia: subgroups of patients with typical comorbidities and sensory symptom profiles. Rheumatology, 49(6), 1146–1152. doi:10.1093/rheumatology/keq066.
Ridner, S. H. (2004). Psychological distress: concept analysis. Journal of Advanced Nursing, 45(5), 536–545. doi:10.1046/j.1365-2648.2003.02938.x.
Rivera, J., & Gonzalez, T. (2004). The fibromyalgia impact questionnaire: a validated Spanish version to assess the health status in women with fibromyalgia. Clinical and Experimental Rheumatology, 22(5), 554–560.
Robinson, M. E., Craggs, J. G., Price, D. D., Perlstein, W. M., & Staud, R. (2011). Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. The Journal of Pain, 12(4), 436–443. doi:10.1016/j.jpain.2010.10.003.
Rodriguez-Raecke, R., Niemeier, A., Ihle, K., Ruether, W., & May, A. (2009). Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. The Journal of Neuroscience, 29(44), 13746–13750. doi:10.1523/JNEUROSCI.3687-09.2009.
Royuela, A., & Macías, J. (1997). Propiedades clinimétricas de la versión castellana del cuestionario de Pittsburgh. Vigilia-Sueño, 9, 81–94.
Sanz, J., García-Vera, M. P., Espinosa, R., Fortún, M., & Vázquez, C. (2005). Adaptación española del Inventario para la Depresión de Beck-II (BDI-II): 3. Propiedades psicométricas en pacientes con trastornos psicológicos. Clínica y Salud, 16(2), 121–142.
Schmahl, C., Bohus, M., Esposito, F., et al. (2006). Neural correlates of antinociception in borderline personality disorder. Archives of General Psychiatry, 63(6), 659–666. doi:10.1001/archpsyc.63.6.659.
Schmidt-Wilcke, T. (2015). Neuroimaging of chronic pain. Best Practice & Research. Clinical Rheumatology, 29(1), 29–41. doi:10.1016/j.berh.2015.04.030.
Schmidt-Wilcke, T., Luerding, R., Weigand, T., Jürgens, T., Schuierer, G., Leinisch, E., & Bogdahn, U. (2007). Striatal grey matter increase in patients suffering from fibromyalgia – a voxel-based morphometry study. Pain, 132(Supplement 1), S109–S116. doi:10.1016/j.pain.2007.05.010.
Schweinhardt, P., & Bushnell, M. C. (2010). Pain imaging in health and disease — how far have we come? Journal of Clinical Investigation, 120(11), 3788–3797. doi:10.1172/JCI43498.
Shillam, C. R., Dupree Jones, K., & Miller, L. (2011). Fibromyalgia symptoms, physical function, and comorbidity in middle-aged and older adults. Nursing Research, 60(5), 309–317.
Smallwood, R. F., Laird, A. R., Ramage, A. E., Parkinson, A. L., Lewis, J., Clauw, D. J., Williams, D. A., Schmidt-Wilcke, T., Farrell, M. J., Eickhoff, S. B., & Robin, D. A. (2013). Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. The Journal of Pain: Official Journal of the American Pain Society, 14(7), 663–675. doi:10.1016/j.jpain.2013.03.001.
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E. J., Johansen-Berg, H., Bannister, P. R., De Luca, M., Drobnjak, I., Flitney, D. E., Niazy, R. K., Saunders, J., Vickers, J., Zhang, Y., De Stefano, N., Brady, J. M., & Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. doi:10.1016/j.neuroimage.2004.07.051.
Spielberger, C. D., Gorsuch, R. L., Lushene, R. E., & Seisdedos Cubero, N. (2011). STAI: Cuestionario de ansiedad estado-rasgo. Madrid: TEA.
Stoffers, D., Altena, E., van der Werf, Y. D., Sanz-Arigita, E. J., Voorn, T. A., Astill, R. G., Strijers, R. L., Waterman, D., & Someren, E. J. W. V. (2014). The caudate: a key node in the neuronal network imbalance of insomnia? Brain, 137(2), 610–620. doi:10.1093/brain/awt329.
Takahashi, R., Ishii, K., Kakigi, T., & Yokoyama, K. (2011). Gender and age differences in normal adult human brain: voxel-based morphometric study. Human Brain Mapping, 32(7), 1050–1058. doi:10.1002/hbm.21088.
Treede, R. D., & Apkarian, A. V. (2010). Nociceptive processing in the cerebral cortex. In The senses: A comprehensive reference (Vol. 5, pp. 669–697).
Vachon-Presseau, E., Roy, M., Martel, M.-O., Caron, E., Marin, M.-F., Chen, J., Albouy, G., Plante, I., Sullivan, M. J., Lupien, S. J., & Rainville, P. (2013). The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain: A Journal of Neurology, 136(Pt 3), 815–827. doi:10.1093/brain/aws371.
Vincent, A., Lahr, B. D., Wolfe, F., Clauw, D. J., Whipple, M. O., Oh, T. H., Barton, D. L., & St. Sauver, J. (2013). Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care & Research, 65(5), 786–792. doi:10.1002/acr.21896.
Ward, B. (2000). AlphaSim: Estimate statistical significance via Monte Carlo simulation — AFNI and NIfTI Server for NIMH/NIH/PHS/DHHS/USA/Earth [ReferenceManual]. Retrieved March 10, 2014, from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.
Weir, P. T., Harlan, G. A., Nkoy, F. L., Jones, S. S., Hegmann, K. T., Gren, L. H., & Lyon, J. L. (2006). The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases, 12(3), 124–128. doi:10.1097/01.rhu.0000221817.46231.18.
Williams, J. R. (2008). The declaration of Helsinki and public health. Bulletin of the World Health Organization, 86(8), 650–652. doi:10.1590/S0042-96862008000800022.
Wolfe, F., Smythe, H. A., Yunus, M. B., Bennett, R. M., Bombardier, C., Goldenberg, D. L., Tugwell, P., Campbell, S. M., Abeles, M., Clark, P., & Sheon, R. P. (1990). The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis & Rheumatism, 33(2), 160–172. doi:10.1002/art.1780330203.
Wolfe, F., Clauw, D. J., Fitzcharles, M.-A., Goldenberg, D. L., Häuser, W., Katz, R. S., Mease, P., Russell, A. S., Russell, I. J., & Winfield, J. B. (2011). Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. The Journal of Rheumatology, 38(6), 1113–1122. doi:10.3899/jrheum.100594.
Wood, P. B., Glabus, M. F., Simpson, R., & Patterson, J. C. (2009). Changes in gray matter density in fibromyalgia: correlation with dopamine metabolism. The Journal of Pain, 10(6), 609–618. doi:10.1016/j.jpain.2008.12.008.
Woolf, C. J. (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain, 152(3), S2–S15.
Acknowledgments
CDP was supported by a FPU grant from the Spanish Ministry of Education (AP 2007-02965) and is currently supported by a UGR Postdoctoral Fellowship (2013 University of Granada Research Plan). Research by CDP is funded by a CEI Biotic grant (V7-2015). Research by GBC is funded by a Spanish Ministry of Economy and Competitiveness grant (EDU2010-21215). Research by AC is funded by a Spanish Ministry of Economy and Competitiveness grant (PSI2012-39292).
We would like to thank Dr. L. L. Di Stasi (Mind, Brain, and Behavior Research Center, University of Granada, Spain) for his suggestions to improve the manuscript and his assistance designing the graphical material. We also thank Dr. M. B. McCamy (Division of Neurobiology, Barrow Neurological Institute, Phoenix, AZ, US) and R. Fernandez-Mendez (School of Health Sciences, University of Nottingham, UK) for his valuable advice and assistance in language edition.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Granada’s Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (Williams 2008). All participants gave written informed consent after a complete description of the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 59 kb)
Video 1
3-D models of subcortical structures showing shape changes related to pain intensity, psychological distress, anxiety, sleep quality, sleepiness, and health status. Red surface represents expansions, whereas blue one contractions
Rights and permissions
About this article
Cite this article
Diaz-Piedra, C., Guzman, M.A., Buela-Casal, G. et al. The impact of fibromyalgia symptoms on brain morphometry. Brain Imaging and Behavior 10, 1184–1197 (2016). https://doi.org/10.1007/s11682-015-9485-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-015-9485-2