Abstract
To clarify the changes in plant photosynthesis and mechanisms underlying those responses to gradually increasing soil drought stress and reveal quantitative relationships between photosynthesis and soil moisture, soil water conditions were controlled in greenhouse pot experiments using 2-year-old seedlings of Forsythia suspensa (Thunb.) Vahl. Photosynthetic gas exchange and chlorophyll fluorescence variables were measured and analyzed under 13 gradients of soil water content. Net photosynthetic rate (P N), stomatal conductance (g s), and water-use efficiency (W UE) in the seedlings exhibited a clear threshold response to the relative soil water content (R SWC). The highest P N and W UE occurred at R SWC of 51.84 and 64.10%, respectively. Both P N and W UE were higher than the average levels at 39.79% ≤ R SWC ≤ 73.04%. When R SWC decreased from 51.84 to 37.52%, P N, g s, and the intercellular CO2 concentration (C i) markedly decreased with increasing drought stress; the corresponding stomatal limitation (L s) substantially increased, and nonphotochemical quenching (N PQ) also tended to increase, indicating that within this range of soil water content, excessive excitation energy was dispersed from photosystem II (PSII) in the form of heat, and the reduction in P N was primarily due to stomatal limitation. While R SWC decreased below 37.52%, there were significant decreases in the maximal quantum yield of PSII photochemistry (F v/F m) and the effective quantum yield of PSII photochemistry (ΦPSII), photochemical quenching (q P), and N PQ; in contrast, minimal fluorescence yield of the dark-adapted state (F 0) increased markedly. Thus, the major limiting factor for the P N reduction changed to a nonstomatal limitation due to PSII damage. Therefore, an R SWC of 37.52% is the maximum allowable water deficit for the normal growth of seedlings of F. suspensa, and a water content lower than this level should be avoided in field soil water management. Water contents should be maintained in the range of 39.79% ≤ R SWC ≤ 73.04% to ensure normal function of the photosynthetic apparatus and high levels of photosynthesis and efficiency in F. suspensa.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photosynthesis is the foundation of plant growth and development and a decisive factor in productivity levels (Xu 2002). Because photosynthesis is highly sensitive to external environmental factors, plant growth and development and their relationship with various environmental factors can be evaluated by measuring the response of plant photosynthesis to environmental stresses (Anselmi et al. 2004). Soil drought is a common, major constraint of plant growth and development and is becoming increasingly severe and frequent in some areas as a result of climate change and thus seriously affecting photosynthesis (Ohashi et al. 2006; Smith et al. 2007; Li et al. 2011; Kebbas et al. 2015).
Numerous studies have investigated the mechanism by which drought stress affects plant photosynthesis (Lawson et al. 2003; Li et al. 2007; Lang and Wang 2015). In general, factors that limit plant photosynthesis under drought stress are either stomatal or nonstomatal, and can be evaluated as changes in the net photosynthetic rate (P N), intercellular CO2 concentration (C i), and stomatal limitation (L s) (Farquhar and Sharkey 1982; Xia et al. 2014). For example, a reduction of P N is attributed to stomatal limitation if C i decreases and L s increases with a decrease in soil water contents within a certain range; in the contrary situation, a reduction of P N is attributed to nonstomatal limitation (Wang et al. 2012; Xia et al. 2014; Lang and Wang 2015). Drought-induced P N decreases have also been suggested to be the joint result of stomatal and nonstomatal factors (Zhang et al. 2010). Despite these differing opinions, the response process and mechanism of plants photosynthesis in drought stress are in association with the genotype and growth stage of the plant, the degree of drought stress, and the selection of measurement indicators (Bray 1993; Lang and Wang 2015). The mechanism limiting photosynthesis under drought stress is complex, especially when drought reaches a certain severity. A simple analysis of leaf gas exchange parameters cannot fully reflect the extent of damage to the photosynthetic apparatus in the blade. As a consequence, there has been a lack of in-depth research on the internal mechanism by which drought stress affects photosynthesis.
Chlorophyll fluorescence can be regarded as an inherent probe for studying the relationship between plant photosynthesis and the environment (Krause and Weis 1991; Lazar 1999) and has been extensively adopted in research addressing the mechanisms underlying the responses of different vegetation types to environmental stress (Masrahi et al. 2015; Yan et al. 2015; Ware et al. 2015). Therefore, it is necessary to study leaf gas exchange and chlorophyll fluorescence parameters simultaneously to obtain an in-depth and systematic understanding of the mechanisms of how environmental stresses inhibits photosynthesis in different plants.
In face of global climate change, the volatility in soil water levels in North China is becoming increasingly more severe and may significantly affect the photosynthesis and growth of plants in the region. Forsythia suspensa (Thunb.) Vahl. is a drought-resistant species used in afforestation and as a common medicinal and ornamental shrub in North China. Research on F. suspensa has mainly focused on its medicinal value (Piao et al. 2008; Hao et al. 2010; Xia et al. 2011; Sung et al. 2016). In contrast, little research has addressed the effect of environmental adversity, such as soil drought stress, on the photo-physiological characteristics of F. suspensa. Nor has research addressed changes in variables in leaf photosynthesis and chlorophyll fluorescence when plants are exposed to a multistage continuous gradient in the soil water level. As a consequence, many physiological problems, including the process and mechanism of photosynthesis reduction in F. suspensa plants under increasing soil drought stress, remain unclear, as do their quantitative relationships with soil water content. In the present study on greenhouse pot experiments using 2-year-old seedlings of F. suspensa under controlled water conditions, we measured changes in leaf photosynthesis variables and chlorophyll fluorescence variables as soil water was gradually decreased. We also investigated the quantitative relationship between plant photosynthesis and soil moisture. This study provides a reference for achieving an in-depth systematic understanding of the effect of drought stress on the photophysiological variables in F. suspensa and guidance for drought-resistant afforestation and rational water management when using F. suspensa.
Materials and methods
Plant and soil
The 2-year-old F. suspensa seedlings selected as plant materials had an average height of 0.45 m and average basal diameter of 0.8 cm. On March 25, 2012, we selected 15 plants from a nursery and planted them in 15 pots with a depth of 60 cm and a diameter of 40 cm. The pots were placed in a controlled greenhouse at the Shandong Key Laboratory of Eco-Environmental Science for the Yellow River Delta, Shandong Province, China (37°23′12″N, 117°59′12″E). In the greenhouse, the light transmittance of the glass exceeded 90%; the average relative air humidity was 45 ± 6%; and the average atmospheric temperature was 25 ± 4 °C. The seedlings were grown under typical conditions for 30 days. On April 25, 2012, three seedlings exhibiting healthy growth of uniform size (plant height and basal diameter) were selected from the 15 plants for measurements of leaf photosynthesis and chlorophyll fluorescence.
Brown soil was collected from Heban Mountain, in Zouping County, Shandong Province. The average soil bulk density (ρ) was 1.25 g cm−3, and the average field water-holding capacity (F C) was 30.95%.
Soil water measurement
A multistage continuous gradient of soil water content was achieved by artificially supplying adequate water, with subsequent natural consumption of water by plants. The plants were irrigated with sufficient water to reach F C at 18:00 hours within the first 2 days (May 1, 2012) of the experiment. Beginning on the 3rd day (May 3, 2012), soil water was reduced through plant transpiration, and the volumetric soil water content (V SWC, %) was measured daily. The soil surface was covered with a plastic film to prevent natural evaporation. A water gradient with adequate water content was achieved based on daily measurements. During each measurement, at 08:00 hours every day, a 6050X3K1B Mini Trase Kit (Soil Moisture Equipment Corp., Santa Barbara, CA, USA) was used to measure V SWC at the 0–60 cm depth. The measurements were continued until leaf wilting and P N = 0. Three plants were measured at each soil water level; three sampling points were chosen for each plant; and three readings were recorded for each sampling point. The mean of the 27 V SWC readings was used as the V SWC of each treatment. The gravitational soil water content (G SWC, %) was calculated as the ratio of V SWC to ρ. The relative soil water content (R SWC, %) was calculated as the ratio of G SWC to F C. A total of 13 soil water levels was obtained, at R SWC of 92.97, 88.09, 73.04, 64.10, 58.96, 51.84, 42.55, 39.79, 37.52, 32.41, 30.44, 27.33, and 23.88%.
Photosynthesis measurements and analyses
Leaf photosynthesis was measured at the same time as the soil water measurements. Before the measurements, three healthy leaves were selected and marked for each plant, i.e., the 3rd to 4th leaves on the middle-upper branches. During each measurement, a LI-6400 portable photosynthetic system (LI-COR, Lincoln, NE, USA) was used to monitor the light response of photosynthesis in the marked leaves at 08:30–11:00 hours every day. The measurements were repeated three times for each leaf. The following variables were measured by the instrument automatically: P N, transpiration rate (E), stomatal conductance (g s), C i, and atmospheric CO2 concentration (C a). Water-use efficiency (W UE) was calculated as W UE = P N/E, and L s was calculated as L s = 1−C i/C a. For the measurement of the light response, the LED light of the photosynthesis system was used to set the photosynthetically active radiation (P AR) to 1800, 1600, 1400, 1200, 1000, 800, 600, 400, 200, 150, 50, 20, and 0 μmol m−2 s−1. Each P AR level was controlled for 120 s to obtain a stable value. A CO2 controller was employed to set the CO2 concentration to 375 ± 6.0 μmol mol−1.
The light saturation point (LSP) at each soil water level was calculated using the measured R SWC and P N data based on the modified rectangular hyperbola model (Ye 2007; Lang et al. 2013). We then obtained values for photosynthesis variables including P N, W UE, g s, C i, and L s at the LSP for each soil water level. Data were analyzed using Excel 2007 (Microsoft, Redmond, WA, USA), SPSS 20.0 (IBM SPSS, Armonk, NY, USA), and Matlab 2010a (MathWorks, Natick, MA, USA). SPSS 20.0 was employed to evaluate the significance of differences between groups through a one-way analysis of variance. The polynomial fittings for P N and W UE at the LSP in response to R SWC were performed using SPSS 20.0. P N and W UE were calculated using Matlab 2010a.
Chlorophyll fluorescence measurements and data analysis
Chlorophyll fluorescence was measured on the marked leaves when photosynthesis was measured. A pulse modulated fluorometer (FMS-2, Hansatech, Norfolk, UK) was applied at different soil water levels. The actual fluorescence (F s) at a P AR level of 1000 μmol m−2 s−1 was measured after 40 min of activation under natural light. Then, fluorescence during the saturating pulse at a steady state (F m′) was measured following an intense flash of light (5000 μmol m−2 s−1; pulse time, 0.7 s). Meanwhile, the leaf was covered, and the light source was turned off for 5 s, followed by dark adaptation for 3 s. Then, the minimal fluorescence yield of the light-adapted state after switching off the actinic light (F 0′) was measured after the far-red light was turned on for 5 s. The minimal fluorescence yield of the dark-adapted state (F 0) was measured after 30 min of dark adaptation. Then, the maximal fluorescence yield of the dark-adapted state (F m) was measured following an intense flash of light (5000 μmol m−2 s−1; pulse time, 0.7 s). F 0 and F m were measured in the early morning before dawn. During the measurements, the blade was evenly pressed in the leaf clamp, and the direction and angle of the probe were kept consistent to ensure that there was no shade and that the blade received light from the same direction. The following variables were calculated according to Roháček (2002): Maximal quantum yield of PSII photochemistry (F v/F m),
Effective quantum yield of PSII photochemistry (Φ PSII),
Nonphotochemical quenching (N PQ),
Photochemical quenching (q P),
where F 0 and F 0′ are the minimal fluorescence yields of the dark-adapted and light-adapted states, respectively; F m is the maximal fluorescence yield of the dark-adapted state; F m′ is the fluorescence during the saturating pulse in a steady state; F s is the actual fluorescence; F v/F m denotes the maximal quantum yield of PSII photochemistry; N PQ is the nonphotochemical quenching; q P is the photochemical quenching; Φ PSII is the effective quantum yield of PSII photochemistry.
Chlorophyll fluorescence varaibles at different soil water levels were analyzed using Excel 2007 and SPSS 20.0. The significance of the differences was evaluated through one-way analysis of variance using SPSS 20.0.
Results
Response of P N, g s and W UE in the leaves of F. suspensa to soil water
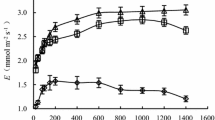
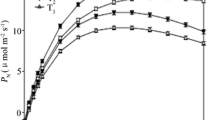
It is presented the threshold responses of P N, g s and W UE in the leaves of F. suspensa to the R SWC in Fig. 1. P N, g s, and W UE gradually increased with a decreasing R SWC within a certain range. After reaching a maximum, the three variables began to decrease with a further decrease of the R SWC. Both P N and g s peaked at R SWC of 51.84%, while the highest W UE was obtained at R SWC of 64.10% (Fig. 1a, b). P N and W UE showed relatively low values at R SWC ≤ 37.52%. At R SWC of 37.5%, P N was reduced by 66.2% compared with its maximum value (at R SWC = 51.84%), while W UE was reduced by 47.4% compared with its maximum (at R SWC = 64.10%). As P N and W UE are often used to characterize the photosynthetic productivity and efficiency, respectively, under different soil water conditions in trees (Zhang et al. 2012; Xia et al. 2013; Lang and Wang 2016), the response of P N and W UE to R SWC indicated that an excessively high or low soil water content, especially drought stress at an R SWC ≤ 37.52%, can reduce the productivity and efficiency in leaves of F. suspensa.
Effect of different soil water conditions on photosynthetic variables in leaves of F. suspense seedlings grown in pots in a greenhouse. Bars indicate ±SE of the mean, n = 27. P N is the net photosynthetic rate, g s is the stomatal conductance, W UE is the water-use efficiency, C i is the intercellular CO2 concentration, L s is the stomatal limitation, R SWC is the relative soil water content
Figure 2a, b illustrate the polynomial fittings of P N and W UE in response to R SWC. The response process of P N to R SWC is fitted well by the polynomial (5) (R 2 = 0.927):
where P N is the net photosynthetic rate, and R SWC is the relative soil water content. As a result, the average P N (\( \bar{P}_{\text{N}} \)) within the range of 23.9% ≤ R SWC ≤ 93.0% was 8.21 calculated by Eq. (6):
where P N is the net photosynthetic rate, and R SWC is the relative soil water content.
According to Eq. (5), within the experimental range of 23.9% ≤ R SWC ≤ 93.0%, when the average P N was 8.21, the corresponding R SWC was 39.5 and 80.0%. Similarly, the corresponding R SWC of average W UE was 35.47 and 79.53%. Thus, for 39.5% ≤ R SWC ≤ 79.53%, F. suspensa plants had higher P N and W UE than the average levels within the experimental soil water range.
Response of C i and L s in the leaves of F. suspensa to soil water
Figure 1c shows the responses of C i and L s in the leaves of F. suspensa were opposite to the direction of R SWC. At R SWC > 37.52%, C i initially increased before decreasing with a decreasing R SWC; however, L s showed the opposite pattern of variation and first decreased before increasing. At R SWC < 37.52%, C i rapidly increased and L s rapidly decreased with a further decrease in the R SWC. These results indicated that within the range from a saturated soil water content to the level at P N = 0 in F. suspensa, the leading factor in reducing photosynthetic productivity and efficiency in leaves of F. suspensa changed from stomatal to nonstomatal limitation with a decreasing water content; the turning point regarding the soil water content was 37.52%.
Response of F 0, F m, F v/F m, and Φ PSII in leaves of F. suspensa to soil water
F 0 is the fluorescence yield at the full opening of the reaction center in photosystem II (PSII), and F m is the fluorescence yield at full closure of the reaction center in photosystem II (PSII). These two variables can reflect electron transport through PSII (Demmig and Björkman 1987; Demmig et al. 1987). In Fig. 3a, d, as R SWC decreased, F 0 initially decreased and then increased; the opposite trend was observed for F m. The lowest F 0 coincided with the highest F m at R SWC = 51.84%. Variance analysis revealed no significant differences in F 0 or F m with varying soil water levels in the range of 39.79% ≤ R SWC ≤ 73.04% (P > 0.05). When R SWC exceeded the above range (R SWC ≥ 88.09% or R SWC ≤ 37.52%), F 0 was significantly higher, while F m was significantly lower (P < 0.05) compared with values at R SWC = 51.84%. This result indicated damage to or reversible inactivation of PSII that inhibited photosynthetic electron transfer activity in its reaction center.
Response of chlorophyll fluorescence variables to different soil water conditions in leaves of F. suspensa seedlings grown in pots in a greenhouse. Bars indicate ±SE of the mean, n = 27. F 0 is the minimal fluorescence yield of the dark-adapted state, F m is the maximal fluorescence yield of the dark-adapted state, F v/F m is the maximal quantum yield of PSII photochemistry, Φ PSII is the effective quantum yield of PSII photochemistry, q P is the photochemical quenching, N PQ is the nonphotochemical quenching, R SWC is the relative soil water content
F v/F m can reflect the efficiency of the primary conversion of light energy in PSII; it is also an excellent indicator for measuring the degree of photoinhibition (Farquhar et al. 1989). Φ PSII is often used to indicate the quantum yield of photosynthetic electron transfer in plants and can also be employed as a relative indicator of the photosynthetic electron transfer rate in plant leaves (Krause and Weis 1991). In Fig. 3b, c, F v/F m was approximately 0.8 in the range of 39.79% ≤ R SWC ≤ 73.04%, with no significant difference between varying soil water levels (P > 0.05). Both F v/F m and Φ PSII were significantly reduced when R SWC was below 37.52%. We inferred that within this range of soil water contents, the leaves of F. suspensa were damaged by light oxidation, and the proportion of opening of the reaction center of PSII was reduced, thus suppressing the electron transfer capability of PSII.
Response of q P and N PQ in leaves of F. suspensa to soil water
Fluorescence quenching, including q P and N PQ, is an important aspect regulating the quantum efficiency of photosynthesis in plants. q P reflects the portion of light energy absorbed by antenna pigments in PSII that is used for photochemical transfer (Van and Snel 1990). N PQ reflects the portion of light energy absorbed by antenna pigments in PSII that is dissipated in the form of heat but is not used for photosynthetic electron transfer (Gilmore and Yamamoto 1991). Figure 3e, f show the threshold response of q P to the R SWC. The highest q P occurred at R SWC = 51.84%. Electron transfer activity was highest in the reaction center of PSII at this water level. There was a reduction in q P when R SWC surpassed 51.84%, and q P was greatly reduced, showing low levels when R SWC ≤ 37.52%. N PQ exhibited a complex response to the R SWC. Within the range of 39.79% ≤ R SWC ≤ 64.10%, the lowest N PQ occurred at R SWC = 51.84% and showed an upward trend with a change in R SWC (increase or decrease). Conversely, N PQ showed a downward trend with an excessively higher or lower R SWC beyond the above range. The reduction in N PQ was particularly significant when R SWC ≤ 37.52% (P < 0.05). These results showed that appropriate water stress can increase heat dissipation from the antenna pigments of PSII in the leaves of F. suspensa and, to some extent, alleviate the effect of water stress on photosynthesis. However, with increasing drought stress (R SWC ≤ 37.52%), the heat dissipation capacity was reduced due to damage to PSII in the leaves.
Discussion
Soil water stress has multiple effects on the photosynthesis of plants. The factors leading to a reduction in P N include stomatal and nonstomatal limitations (Wang et al. 2012; Xia et al. 2014; Lang and Wang 2015). Farquhar and Sharkey (1982) suggested using C i and L s to determine whether stomatal or nonstomatal limitations are the cause of a reduction of P N. For most plants, such as Robinia pseudoacacia, Platycladusorientalis, Prunus sibirica L. (Zhang et al. 2012; Lang et al. 2013), stomatal closure and the reduction of g s in the early stage of drought stress can prevent CO2 from entering the blade and thus reduce P N via stomatal limitation; when the degree or duration of drought stress is increased, P N is reduced via nonstomatal limitation, i.e., through structural and functional impairment of the leaf photosystem. A similar trend was found in the current study, as revealed through analysis of the response of photosynthetic variables in leaves of F. suspensa under different levels of drought stress. Within the range of 37.52% < R SWC ≤ 51.84%, the reduction in P N was associated with a decrease in C i and increase in L s. Additionally, within the same range of soil water content, F 0 was relatively low; both F v/F m and Φ PSII were relatively high, showing no differences between different water levels; and N PQ increased with a decrease of the R SWC. These observations showed that F. suspensa plants protect the photosynthetic apparatus by enhancing thermal dissipation, and the reduction in P N within the range of 37.52% < R SWC ≤ 51.84% was due to stomatal limitation. When drought stress was further increased (R SWC ≤ 37.52%), P N was further reduced. Additionally, there was a significant increase in C i, in contrast to a substantial reduction in L s. These results indicated that the primary cause of the reduction of P N in the leaves of F. suspensa changed to nonstomatal limitation.
Nonstomatal limitation inducing a reduction of P N under drought stress is associated with damage to the photosynthetic apparatus as well as photosynthetic electron transport activity and the efficiency of light energy conversion (Guo et al. 2009). It is possible to infer the condition of the reaction center based on the change in F 0: nonphotochemical energy dissipation of antenna pigments in PSII often leads to a reduction in F 0, while the destruction or irreversible inactivation of reaction centers in PSII often causes an increase in F 0 (Krause 1988). Under normal circumstances, the light energy absorbed by chlorophyll is mainly consumed through electron transfer, chlorophyll fluorescence, and heat dissipation; there is an inversely proportional relationship between the three pathways (Wu et al. 2003). Photosynthesis and heat dissipation can be analyzed by estimating q P and N PQ (Peterson et al. 1998). The greater the q P value, the higher the electron transport activity in PSII (Guo et al. 2009). N PQ reflects the portion of light energy adsorbed by antenna pigments that is dissipated in the form of heat. Excessive light energy in the reaction center of PSII will destroy the photosynthetic apparatus, if it is not dissipated in a timely manner (Gilmore and Yamamoto 1991). In the present study, we found a substantially higher F 0 and lower F m, F v/F m, Φ PSII, q P, and N PQ under drought stress when R SWC ≤ 37.52%. This result indicated that PSII had been damaged and that the heat dissipation capacity was reduced in the leaves of F. suspensa. Excessive accumulation of light energy in the reaction center caused further damage to the photosynthetic apparatus. This mechanism inhibited photosynthetic electron transfer from the reaction center of PSII to the QA, QB and PQ pools, thus leading to a significant reduction in P N. Therefore, R SWC = 37.52% is the turning point of the soil water content for stomatal versus nonstomatal limitation of photosynthesis in leaves of F. suspensa and the maximum allowable water deficit for normal growth of F. suspensa (Zhang et al. 2012).
In recent years, much research has been done to investigate the quantitative relationship between soil moisture and photosynthesis of plants (Zhang et al. 2010, 2012; Xia et al. 2013; Lang and Wang 2016). In most of these studies, P N and W UE are frequently used to characterize the productivity and efficiency of plant photosynthesis in relation to soil water levels, to evaluate the ranking of soil water productivity (Zhang et al. 2012; Xia et al. 2013; Lang and Wang 2016). However, because chlorophyll fluorescence is an inherent probe for studying the relationship between plant photosynthesis and the environment (Krause and Weis 1991; Lazar 1999), chlorophyll fluorescence should be evaluated at a soil water range appropriate for photosynthetic productivity in the plant. The current study showed that within the range from a saturated soil water content to the level at P N = 0, P N and W UE in leaves of F. suspensa showed a clear threshold response to R SWC. The highest P N and W UE were obtained at R SWC of 51.84 and 64.10%, respectively. In the range of 39.5% ≤ R SWC ≤ 79.53%, both P N and W UE were higher than their average levels within the experimental range of soil water contents. The response of the fluorescence variables in leaves of F. suspensa to soil water levels showed that within the range of 39.79% ≤ R SWC ≤ 73.04%, F 0 was stable at low levels, while N PQ was higher, and both F v/F m and Φ PSII maintained high levels (F v/F m > 0.8, Φ PSII ≥ 0.6). These results indicated that within this range of soil water content (39.79% ≤ R SWC ≤ 73.04%), F. suspensa plants dissipate excessive excitation energy in the form of heat, effectively protecting the photosynthetic apparatus and contributing to efficient photosynthetic light reactions. Thus, F. suspensa can maintain the normal function of the photosynthetic apparatus and higher levels of photosynthetic productivity and efficiency (higher than average P N and W UE) at 39.79% ≤ R SWC ≤ 73.04%. Hence, this range of soil water content (39.79% ≤ R SWC ≤ 73.04%) should be chosen to achieve high photosynthetic productivity of F. suspensa.
Conclusions
As the climate changes, soil droughts in some areas are increasingly severe and frequent, serious affecting photosynthesis. However, there has been a lack of in-depth research regarding the internal mechanisms by which drought stress affects photosynthesis. Here we measured and analyzed changes in photosynthetic gas exchange and chlorophyll fluorescence variables under increasing soil drought in leaves of F. suspensa. Photosynthetic efficiency variables such as P N, W UE, F v/F m and Φ PSII, exhibited a clear threshold response to soil moisture. When soil water contents were in the range of 39.79% ≤ R SWC ≤ 73.04%, both P N and W UE were higher than the average levels, and the photosynthetic apparatus of F. suspensa maintained normal function.
Soil drought stress (R SWC < 51.84%) reduced photosynthesis in F. suspensa in association with soil water levels; within the range of 37.52% < R SWC < 51.84%, P N was reduced mainly because of the decrease in leaf stomatal conductance and subsequent blocking of the CO2 supply. With increasing drought stress (R SWC ≤ 37.52%), P N was reduced mainly because of nonstomatal limitation, as reflected by the damage to PSII. As a result, R SWC of 37.52% is the maximum allowable water deficit for the normal growth of F. suspensa.
In our analysis of the complex process and mechanism of photosynthesis in response to different soil drought conditions, we revealed the appropriate and lowest threshold of soil water conditions of F. suspensa. These results can guide practices for drought-resistant afforestation and field soil water management of trees in water deficit areas and aid the development of a system of drought-adaptive measures in the background of climate change.
References
Anselmi S, Chiesi M, Giannini M, Manes F, Maselli F (2004) Estimation of mediterranean forest transpiration and photosynthesis through the use of an ecosystem simulation model driven by remotely sensed data. Global Ecol Biogeogr 13(4):371–380
Bray EA (1993) Molecular responses to water deficit. Plant Physiol 103(4):1035–1040
Demmig B, Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence and photon yield of O2 evolution in leaves of higher plants. Planta 171(2):171–184
Demmig B, Winter K, Krüger A, Czygan C (1987) Photoinhibition and zeaxanthin formation in intact leaves a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84(2):218–224
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33(1):317–345
Farquhar GD, Wong SC, Evans JR, Hubick KT (1989) Photosynthesis and gas exchange. In: Jones HG, Flowers TJ, Jones MB (eds) Plants under stress. Cambridge University Press, Cambridge, pp 47–69
Gilmore AM, Yamamoto HY (1991) Zeaxanthin formation and energy dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol 96(2):635–643
Guo CF, Sun Y, Tang YH, Zhang MQ (2009) Effect of water stress on chlorophyll fluorescence in leaves of tea plant (Camellia sinensis). Chin J Eco-Agric 17(3):560–564
Hao Y, Li DF, Piao XL (2010) Forsythia suspensa extract alleviates hypersensitivity induced by soybean β-conglycinin in weaned piglets. J Ethnopharmacol 128(2):412–418
Kebbas S, Lutts S, Aid F (2015) Effect of drought stress on the photosynthesis of Acacia tortilis subsp. raddiana at the young seedling stage. Photosynthetica 53(2):288–298
Krause GH (1988) Photoinhibition of photosynthesis: an evaluation of damaging and protective mechanisms. Physiol Plant 74:566–574
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Lang Y, Wang M (2015) Effects of soil water on photosynthesis of Forsythia suspensa (Thunb.) Vahl. in spring and summer. Acta Ecol Sin 35(9):3043–3051
Lang Y, Wang M (2016) Threshold effect of photosynthesis in Forsythia suspense to soil water and its photosynthetic productivity grading in spring and summer. Sci Silva Sin 52(2):38–46
Lang Y, Wang M, Zhang GC, Zhao QK (2013) Experimental and simulated light responses of photosynthesis in leaves of three tree species under different soil water conditions. Photosynthetica 51(3):370–378
Lawson T, Oxborough K, Morison JL, Baker NR (2003) The responses of guard and mesophyll cell photosynthesis to CO2, O2, light, and water stress in a range of species are similar. J Exp Bot 54(388):1743–1752
Lazar D (1999) Chlorophyll a fluorescence induction. Biochim Biophys Acta 1412(3):1–28
Li WR, Zhang SQ, Shan L (2007) Responsibility of nonstomatal limitations for the reduction of photosynthesis—response of photosynthesis and antioxidant enzyme characteristics in alfalfa (Medicago sativa L.) seedlings to water stress and rehydration. Front Agric China 1(3):255–264
Li ZQ, Niu F, Fan JW, Liu YG, Rosenfeld D, Ding YN (2011) Long-term impacts of aerosols on the vertical development of clouds and precipitation. Nat Geosci 4(12):888–894
Masrahi YS, Al-Turki TA, Sayed OH (2015) Photosynthetic adaptation and survival strategy of Duvalia velutina in an extremely arid environment. Photosynthetica 53(4):555–561
Ohashi Y, Nakayama N, Saneoka H, Fujita K (2006) Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol Plant 50(1):138–141
Peterson RB, Sivak MN, Walker DA (1998) Relationship between steady-state fluorescence yield and photosynthetic efficiency in spinach leaf tissue. Plant Physiol 88:158–163
Piao XL, Jang MH, Cui J, Piao XS (2008) Lignans from the fruits of Forsythia suspensa. Bioorg Med Chem Lett 18(6):1980–1984
Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning and mutual relationships. Photosynthetica 40(1):13–29
Smith DM, Cusack S, Colman AW, Folland CK, Harris GR, Murphy JM (2007) Improved surface temperature prediction for the coming decade from a global climate model. Science 317(5839):796–799
Sung YY, Lee AY, Kim HK (2016) Forsythia suspensa fruit extracts and the constituent matairesinol confer anti-allergic effects in an allergic dermatitis mouse model. J Ethnopharmacol 187:49–56
Van KO, Snel JFH (1990) The use of chlorophyll nomenclature in plant stress physiology. Photosynth Res 25:147–150
Wang ZX, Chen L, Ai J, Qin HY, Liu YX, Xu PL, Jiao ZQ, Zhao Y, Zhang QT (2012) Photosynthesis and activity of photosystem II in response to drought stress in Amur Grape (Vitis amurensis Rupr.). Photosynthetica 50(2):189–196
Ware MA, Belgio E, Ruban AV (2015) Photoprotective capacity of nonphotochemical quenching in plants acclimated to different light intensities. Photosynth Res 126(2):261–274
Wu CA, Meng QW, Zou Q, Zhao SJ, Wang W (2003) Comparative study on the photooxidative response in different wheat cultivar leaves. Acta Agron Sin 29(3):339–344
Xia EQ, Ai XX, Zang SY, Guan TT, Xu XR, Li HB (2011) Ultrasound-assisted extraction of phillyrin from Forsythia suspensa. Ultrason Sonochem 18(2):549–552
Xia JB, Zhang SY, Zhao ZG, Zhao YY, Gao Y, Gu GY, Sun JK (2013) Critical effect of photosynthetic efficiency in Salix matsudana to soil moisture and its threshold grade in shell ridge island. Chin J Plant Ecol 37(9):851–860
Xia JB, Zhang GC, Wang RR, Zhang SY (2014) Effect of soil water availability on photosynthesis in Ziziphus jujuba var. spinosus in a sand habitat formed from seashells: Comparison of four models. Photosythetica 52(2):253–261
Xu DQ (2002) Photosynthetic Efficiency. Shanghai Scientific & Technological Press, Beijing, pp 4–6
Yan N, Zhang YL, Xue HM, Zhang XH, Wang ZD, Shi LY, Guo DP (2015) Changes in plant growth and photosynthetic performance of Zizania latifolia exposed to different phosphorus concentrations under hydroponic condition. Photosynthetica 53(4):630–635
Ye ZP (2007) A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 45(4):637–640
Zhang SY, Zhang GC, Gu SY, Xia JB, Zhao JK (2010) Critical responses of photosynthetic efficiency of goldspur apple tree to soil water variation in semiarid loess hilly area. Photosynthetica 48(4):589–595
Zhang GC, Xia JB, Shao HB, Zhang SY (2012) Grading woodland soil water productivity and soil bioavailability in the semi-arid loess plateau of China. CLEAN–Soil Air Water 40(2):148–153
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: This study was supported by the National Natural Science Foundation of China (Nos. 41621061, 31500511) and the Natural Science Foundation of Shandong Province of China (No. ZR2015CL044).
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong
Rights and permissions
About this article
Cite this article
Lang, Y., Wang, M., Xia, J. et al. Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspensa . J. For. Res. 29, 45–53 (2018). https://doi.org/10.1007/s11676-017-0420-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-017-0420-9