Abstract
The individual effects of Al2O3, CaO and MgO on gas/slag/matte/spinel equilibria in the Cu-Fe-O-S-Si-(Al, Ca, Mg) system at 1473 K (1200 °C) and p(SO2) = 0.25 atm. have been experimentally measured for a range of oxygen partial pressures and matte compositions. The experimental methodology has included the high temperature equilibration of individual samples on a spinel primary phase substrate under controlled gas atmospheres (CO/CO2/SO2/Ar), followed by rapid quenching of the equilibrium condensed phases and direct measurement of the phase compositions using electron probe x-ray microanalysis. The experimental results show that the presence of Al2O3, CaO and MgO reduce the iron, sulphur and copper concentrations in the slag phase. Present study is undertaken as part of an integrated approach involving thermodynamic modelling and experimental measurements. The experimental data are compared with predictions obtained using the current thermodynamic database for the Cu-Fe-O-S-Si-(Al, Ca, Mg) system in order to further improve thermodynamic parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Cu-Fe-O-S-Si-(Al, Ca, Mg) system describes the principal chemical components present in the pyrometallurgical copper smelting and converting systems. The Al2O3, CaO and MgO are introduced into these processes through their presence in the concentrate feed and flux materials or as a result of material dissolved from refractories during the operations. For example, in industrial practice, fayalite based copper smelting slags (FeO-Fe2O3-SiO2) contain typically 2-5 wt.% Al2O3, 1-4 wt.% CaO and 1-2 wt.% MgO.[1] The presence of these elements has an effect on the phase equilibria and the distribution of major components between the gas, slag, matte and solid oxide phases (such as spinel or tridymite). The availability of accurate information about multiphase equilibria in the system is important for the optimisation of existing processes and development of new industrial processes.
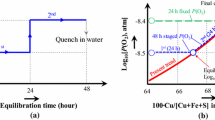
The thermodynamic equilibria between the slag and matte in the Cu-Fe-O-S-Si system have previously been reported.[2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19] In a recent series of studies, an integrated approach combining experimental work and thermodynamic modelling has been implemented to investigate phase equilibria and distribution of elements in the Cu-Fe-O-S-Si-(Al, Ca, Mg) system under controlled oxygen and sulphur dioxide partial pressures.[20,21,22,23,24,25,26,27,28,29,30] The experimental technique included high temperature equilibration experiments using a spinel or tridymite substrate in controlled gas atmosphere (CO/CO2/SO2/Ar), quenching of sample and accurate measurements of compositions of coexisting phases using electron probe x-ray microanalysis (EPMA). The application of EPMA for locally equilibrated slag/matte samples eliminates the possibility of measuring entrained droplets. Combining EPMA with SEM allows identification of the local area of phases closest to equilibrium and measurements the compositions of coexisting phases with high accuracy. Decreased mass of samples, 0.5-0.7 g, ensures the achievement of equilibrium state within reasonable experimental time. Typically, equilibration takes 24 h at 1473 K (1200 °C). Small mass allows faster quenching, which is particularly important for investigating slag-solid equilibrium, the information about the liquidus at high temperature. Using the primary phase (spinel or tridymite) as a substrate for matte/slag/gas equilibria prevents the contamination of the sample with side elements and leads to more accurate Al2O3, CaO and MgO determination in the slag phase. Conditions of the experiments are calculated using the thermodynamic database, thus decreasing the overall number of experiments, while making sure that resulting information is sufficient to fix the values of certain model parameters.
The results of these later studies and earlier publications were assessed by Shishin (Ref 20). Thermodynamic modelling of the effects of Al2O3, CaO and MgO on slag–matte equilibria in the Cu-Fe-O-S-Si-(Al, Ca, Mg) system was conducted in Ref 28. The model reproduced individual effects of Al2O3, CaO and MgO on spinel and tridymite substrate. Model predictions were verified using the data for combined Al2O3 + CaO + MgO effect on slag/matte/tridymite equilibrium, corresponding to low Fe/SiO2 in slag. Good agreement was obtained. The combined effect of Al2O3 + CaO + MgO on slag/matte/spinel equilibrium, i.e. for high Fe/SiO2, has not been accurately measured yet.
The present paper summarizes the outcomes of the study of the effects of Al2O3, CaO and MgO individually on phase equilibria in the presence of gas, slag, matte and spinel phases, and the elemental distributions between the condensed phases.
2 Experimental Technique and Procedure
The experimental methodology used in this study is based on the general approach developed by the authors (Ref 22) further adapted to the study of the gas/slag/matte equilibria with the spinel substrate.[24,28]. This involves high-temperature equilibration of samples on a spinel substrate in a vertical tube furnace under selected and carefully controlled gas atmospheres (Ar-CO-CO2-SO2), rapid quenching of the equilibrated phases, metallographic preparation of samples, and direct measurement of the compositions of the equilibrated phases by electron-probe x-ray microanalysis (EPMA). The spinel primary phase substrate shape was in the form of an envelope with open ends.[24,28] Based on the results of preliminary experiments an equilibration time of 24 h was selected for all experiments. Details of the EPMA standards and measurement technique used are provided by Sineva et al. (Ref 30). Only the concentrations of metal cations and sulphur were measured by EPMA in the present study. All concentrations in the liquid slag, spinel and matte phases were recalculated to selected oxidation states (i.e. Cu2O, FeO, SiO2 and S) and normalised for presentation purposes only. The normalised phase compositions and original sums of elements or oxides from the EPMA analyses are provided in the tabular form.
The thermodynamic calculations have been undertaken using the FactSage computer package with advanced thermodynamic solution models [31,32] and an internal thermodynamic database.[33]
3 Results
3.1 Effects of Al2O3 on the Gas/Slag/Matte/Spinel Equilibria at 1200 °C and p(SO2) = 0.25 atm
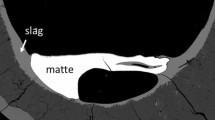
Figure 1 shows an example of the sample microstructure from an equilibration experiment involving gas/slag/matte/spinel phases at 1200 °C and p(SO2) = 0.25 atm for the Al2O3-containing system (Cu-Fe-O-S-Si-Al). Table 1 summarizes the compositions of the phases present at selected oxygen partial pressures. It has been found that the concentrations of aluminium in matte are below the detection limits in all conditions examined. The Al2O3 in the samples is distributed between slag and spinel phases. The experimental data are displayed in Fig. 2(a)–(h) as a function of 100 Cu/[Cu + Fe + S] wt pct in matte that is close to the matte grade (wt.% Cu in matte). The predicted trends calculated for fixed % Al2O3 in slag are included in the figures. Note that the experimental slag compositions vary slightly from the target fixed values used in the calculations due to the partitioning of the components between the condensed phases. These experimental points should not therefore be expected correspond exactly to those calculated for the selected fixed % Al2O3 in slag.
Set of graphs describing the effects of Al2O3 on gas/slag/matte/spinel equilibria in the Cu-Fe-O-S-Si-Al system at 1473 K (1200 °C) and p(SO2) = 0.25 atm as a function of Cu/(Cu + Fe + S) in matte. (a) Oxygen partial pressure (log10[p(O2), atm]); (b) concentration of sulphur in matte; (c) dissolved oxygen in matte; (d) concentration of “FeO” in slag; (e) concentration of sulphur in slag; (f) concentration of Cu in slag; (g) ferric iron to total iron ratio in slag; (h) Al2O3 concentration in slag. Solid lines are calculated using FactSage 7.2 [31,32] and internal database,[33] symbols are experimental data for systems containing alumina are obtained in the present study
Figure 2(a) shows that the oxygen partial pressure increases with increasing matte grade. The experimental data indicate this trend is independent of alumina in slag and are lower than the model predictions for all matte grades. Systematic discrepancy is observed between calculated and experimental p(O2)s for a given matte grade, temperature and p(SO2). The difference is about 0.2 in log10 p(O2). The reasons of the mentioned inconsistency are discussed by of Shishin et al. (Ref 20) in details, but didn’t resolved yet.
Effect of alumina on sulphur concentration in matte is observed at low matte grades with copper concentration lower than 50 wt.% according to calculated trends (Fig. 2b). For instance, at 55 wt.% matte grade addition of 7.5 wt.% Al2O3 increases sulphur concentration in matte at 0.5 wt.% of sulphur. However, the sensitivity of experiment was not enough to establish the mentioned effect on sulphur concentration in matte.
The oxygen concentrations in matte have not been measured in this study; the trends are predicted using thermodynamic database (see Fig. 2c). Within the range of matte grades studied, increasing the Al2O3 in slag and matte grade are shown, by both the experimental data and the model predictions, to significantly decrease %FeO in slag (Fig. 3d), %S in slag (Fig. 3e) and %Cu in slag (Fig. 3f). Note these are concentrations of dissolved copper in slag; the use of the EPMA technique enables dissolved and entrained copper to be clearly distinguished. The ratio of ferric iron to total iron ratio in slag is predicted, using the thermodynamic database, to decrease with increasing %Al2O3 in slag; no experimental data was obtained on this parameter using the experimental technique employed in the present study. As indicated in the above text the experimental values of %Al2O3 in slags vary slightly from the values selected for the model predictions; these differences are illustrated in Fig. 2(e).
3.2 Effects of CaO on the Gas/Slag/Matte/Spinel Equilibria at 1200 °C and p(SO2) = 0.25 atm
Figure 3 shows an example of the microstructure of a gas/slag/matte/spinel sample equilibrated at 1200 °C and p(SO2) = 0.25 atm for the CaO-containing system (Cu-Fe-O-S-Si-Ca). Table 2 and Fig. 4 summarize the compositions of the phases at various oxygen partial pressures. It has been found that the concentrations of Ca in matte and spinel phases are low, and, effectively, all CaO present in the mixture is dissolved into the slag phase.
Set of graphs describing the gas/slag/matte/spinel equilibria in the Cu-Fe-O-S-Si-Ca system at 1473 K (1200 °C) and p(SO2) = 0.25 atm as a function of Cu/(Cu + Fe + S) in matte. (a) Oxygen partial pressure (log10[p(O2), atm]); (b) concentration of sulphur in matte; (c) dissolved oxygen in matte; (d) concentration of “FeO” in slag; (e) concentration of sulphur in slag; (f) concentration of Cu in slag; (g) ferric iron to total iron ratio in slag; (h) CaO concentration in slag. Solid lines are calculated using FactSage 7.2 [31,32] and internal database,[33] symbols are experimental data obtained in the present study
The trends of the matte grade versus P(O2) (Fig. 4a), sulphur in matte versus matte grade (Fig. 4b) and oxygen in matte versus matte grade (Fig. 4c) for the CaO-containing system are similar to those of the CaO-free system (Cu-Fe-O-S-Si). The experimental data and the model predictions show that increasing the CaO concentration in slag and increasing matte grade both result in significant decreases in %FeO in slag (Fig. 4d), %S in slag (Fig. 4e) and %Cu in slag (Fig. 4f).
3.3 Effects of MgO on the Gas/Slag/Matte/Spinel Equilibria at 1200 °C and p(SO2) = 0.25 atm
Figure 5 shows an example of the microstructure of a sample containing gas/slag/matte/spinel/olivine equilibrated at 1200 °C and p(SO2) = 0.25 atm for the MgO-containing system (Cu-Fe-O-S-Si-Mg). Note the figure shows the presence of the olivine phase in addition to the spinel. The olivine was observed to form in the sample containing 1.35 wt.% MgO at 44.8 wt.% Cu in matte. Table 3 and Fig. 6 summarize the compositions of phases at selected oxygen partial pressures. The MgO in the mixture is distributed between slag and spinel phases, and the dissolution of Mg in matte is below the EPMA detection limit.
Set of graphs describing the gas/slag/matte/spinel equilibria in the Cu-Fe-O-S-Si-Mg system at 1473 K (1200 °C) and p(SO2) = 0.25 atm as a function of Cu/(Cu + Fe + S) in matte. (a) Oxygen partial pressure (log10[p(O2), atm]); (b) concentration of sulphur in matte; (c) dissolved oxygen in matte; (d) concentration of “FeO” in slag; (e) concentration of sulphur in slag; (f) concentration of Cu in slag; (g) ferric iron to total iron ratio in slag; (h) MgO concentration in slag. Solid lines are calculated using FactSage 7.2 [33] and internal database,[32] symbols are experimental data obtained in the present study
The trends of the matte grade versus p(O2) (Fig. 6a), sulphur in matte versus matte grade (Fig. 6b) and oxygen in matte versus matte grade (Fig. 6c) of the MgO-containing system are similar to that of MgO-free system (Cu-Fe-O-S-Si). Increasing the concentration of MgO in slag for a fixed matte grade decreases the FeO concentration in the slag (see Fig. 6d), addition of MgO slightly decreases the %S (Fig. 6e) and %Cu (Fig. 6f) in slag especially at low matte grade area.
4 Discussion
The experimental data obtained in the present study clearly show that the presence of individual components Al2O3, CaO and MgO influences the compositions of the coexisting condensed phases at gas/slag/matte/spinel equilibria for fixed temperature, oxygen and sulphur potentials. The concentrations of these components in the matte phase were, in all cases, below the limits that could be accurately measured using the present EPMA technique. Whilst the decrease in %FeO in slag can be partially attributed to the dilution due to the addition of the Al2O3, CaO and MgO components, all three, to different extents, are distributed between slag and spinel phases, and those are not linear correlations. The presence of Al2O3, CaO and MgO result in decreases in the %S and %Cu in slag. The effects of the additional components appear to increase with decreasing matte grade, i.e. the effects are highest for lower matte grades. Experimentally, it is very difficult at this stage to accurately quantify the distribution of the components and their effects for matte grades above approximately 72% Cu. The trends in behaviour are consistent with the current thermodynamic database predictions, however some adjustment to model parameters will be necessary to account for equilibria at lower matte grades.
5 Summary
Experimental measurements of the gas/slag/matte/spinel equilibria at 1473 K (1200 °C) and p(SO2) = 0.25 atm have provided new detailed data for the Cu-Fe-S-Si-O-Al-Ca-Mg system. It has been shown that, increasing the Al2O3, CaO and MgO concentrations in slag,
-
Do not measurably effect the relations between the p(O2) and matte composition.
-
Decrease of iron concentration in slag.
-
Decrease the sulphur and dissolved copper concentrations in slag (the trends for the effect of magnesia are not significant).
In general, there is good agreement between the experimental data and the current thermodynamic database, demonstrating the value of the tool in predicting process trends.
References
W.G.I. Davenport et al., Extractive Metallurgy of Copper, 4th ed., Pergamon Press, Oxford, 2002, p 155-171
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. III. Partial Liquidus Diagram for Cu2S-FeS-FeO System, Technol. Rep. Tohoku Univ., 1955, 19(2), p 239-250
A. Yazawa and M. Kameda, Fundamental Studies on Copper Smelting. IV. Solubility of FeO in Copper Matte from SiO2-saturated FeO-SiO2 Slag, Technol. Rep. Tohoku Univ., 1955, 19(2), p 251-261
Kameda, M. and A. Yazawa. The Oxygen Content of Copper Mattes, in Physical Chemistry of Process Metallurgy, Part 2 (TMS Conference Proceedings, Interscience, NY, 1961)
N. Korakas, Etude thermodynamic de l’équilibre entre scories ferro-siliceuses et mattes de cuivre. Application aux problèmes posés par la formation de magnetite lors du traitement des minerais sulfurés de cuivre (Univirsité de Liège, 1964)
U. Kuxmann and F.Y. Bor, Studies on the Solubility of Oxygen in Copper Mattes under Ferric Oxide Slags Saturated with Silica, Erzmetall, 1965, 18, p 441-450
F.Y. Bor and P. Tarassoff, Solubility of Oxygen in Copper Mattes, Can. Metall. Q., 1971, 10(4), p 267-271
A. Geveci and T. Rosenqvist, Equilibrium Relations Between Liquid Copper, Iron-Copper Matte, and Iron Silicate Slag at 1250 °C, Trans. Inst. Min. Metall., 1973, 82, p C193-C201
M. Nagamori, Metal Loss to Slag: Part I. Sulfidic and Oxidic Dissolution of Copper in Fayalite Slag from Low Grade Matte, Metall. Trans. B, 1974, 5(3), p 531-538
F.J. Tavera and W.G. Davenport, Equilibrations of Copper Matte and Fayalite Slag under Controlled Partial Pressures of Sulfur Dioxide, Metall. Trans. B, 1979, 10B(2), p 237-241
G.H. Kaiura, K. Watanabe, and A. Yazawa, The Behavior of Lead in Silica-Saturated Copper Smelting Systems, Can. Metall. Q., 1980, 19(2), p 191-200
H. Jalkanen, Copper and Sulfur Solubilities in Silica-Saturated Iron Silicate Slags from Copper Mattes, Scand. J. Metall., 1981, 10(4), p 177-184
A. Yazawa, S. Nakazawa, and Y. Takeda, Distribution Behavior of Various Elements in Copper Smelting Systems, Adv. Sulfide Smelt., 1983, 1, p 99-117
R. Shimpo et al., A Study on the Equilibrium Between Copper Matte and Slag, Can. Metall. Q., 1986, 25(2), p 113-121
F.J. Tavera and E. Bedolla, Distribution of Copper, Sulfur, Oxygen and Minor Elements Between Silica-Saturated Slag, Matte and Copper-Experimental Measurements, Int. J. Miner. Process., 1990, 29(3–4), p 289-309
H. Li and W.J. Rankin, Thermodynamics and Phase Relations of the Fe-O-S-SiO2(sat) System at 1200 °C and the Effect of Copper, Metall. Trans. B, 1994, 25B(1), p 79-89
Y. Takeda, Oxygen Potential Measurement of Iron Silicate Slag–Copper–Matte System, in Proceedings International Conference on Molten Slags, Fluxes Salts ‘97, 5th (Iron and Steel Society, Warrendale, 1997)
Y. Takeda, Copper Solubility in Matte Smelting Slag, in Proceeding International Conference on Molten Slags, Fluxes Salts ‘97, 5th (Iron and Steel Society, Warrendale, 1997)
J.M. Font et al., Solubility of Copper or Nickel in Iron-Silicate Base Slag Equilibrated with Cu2s-FeS or Ni3S2-FeS Matte Under High Partial Pressures of SO2, Metall. Rev. MMIJ, 1998, 15(1), p 75-86
D. Shishin, S.A. Decterov, and E. Jak, Thermodynamic Assessment of Slag–Matte–Metal Equilibria in the Cu-Fe-O-S-Si System, J. Phase Equilib. Diffus., 2018, 39(5), p 456-475
D. Shishin, P.C. Hayes, and E. Jak. Multicomponent Thermodynamic Databases for Complex Non-ferrous Pyrometallurgical Processes, in Extraction 2018 (Springer, Ottawa, 2018).
E. Jak et al., Integrated Experimental Phase Equilibria and Thermodynamic Modelling Studies for Copper Pyrometallurgy, in 9th International Copper Conference, Kobe, Japan (2016), pp. 1316–1331
T. Hidayat et al., Experimental Investigation of Gas/Slag/Matte/Spinel Equilibria in the Cu-Fe-O-S-Si System at T = 1250 °C and p(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2018, 49(4), p 1732-1739
Hidayat, T., et al., Experimental Investigation of Gas/Slag/Matte/Spinel Equilibria in the Cu-Fe-O-S-Si System at T = 1200 °C and p(SO2) = 0.25 atm. Metall. Mater. Trans. B, 2018. 49(4): p. 1750-1765.
A. Fallah-Mehrjardi, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200 °C] and p(SO2) = 0.1 atm, Int. J. Mater. Res., 2018, 49, p 1732-1739
A. Fallah-Mehrjardi et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Experimental Results at T = 1473 K [1200 °C] and p(SO2) = 0.25 atm, Metall. Mater. Trans. B, 2017, 48(6), p 3017-3026
A. Fallah-Mehrjardi et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si system in Controlled Gas Atmospheres: Development of Technique, Metall. Mater. Trans. B, 2017, 48(6), p 3002-3016
T. Hidayat, P.C. Hayes, and E. Jak, Experimental Investigation of Gas/Matte/Spinel Equilibria in the Cu-Fe-O-S System at 1473 K (1200 °C) and p(SO2) = 0.25 atm, J. Phase Equilib. Diffus., 2018, 39(2), p 138-151
D. Shishin et al., Integrated Experimental and Thermodynamic Modelling Study of the Effects of Al2O3, CaO and MgO on Slag-Matte Equilibria in the Cu-Fe-O-S-Si-(Al, Ca, Mg) System, J. Phase Equilib. Diffus., 2018, 40, p 445-461
S. Sineva et al., Experimental Investigation of Gas/Slag/Matte/Tridymite Equilibria in the Cu-Fe-O-S-Si-Al-Ca-Mg System in Controlled Gas Atmosphere: Experimental Results at 1473 K (1200 °C), 1573 K (1300 C) and p(SO2) = 025 atm, J. Phase Equilib. Diffus., 2020, 41, p 243-256. https://doi.org/10.1007/s11669-020-00810-8
C.W. Bale et al., FactSage Thermochemical Software and Databases, Calphad, 2002, 26(2), p 189-228
C.W. Bale et al., FactSage Thermochemical Software and Databases, 2010–2016, Calphad, 2016, 54, p 35-53
D. Shishin, P.C. Hayes, and E. Jak, Development and Applications of Thermodynamic Database in Copper Smelting, in Copper’19 Conference (Vancouver, 2019)
Acknowledgments
The authors would like to thank Australian Research Council Linkage program LP140100480, Altonorte Glencore, AngloAmerican Platinum, Atlantic Copper, Aurubis, Boliden, Olympic Dam Operation BHP Billiton, Kazzinc Glencore, Kennecott Rio Tinto, Outotec Oy (Espoo), PASAR Glencore, Umicore, and for the financial support for this study. The authors would like to thank Dr Denis Shishin and Dr Maksym Maksym Shevchenko for assistance in preparation of this paper. The authors would also like to thank the staff of the Centre for Microscopy and Microanalysis, University of Queensland for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sineva, S., Fallah-Mehrjardi, A., Hidayat, T. et al. Experimental Study of the Individual Effects of Al2O3, CaO and MgO on Gas/Slag/Matte/Spinel Equilibria in Cu-Fe-O-S-Si-Al-Ca-Mg System at 1473 K (1200 °C) and p(SO2) = 0.25 atm. J. Phase Equilib. Diffus. 41, 859–869 (2020). https://doi.org/10.1007/s11669-020-00847-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00847-9