Abstract
The thermodynamic properties of vanadium have been evaluated to 3700 K. Selected values include an enthalpy of sublimation of 515 ± 2 kJ/mol at 298.15 K and a boiling point at one atmosphere pressure of 3694 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
This work is one of a series on thermodynamic properties of the elements to appear in the Journal of Phase Equilibria and Diffusion. The articles are expected to be especially useful to those who are updating databases for phase diagram prediction and for other applications as well. Each article in the series will list the properties of a single element, including Ag,[1] Au,[2] Be,[3] Cr, Cu,[4] Hf,[5] Mo, Nb, Ta, Ti, V and W. References are given for papers which have already been published. In addition, the series will also include two summary papers on selected values of the melting points and boiling points of the elements and on the enthalpies and entropies of fusion and transition of the elements.
Previous comprehensive reviews on vanadium were by Hultgren et al.,[6] Smith[7] Gurvich et al.[8] JANAF[9] and Desai[10] and reviews on the solid only by Maglić.[11] A further review is justified by including the high temperature enthalpy measurements of Lin and Frohberg.[12]
A superconducting transition temperature (TC) of 5.435 K as determined by Leupold et al.[13] was accepted because of the high purity of the material used. After correction to the ITS-90 temperature scale the melting point determined by Rudy and Windisch[14] at 2201 ± 6 K was also accepted since this was confirmed by more recent values of 2200 K as determined by Hiernaut et al.[15] and 2199 K as determined by Pottlacher et al.[16]
Wherever possible values have been corrected to the currently accepted atomic weight of 50.9415 ± 0.0001[17] and to the ITS-90 temperature scale using correction factors of Douglas,[18] Rusby,[19], Rusby et al.,[20] and Weir and Goldberg.[21]
It was suggested by Westlake[22] that numerous anomalies found in the measurements of physical properties of vanadium between 180 K and 250 K were due to the precipitation of impurity hydrogen as vanadium hydride. Subsequently, Westlake et al.[23] dehydrogenated samples at 1073 K in high vacuum and found that electrical resistivity and elastic constants measurements no longer showed evidence of such anomalies. However, based on dilatometric measurements on high purity materials containing low hydrogen levels, Bollinger et al.[24] suggested that all of the Group 5 metals did in fact show evidence of a structural distortion below 300 K which was martensitic in character and lead to a structure which was not cubic. They further suggested that the vacuum used in the heat treatment process to remove hydrogen was not high enough to prevent a small amount of oxidation which led to the introduction of interstitial impurities which were enough to completely suppress the martensitic transformation, and this was the reason for the removal of the anomalies and not the removal of hydrogen as suggested by Westlake.[22] However, in spite of this speculation, it is noted that Jung et al.[25] had carried out electrical resistivity measurements on samples containing less than 1 wt ppm hydrogen and less than a total of 100 at ppm of oxygen + carbon + nitrogen and found no evidence of anomalies between 6 K and 300 K. Further, Smirnov and Finkel,[26] Schmitz-Pranghe and Dünner[27] and Henry et al.[28] all confirmed that the structure remained as body-centred cubic below 120 K. Even an apparent transition to a tetragonal structure at 230 K observed by Finkel’ et al.[29] was shown by Westlake et al.[23] to be due to a mis-interpretation and the correct interpretation indicated that the structure remained in the body-centred cubic form. In view of these findings, it is assumed that until the measurements of Bollinger et al.[24] are independently verified and that the supposed new low temperature structure is fully characterised then the structure is considered to be body-centred cubic over the whole temperature range (Fig. 1, 2, 3).
Low temperature specific heat of solid vanadium, from 6 K to 298.15 K, taken from Table 17
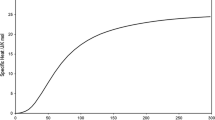
Specific heat of vanadium for 300 < T < 3700 K, taken from Table 18
High temperature thermodynamic properties of vanadium for 300 < T < 3700 K, taken from Table 18
2 Solid Phase
2.1 Superconducting State: Range 0 to 5.435 K
In the description of the electronic structure of transition metals in terms of two electronic conduction bands, if the scattering of electrons between the two bands is sufficiently weak, then both may separately undergo a transition into the superconducting state with the resultant formation of two energy gaps, which is a Type II superconductor. Anomalies which appeared to confirm this behaviour were observed for vanadium in the specific heat measurements of Shen[30] and Radebaugh and Keesom[31] However for niobium, Sellers et al.[32] showed that the anomalies were due to hydrogen contamination and that with hydrogen removed niobium behaved as a typical Type I superconductor. Therefore, it is considered that vanadium would behave similarly and the anomalies found in the measurements of Shen[30] and Radebaugh and Keesom[31] were ignored. Therefore, below Tc/T = 2 the remaining specific heat values from these two sets of measurements were averaged and then fitted to the Bardeen-Cooper-Schrieffer equation and a lattice term after correcting to the selected values given in the Normal State review of γ = 9.67 mJ/(mol K2), and Tc = 5.435 K and a lattice contribution equal to the selected value of θ D = 397.2 K. The full representation of the specific heat (Cs) below 2.718 K (Tc/2) as then given by Eq 1:
Above a temperature of Tc/T = 2, it is a requirement that the entropies of both the superconducting state and the normal state are equal at Tc. At Tc, a value of Cs = 135.9 mJ/(mol K) is estimated from the graphical representation of Leupold et al.[13] and this value was combined with the value of 26.52 mJ/(mol K) at 2.718 K obtained from Eq 1 and an intermediate value of 69.944 mJ/(mol K) at the mid-point temperature of 4.076 K obtained by iteration so that at Tc the entropy values of the superconducting state and the normal state are equal. This gives Eq 2 for Cs over the range 2.718 K to Tc:
Derived thermodynamic values based on Eq 1 and 2 are given in Table 16 whilst differences from the selected values of other measurements in this region are given in Table 10.
2.2 Normal State: 0 to 298.15 K
Low temperature specific heat for vanadium in the normal state is given in terms of the modified Debye equation: Cp = D/T2 + γ T + A T3 where D is the nuclear quadrupole coefficient, γ is the electronic coefficient and A is the lattice contribution, which is usually represented in terms of a limited Debye temperature, θ D , which can be determined by the equation θ 3 D = (12/5) π 4 R/A = 1943.770/A where R is the Gas Constant and A is given in units of J (mol K4). Desai[10] listed a large number of determinations of the values of γ and θ D but only those where the values of θ D approach the values determined from elastic constants values of 399.3 K determined by Alers[33] and 403 K determined by Bolef et al.[34] are considered, which consists of the measurements of Shen[30] (<4.2 K), Radebaugh and Keesom[31] (0.4-5.1 K) and Leupold et al.[13] (2-20 K) with values given in Table 1. Because of the very high purity of the samples used, the values of Leupold et al. were selected for γ and θ D and the values of Shen for D. It is noted that the inclusion of D has little effect on the values of Leupold et al. above 2.5 K.
The values selected in Table 1 apply up to 7.47 K at which temperature Leupold et al.[13] showed an abrupt change in the slope of the specific heat values, which was due to the presence of a Kohn anomaly in the phonon density of states. The further measurements of Leupold et al. were selected above this temperature and up to the experimental limit at 20 K.
From 55 to 190 K the specific heat values of Anderson[35] (54.2-296.5 K) were selected since these gave the best correlation with the values of Leupold et al.[13] when interpolating in the range 20 to 55 K. However above 190 K the specific heat values of Takahashi et al.[36] (82-991 K) were selected in order to give continuity with selected high temperature values. Representative equations for the range 20-298.15 K are given in Table 6.
Normal state thermodynamic values below the superconducting temperature are also given in Table 16 and above this temperature in Table 17. The deviations of other normal range specific heat values are given in Table 11 except for the measurements of Chernoplekov et al.[37] (1.5-40 K) which were shown only in the form of a small graph.
2.3 Solid: Range 298.15-2201 K
In the overlap region the smoothed drop calorimetry enthalpy measurements of Berezin and Chekhovskoi[38] (1345-2173 K) and Lin and Frohberg[12] (1809-2176 K in the solid region) initially agree at 1800 K but close to the experimental limit the deviation increases to 2.0%, which is considered to be a poor agreement for consideration in the evaluation. Therefore selected values are based on the closely agreeing smoothed specific heat values of Takahashi et al.[36] (82-991 K) and Cezairliyan et al.[39] (1500-2100 K) which give an exact fit if the specific heat is represented by two equations with a crossover temperature of 1478 K:
Based on the original experimental values, Eq 3 has an overall accuracy of 0.3% and Eq 4 0.9%. Derived enthalpy and entropy equations are given in Table 7.
Compared to the solid enthalpy equations given in Table 7 the smoothed enthalpy values of Berezin and Chekhovskoi[38] are initially 2.1% low then converge to the selected values being only 0.3% high close to the melting point whilst the smoothed values of Lin and Frohberg[12] trend from 0.8% low to 1.7% low.
Derived values are given in Table 18 whilst deviations of high temperature solid specific heat values are given in Table 12 and deviations of solid enthalpy values in Table 13 except for the specific heat values of Bendick and Pepperhoff[40] (350-1700 K) which were shown only in the form of a small graph and were not included. All values were compared without correction for possible temperature scale effects.
2.4 A Comparison of Selected Values at 298.15 K
The comparison is given in Table 2. The selected enthalpy and entropy values of Desai[10] and in the present review are similar since they are based on the same data sources.
3 Liquid Phase
After correction to the selected melting point, the enthalpy of fusion determined by Berezin and Chekhovskoi[38] corrected to 23013 ± 516 J/mol, and the value determined by Lin and Frohberg[12] corrected to 23033 ± 618 J/mol, were averaged to 23023 ± 400 J/mol, which is selected and fixes the liquid enthalpy at the melting point as H°T − H°298.15 = 85,738 J/mol.
Liquid enthalpy values of Berezin et al.[41] (2084-2325 K) and Lin and Frohberg[12] (2140-2632 K) were re-evaluated leading to liquid specific heat values of 46.225 ± 1.41 J/(mol K) and 46.717 ± 0.58 J/(mol K) respectively and averaging to 47.471 ± 0.71 J/(mol K). In combination with the enthalpy value at the melting point the enthalpy of the liquid can then be represented by the selected equation:
The selected specific heat is in satisfactorily agreement with the later drop calorimetric value of Schaefers et al.[42] (2016-2403 K) as given in Table 3. Thermodynamic properties derived from Eq 5 are also given in Table 18 and deviations of liquid enthalpy values from the selected values are given in Table 14.
4 Gas Phase
4.1 Thermodynamic Properties of the Gas Phase
Selected values are based on the 544 energy levels selected by Thorne et al.[48] The thermodynamic properties were calculated using the method of Kolsky et al.[49] and the 2014 Fundamental Constants (Mohr et al.[50,51]). Derived thermodynamic values are given in Table 18.
4.2 Enthalpy of Sublimation
Because of a general lack of detail as to what temperature scales were used and problems associated with the exact measurement of temperature then no attempt was made to correct vapor pressure measurements to ITS-90 from what would have been contemporary scales. Values are summarised in Table 4 where the selected value of 515 ± 2 kJ/mol is an average of the last four Third Law determinations.
5 Vapor Pressure
The vapor pressure equations as given in Table 5 was evaluated for the solid from free energy functions for the solid and the gas at 50 K intervals from 1150 K to 2200 K and the melting point and for the liquid at the melting point and at 50 K intervals from 2250 to 3700 K and were fitted to the following equation:
6 Comments on Previous Reviews of the Thermodynamic Properties
Other reviews are considered by comparing selected solid specific values in the high temperature region. As shown in Table 15 older reviews such as those of Hultgren et al.,[6] Smith,[7] Gurvich et al.[8] and JANAF (Chase[9]) tended to show maximum high deviations in the region of 1500-1700 K and then tended to show a marked deviation low in the region of the melting point. More recent reviews such as those of Desai[10] and Maglić[11] tended to show better agreement with the presently selected values.
7 Summary of Representative Equations
Low temperature specific heat equations are given in Table 6 and high temperature representative equations are given in Table 7, free energy equations are given in Table 8 and transition values involved with the free energy equations in Table 9.
8 Deviations from the Selected Values
Deviations of superconducting specific heat values are given in Table 10 and low temperature normal state specific heat in Table 11. Deviations of high temperature specific heat in Table 12 and enthalpy values in the solid and liquid ranges in Tables 13 and 14 respectively. Deviations of other selected specific heat values are given in Table 15.
9 Thermodynamic Tables
Low temperature thermodynamic properties of the solid are given in Tables 16 and 17 and of the high temperature thermodynamic properties of the condensed phases in Table 18. Thermodynamic properties of the gas are given in Table 19 whilst the vapor pressure summary is given in Table 20.
References
J.W. Arblaster, Thermodynamic Properties of Silver, J. Phase Equilib. Diffus., 2015, 36(6), p 573-591
J.W. Arblaster, Thermodynamic Properties of Gold, J. Phase Equilib. Diffus., 2016, 37(2), p 229-245
J.W. Arblaster, Thermodynamic Properties of Beryllium, J. Phase Equilib. Diffus., 2016, 37(5), p 581-591
J.W. Arblaster, Thermodynamic Properties of Copper, J. Phase Equilib. Diffus., 2015, 36(5), p 422-444
J.W. Arblaster, Thermodynamic Properties of Hafnium, J. Phase Equilib. Diffus., 2014, 35(4), p 490-501
R. Hultgren, P.D. Desai, D.T. Hawkins, M. Gleiser, K.K. Kelley, and D.D. Wagman, Selected Values of the Thermodynamic Properties of the Elements, American Society for Metals, Metals Park, 1973
J.F. Smith, The V (Vanadium) System, Bull. Alloy Phase Diag., 1981, 2, p 40-41
L.V.Gurvich, I.V.Veits, V.A.Medvedev, G.A.Bergman, V.S.Yungman, G.A.Khachkuruzov, V.S.Yorish, o.v.Dorofeeva, E.L.Osina, P.I.Tolmach, I.N.Przhevak’skii, I.I.Nazarenko, N.M.Aristova, E.A.Shenyavskaya, L.N.Gorokhov, A.L.Rogatskii, M.E.Efimov, V.Ya.Leonidov, Y.G. Khait, A.G. Efimova, S.E. Tomberg, A.V. Gusarov, N.E. Khandamirova, G.N. Yurkov, L.R. Fokin, L.F. Kuratova and A.D. Gol’dshtein, Thermodynamic Properties of Individual Substances, V.P. Glushko, L.V. Gurvich, G.A. Bergman, I.V. Veits, V.A. Medvedev, G.A. Khachkuruzov and V.S. Yungman (Ed.), “Nauka”, Moscow, Vol. 4, 1982
M.W. Chase Jr., NIST-JANAF Thermochemical Tables, 4th edn, J. Phys. Chem. Ref. Data, Monograph No. 9, 1998
P.D. Desai, Thermodynamic Properties of Vanadium, Int. J. Thermophys., 1986, 7, p 213-228
K.D. Maglić, Recommended Specific Heat Capacity Functions of Group VA Elements, Int. J. Thermophys., 2003, 24, p 489-500
R. Lin and M.G. Frohberg, Enthalpy Measurements on Solid and Liquid Vanadium by Levitation Calorimetry, Z. Metallkde, 1991, 82, p 48-52
H.A. Leupold, G.J. Iafrate, F. Rothwarf, J.T. Breslin, D. Edmiston, and T.R. AuCoin, Low-Temperature Specific Heat Anomalies in the Group V Transition Metals, J. Low Temp. Phys., 1977, 28, p 241-261
E. Rudy and S. St.Windisch, The Phase Diagrams Hafnium-Vanadium and Hafnium-Chromium, J. Less Common Metals, 1968, 15, p 13-27
J.P. Hiernaut, F. Sakuma, and C. Ronchi, Determination of the Melting Point and the Emissivity of Refractory Metals with a Six-Wavelength Pyrometer, High Temp High Pressures, 1989, 21, p 139-148
G. Pottlacher, T. Hüpf, B. Wilthan, and C. Cagran, Thermophysical Data of Liquid Vanadium, Thermochim. Acta, 2007, 461, p 88-95
Commission on Isotopic Abundances and Atomic Weights (CIAAW), Atomic Weights of the Elements 2015, ciaaw.org/atomic-weights.htm, Aug. 2015
T.B. Douglas, Conversion of existing calorimetrically determined thermodynamic properties to the basis of the international practical temperature Scale of 1968, J. Res. Natl. Bur. Stand., 1969, 73A, p 451-470
R.L. Rusby, The Conversion of Thermal Reference Values to the ITS-90, J. Chem. Thermodyn., 1991, 23, p 1153-1161
R.L. Rusby, R.P. Hudson, and M. Durieux, Revised Values for (t90-t68) from 630 C to 1064 C, Metrologia, 1994, 31, p 149-153
R.D. Weir and R.N. Goldberg, On the Conversion of Thermodynamic Properties to the Basis of the International Temperature Scale of 1990, J Chem Thermodynamics, 1996, 28, p 261-276
D.G. Westlake, Anomalies in the Physical Properties of Vanadium, The Role of Hydrogen, Philos. Mag., 1967, 16, p 905-908
D.G. Westlake, S.T. Ockers, M.H. Mueller, and K.D. Anderson, Reexamination of Vanadium for Allotropy, Metall. Trans., 1972, 3, p 1711-1713
R.K. Bollinger, B.D. White, J.J. Neumeier, H.R.Z. Sandim, Y. Susuki, C.A.M. dos Santos, R. Avci, A. Migliori, and J.B. Betts, Observation of a Martensitic Structural Distortion in V, Nb and Ta, Phys. Rev. Lett., 2011, 107, p 075503-1-075503-4
W.D. Jung, F.A. Schmidt, and G.C. Danielson, Thermal Conductivity of High-Purity Vanadium, Phys. Rev. B, 1977, 15, p 659-665
Y.M. Smirnov and V.A. Finkel’ Crystal Structure of Tantalum, Niobium and Vanadium at 110 to 400°K, Zh. Eksp. Teor. Fiz., 1965, 49, p 1077-1082 (Sov. Phys. JETP 1966, 22 p 750-753)
N. Schmitz-Pranghe and P. Dünner, Gitterstruktur und Thermische Ausdechung der Übergangsmetalle Scandium, Titan, Vanadin und Mangan, Z. Metallkde, 1968, 59, p 377-382
J.L. Henry, S.A. O’Haire, R.A. McCune, and M.P. Krug, The vanadium-oxygen system: phase relations in the vanadium-rich region below 1200 C, J. Less Common Metals, 1970, 21, p 115-135
V.A. Finkel’, V.I. Glamazda, and G.P. Kovtun, A Phase Transition in Vanadium, Z. Eksp. Teor. Fiz., 1969, 57, p 1065-1068 (Sov. Phys. JETP, 1970, 30, p 581-583)
Y.L. Shen, Low Temperature Heat Capacities of Vanadium, Niobium and Tantalum, Ph.D. Thesis, University of California, Lawrence Radiation Laboratory, Berkeley, California. U.S. Atomic Energy Commission Rept. UCRL-16117, 1965
R. Radebaugh and P.H. Keesom, Low-Temperature Thermodynamic Properties of Vanadium. I. Superconducting and Normal States, Phys. Rev., 1966, 149, p 209-216
G.J. Sellers, A.C. Anderson, and H.K. Birnbaum, The Anomalous Heat Capacity of Superconducting Niobium, Phys. Lett. A, 1973, 44, p 173-174
G.A. Alers, Elastic Moduli of Vanadium, Phys. Rev., 1960, 119, p 1532-1535
D.I. Bolef, R.E. Smith, and J.G. Miller, Elastic Properties of Vanadium. I. Temperature Dependence of the Elastic Constants and the Thermal Expansion, Phys. Rev. B, 1972, 3, p 4100-4108
C.T. Anderson, The Heat Capacities of Vanadium, Vanadium Trioxide, Vanadium Tetroxide and Vanadium Pentoxide at Low Temperatures, J. Am. Chem. Soc., 1936, 58, p 564-566
Y. Takahashi, J.-I. Nakamura, and J.F. Smith, Laser Flash Calorimetry. III. Heat Capacity of Vanadium from 80 to 1000 K, J. Chem. Thermodyn., 1982, 14, p 977-982
N.A. Chernoplekov, G.K. Panova, B.N. Samoĭlov, and A.A. Shikov, Change of the Vanadium Phonon Spectrum Following Introduction of Tantalum Admixtures, Zh. Eksp. Teor. Fiz., 1972, 63, p 1381-1389 (Sov. Phys. JETP, 1973, 36, p 731-735)
B.Y. Berezin and V.Y. Chekhovskoi, Enthalpy and Heat Capacity of Niobium and Vanadium in the Region from 298.15°K to the Melting Point, Teplofiz. Vys. Temp., 1977, 15, p 772-778 (High Temp., 1977, 15,p 651-656)
A. Cezairliyan, F. Righini, and J.L. McClure, Simultaneous Measurements of Heat Capacity, Electrical Resistivity and Hemispherical Total Emittance by a Pulse Heating Technique: vanadium, 1500 to 2100 K, J. Res. Natl. Bur. Stand., 1974, 78A, p 143-147
W. Bendick and W. Pepperhoff, The Heat Capacity of Ti, V and Cr, J. Phys.F, Met. Phys., 1982, 12, p 1085-1090
B.Y. Berezin, V.Y. Chekhovskoy, and A.E. Sheindlin, The Enthalpy and Specific Heat of Molten Vanadium, High Temp. Sci., 1972, 4, p 478-486
K. Schaefers, M. Rösner-Kuhn, and M.G. Frohberg, Enthalpy Measurements of Undercooled Melts by Levitation Calorimetry: the Pure Metals Nickel, Iron, Vanadium and Niobium, Mater. Sci. Eng. A, 1995, 197, p 83-90
U. Seydel, H. Bauhof, W. Fucke, and H. Wadle, Thermophysical Data for Various Transition Metals at High Temperatures Obtained by a Submicrosecond-Pulse-Heating Method, High Temp High Press, 1979, 11, p 635-642
G.R. Gathers, J.W. Shaner, R.S. Hixon, and D.A. Young, Very High Temperature Thermophysical Properties of Solid and Liquid Vanadium and Iridium, High Temp. High Press, 1979, 11, p 653-668
J.A. Treverton and J.L. Margrave, Thermodynamic Properties by Levitation Calorimetry. III. The Enthalpies of Fusion and Heat Capacities for the Liquid Phases of Iron, Titanium and Vanadium, J. Chem. Thermodyn., 1971, 3, p 473-481
B.Y. Berezin, V.Y. Chekhovskoi and A.E. Sheindlin, Heat of Fusion of Vanadium, Dokl.Akad.Akad.Nauk SSSR, 1971, 201, p 583-585 (Sov.Phys. Doklady, 1972, 16, p 1007-1009)
P.-F. Paradis, T. Ishikawa, T. Aoyama, and S. Yoda, Thermophysical Properties of Vanadium at High Temperatures Measured with an Electrostatic Levitation Furnace, J. Chem. Thermodyn., 2002, 34, p 1929-1942
A.P. Thorne, J.C. Pickering, and J. Semeniuk, The Spectrum and Term Analysis of VI, Astrophys. J. Suppl. Ser., 2011, 192, p 111-1111
H.G. Kolsky, R.M. Gilmer and P.W. Gilles, The Thermodynamic Properties of 54 Elements Considered as Ideal Monatomic Gases. U.S. Atomic Energy Commission Rept. LA 2110, 1957
P.J. Mohr, D.B. Newell, and B.N. Taylor, CODATA Recommended Values of the Fundamental Physical Constants: 2014, Rev Mod Phys, 2016, 88, p 035009-1-035009-73
P.J. Mohr, D.B. Newell, and B.N. Taylor, CODATA Recommended Values of the Fundamental Physical Constants: 2014, J. Phys. Chem. Ref. Data, 2016, 45, p 043102-1-043102-74
E.K. Storms, A. Lowe, E. Baca, and J. Griffin, The Vaporization Behaviour of the Defective Carbides. III. The Vanadium-Carbon System, High. Temp. Sci., 1973, 5, p 276-290
R.H. Moore, D. Robinson, and B.B. Argent, The Use of High Resolution Mass Spectrometry in the Measurement of Thermodynamic Properties of Metallic Systems, J. Phys. E: Sci. Instrum., 1975, 8, p 641-648
J.W. Edwards, H.L. Johnston, and P.E. Blackburn, The Vapor Pressure of Inorganic Substances. VI. Vanadium Between 1666 °K and 1882 °K, J. Am. Chem. Soc., 1951, 73, p 4727-4729
R.K. Saxer, The Chemical Activities of Iron and Vanadium in Binary Iron-Vanadium Alloys and the Vapor Pressures of Pure Cobalt, Iron and Vanadium, The Ohio State University, Columbus, Ohio, Ph.D.Thesis, 1962
M. Farber and R.D. Srivastava, Effusion-Mass Spectrometric Study of Thermodynamic Properties of Vanadium and Vanadium Nitride, J. Chem. Soc., Faraday Trans., 1973, 69, p 390-398
W.S. Corak, B.B. Goodman, C.B. Satterthwaite, and A. Wexler, Atomic Heats of Normal and Superconducting Vanadium, Phys. Rev., 1956, 102, p 656-661
K. Clusius, P. Franzosini and U. Piesbergen, Ergebnisse der Tieftemperaturforschung. XXXII. Die Atom- und Elektronwärme des Vanadins und Niobs zwischen 10° and 273°K, Z.Naturforschg., 1960, 15a, p 728-734
Bieganski and B.Stalinski, Heat Capacities and Thermodynamic Functions of Vanadium and Vanadium Hydride within the Range 24 to 340°K. The Hydrogen Contribution to the Heat Capacity of Transition Metal Hydrides, Bull. Acad. Polon. Sci. Ser. Sci. Chim., 1961, 9, p 367-372
J.H. Boggs, R.A. Knezek and J.A. Wiebelt, Status Report on a Study of the Use of Furnace Calorimetry for the Rapid Determination of Specific Heats of Solids at High Temperature, U.S. Atomic Energy Commission, Rept. AECU-4282, 1959
R. Kohlhaas, M. Braun and O. Vollmer, Die Atomwärme von Titan, Vanadin und Chrom im Berich Hoher Temperaturen, Z.Naturforschg, 1965, 20a, p 1077-1079
L.P. Filippov and R.P. Yurchak, High Temperature Investigation of the Thermal Properties of Solids, Inzh. Fiz. Zh., 1971, 21, p 561-577 (J. Eng. Phys., 1971, 21, p 1209-1220)
L.P. Filippow, Untersuchung der Thermischen Eigenschaften im Stoff an der Moskauer Universität, Int. J. Heat Mass Transfer, 1973, 16, p 865-885
V.E. Peletskii, V.P. Druzhinin and Y.G. Sobol’, Thermophysical Properties of Vanadium at High Temperatures, High Temp. High Press., 1971, 3, p 153-159
V.Y. Chekhovskoi and R.G. Kalinkina, The True Specific Heat of Vanadium in the Temperature Range 300-900°K, Teplofiz.Vys.Temp., 1973, 11, p 885-886 (High Temp., 1973, 11, p 796-797)
B.E. Neimark, P.E. Belyakova, B.R. Brodskii, L.K. Voronin, S.F. Korytina and A.N. Merkul’ev, Physical Properties of Vanadium, Heat Transfer Sov.Res., 1973, 5, p 141-145
A.A. Kulish and L.P. Filippov, Determination of the Physical Properties of Group V Metals at High Temperatures by Means of a Study of Deformation Vibrations of Plates, Teplofiz.Vys.Temp., 1978, 16, p 602-610 (High Temp., 1978, 16, p 512-519)
A. Stanimirović, G. Vuković, and K. Maglić, Thermophysical and Thermal Optical Properties of Vanadium by Millisecond Calorimetry Between 300 and 1900 K, Int. J. Thermophys., 1999, 20, p 325-332
G.C. Beakley, A Comparative Method of Specific Heat Determination, Ph.D. Thesis, Oklahoma State University, Stillwater, Oklahoma, 1956
R.A. Knezek, Development and Evaluation of a Coating Material for Use in the Comparative Calorimetry Method of Determining Specific Heats of Solids, M.S. Thesis, Oklahoma State University, Stillwater, Oklahoma, 1959
F.M. Jaeger and W.A. Veenstra, The exact Measurement of the Specific Heats of Solid Substances at High Temperatures. VI. The Specific Heats of Vanadium, Niobium, Tantalum and Molybdenum, Rec. Trav. Chim., 1934, 53, p 677-687
I.B. Fieldhouse and J.I. Lang, Measurement of Thermal Properties, Wright Air Development Division, Air Research and Development Command, United States Air Force, Wright-Patterson Air Force Base, Ohio, WADD Tech.Rept. 60-904, 1961
Y.M. Goluvtin and T.M. Kozlovskaya, Variation of the Enthalpy and Heat Capacity in the Vanadium-Silicon System, Zh. Fiz. Khim., 1962, 36, p 362-364 (Russ. J. Phys. Chem., 1962, 36, p 183-184)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arblaster, J.W. Thermodynamic Properties of Vanadium. J. Phase Equilib. Diffus. 38, 51–64 (2017). https://doi.org/10.1007/s11669-016-0514-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-016-0514-7