Abstract
The Al-Ba was thermodynamically optimized with the help of CALPHAD method. The solution phases such as liquid, fcc and bcc phases were modeled as substitutional solution phases. The excess Gibbs energies of these phases were treated with Redlich-Kister polynomial functions. A set of self-consistent thermodynamic parameters for describing various phases in the Al-Ba system was obtained, which can well reproduce the corresponding experimental data. The Al-Ba-Ni ternary system were also extrapolated based on the present binary system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum amorphous alloys caused tremendous research interest due to their high specific strength, high toughness and high corrosion resistance.[1–4] However, small size of Al based amorphous alloy limits its application in the industry area. Improving the glass formation ability (GFA) of aluminum alloy is an important subject to obtain the large size amorphous alloys for our material researchers. It was proved that the addition of large atoms (such as rare earth elements) in the alloys can increase the glass formation ability (GFA).[5–7] The GFA of alloys were also improved when the addition of Ca, Sr, and Ba elements in the Al-Y-Ni-Co alloys.[8] Phase diagram of Al-Y-Ni-Co-(Ca, Sr, Ba) systems will be a fundamental information for us to understand the experimental phenomenon.[9,10]
The aim of this work is to establish a thermodynamic database of Al-Ba system based on the CALculation of PHAse Diagram (CALPHAD) technique, which is a sub-system of Al-Y-Ni-Co-(Ca, Sr, Ba) systems. With the help of this database, the phase diagram information can be obtained to investigate the glass formation ability (GFA) of Al alloys.[11]
Experiments Information
The Binary Systems
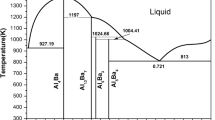
The thermodynamic describe for the Al-Ni and Ba-Ni systems are from literatures,[12,13] as shown in Fig. 1 and 2. The phase diagram information of Al-rich region (0 to 10 at.% Al) in the Al-Ba system were reported by Alberti[14] with the help of thermal and microscopic studies. And a eutectic reaction between liquid, Fcc_Al and Al4Ba at 934 K was indicated in this work.
The calculated phase diagram of the Al-Ni system[12]
The calculated phase diagram of the Ba-Ni system[13]
Based on the thermal and x-ray analysis, the phase relations of Ba-rich region (44 to 100 at.%) were investigated by Flanigen.[15] The Ba-rich region compound was determined as Al2Ba, which formed from pertectic reaction at 1118 K. And the eutectic reaction between liquid, Al2Ba and Ba at 811 K was confirmed in his work.
With the above phase information, Iida[16] used thermal analysis and microscopic analysis of 13 alloys to set up the whole phase diagram of the Al-Ba system. However, only Al4Ba phase was reported in his work.
The Al-Ba binary system was assessed by Elliott and Shunk,[17] three intermediate phases: Al4Ba, Al2Ba, AlBa had been reported in their work.[17] Structure analysis Al2Ba compound was not completed, but the formula Al2Ba was assigned to it based only on the DTA data. It was also reported that about 5.4 at.% Al dissolved in Bcc_Ba and No appreciable solid solubility of Ba in terminal phase Al was observed.
Bruzzone and Merlo[18] published a phase diagram of this system similar with that of Elliott and Shunk.[17] After their results, a reexamination was conducted by Fornasini,[19] this new experiments show that the formula of Al2Ba and AlBa compounds are more appropriate as Al13Ba7 and Al5Ba4. No solid solubility of Al and Ba in terminal phase was found in this work.
The enthalpy of mixing of A1-Ba alloys at 1215 K from 0 to 47 at.% Ba was determined by drop calorimetry.[20] Unfortunately, due to the temperature at 1215 K, only in the region of 0.0129<XBa<0.0450 and 0.3439<XBa<0.4664, their alloys were pure liquid. So only the enthalpies of mixing of the alloy compositions in these areas were accepted in our optimization. The activities of Ba in liquid Al-Ba alloys at 1373 K were determined with a combination of Knudsen effusion-mass and pseudo-isopiestic measurements.[21]
The Ternary System
The Al9BaNi2 ternary compound with hexagonal structure was reported by Turban and Schafer.[22] With the help of metallography, X-ray diffraction analysis and microhardness measurements, the isothermal section at 773 K of Al-Ba-Ni system was determined by Ainutdinov et al.[23] as shown in Fig. 3, and the Al9BaNi2 was also confirmed in their work. The solubility of Ba in the Al-Ni intermetallic compounds and of Ni in the Al-Ba intermetallic compounds is less than 3 at.%. In the present work, the solubility was ignored.
The experimental isothermal section of Al-Ba-Ni system at 773 K[23]
Up to now, there is no experimental or calculated thermochemistry of the Al-Ba-Ni system available in the literature. Therefore, the Al-Ba-Ni system has been extrapolated on basis of the above ternary phase diagram information.
Thermodynamic Models
The CALPHAD approach[24] was applied in the Al-Ba-Ni system, each phase in this system has a Gibbs energy function. For solution phase (φ) such as liquid, fcc, bcc and hcp, the Gibbs energy was described as followed:
where
where φ denotes the solution phases, x i (i = Al, Ba and Ni) denotes mole fraction of component i, and \(^{0} G_{i}^{\varphi }\) is the molar Gibbs energy of pure element i in the structural state of φ.
\(^{\left( j \right)} L_{{{\text{Al}},{\text{Ni}}}}^{\varphi }\) and \(^{\left( j \right)} L_{{{\text{Ba}},{\text{Ni}}}}^{\varphi }\) are taken from Ref 12 and 13 is describing as,
A j , B j and Cj are the adjusted parameters being optimized in the present work. \(L_{\text{Al,Ba,Ni}}^{\varphi }\) is ternary interaction parameters. Due to lack of experiment data, it is set to be zero.
For the intermetallic compounds (Al4Ba, Al13Ba7 and Al5Ba4), the Gibbs enegy function is :
The parameters A and B need to be optimized in this work.
For the ternary compound Al9BaNi2, it is modeled as stoichiometric phase AlxBayNiz, The Gibbs energy expression for each of these stoichiometric compounds is written as
where x, y and zare the stoichiometry ratios of the sublattices. A and B are the adjusted parameters to be optimized in the present work.
Results and Discussion
On the basis of lattice stabilities cited from Dinsdale,[25] the optimization of the Al-Ba system is carried out using the Parrot module in the Thermo-Calc program.[26] The parameters of liquid phase were first optimized from the enthalpies of mixing and activity data of the liquid alloys. Then, phase diagram information was introduced to obtain the parameters of solid phases. Finally, all parameters were evaluated together to make sure self-consistent. All the evaluated parameters are listed in Table 1.
Figure 4 shows the calculated phase diagram of Al-Ba system compared with experimental data,[14,16,18] while Table 2 lists the invariant reactions in the Al-Ba system. An agreement within 10 K between the calculated and experimentally determined temperature are achieved.
As shown in Fig. 5, the calculated mixing enthalpy is reasonably agreement with the experiment data.[20] The comparison of the experimental activity[21] and the calculated one are presented in Fig. 6. It is demonstrated that the experimental data of thermodynamically properties can be well described by the present calculation within the experimental errors.
The calculated enthalpy of mixing of liquid at 1215 K in Al-Ba system with the experimental data.[20] Reference states: liquid Al and liquid Ba
The calculated activity of Ba in liquid alloy at 1373 K in Al-Ba system with experimental data.[21] Reference states: liquid Ba
The calculated Al-Ba-Ni isothermal section at 773 K is shown in Fig. 7. The calculated isothermal section results have well reproduced the experimental data[23] except the phase between Al4Ba and AlNi. From the results of Ainutdinov et al.,[23] the two phase region of Al4Ba+AlNi existed. However, in our calculated results, the two phase region in this area is Al13Ba7+Al3Ni2. we found that it was very difficult to fit the result of Ainutdinov et al.[23] in our optimization, such two-phase region were not available even after introducing the solubilities of Ba in AlNi phase. Moreover, comparing with the other isothermal of Al-Ni-Ca and Al-Ni-Si systems[27,28] at 773 K, we found there are no such two phase regions (Al4Ca+AlNi and Al4Sr+AlNi), therefore we think our calculation is reasonable. Further experimental verification is still needed.
Conclusions
The Al-Ba system has been assessed thermodynamically based on reported experimental phase diagram and thermodynamic properties. Reasonable agreement between calculated and experimental data has been obtained and thermodynamic parameters for various phases in this system have been obtained.
References
A. Inoue, B. Shen, H. Koshiba, H. Kato, and A.R. Yavari, Cobalt-Based Bulk Glassy Alloy with Ultrahigh Strength and Soft Magnetic Properies, Nat. Mater., 2003, 2, p 661-663
J. Schroers and W.L. Johnson, Ductile Bulk Metallic Glass, Phys. Rev. Lett., 2004, 93, p 255506
B. Zhang, M.X. Pan, D.Q. Zhao, and W.H. Wang, “Soft” Bulk Metallic Glasses Based on Cerium, Appl. Phys. Lett., 2004, 85, p 61-63
Y.H. Liu, G. Wang, R.J. Wang, D.Q. Zhao, M.X. Pan, and W.H. Wang, Super Plastic Bulk Metallic Glasses at Room Temperature, Science, 2007, 315, p 1385-1388
Z.P. Lu and C.T. Liu, Role of Minor Alloying Additions in Formation of Bulk Metallic Glasses: A Review, J. Mater. Sci., 2004, 39(12), p 3965-3974
C.T. Liu and Z.P. Lu, Effect of Minor Alloying Additions on Glass Formation in Bulk Metallic Glasses, Intermetallics, 2005, 13, p 415-418
W.H. Wang, Roles of Minor Additions in Formation and Properties of Bulk Metallic Glasses, Prog. Mater. Sci., 2007, 52, p 540-596
J.Q. Wang, Y.H. Liu, S. Imhoff, N. Chen, D.V. Louzguine-Luzgin, A. Takeuchi, M.W. Chen, H. Kato, J.H. Perepezko, and A. Inoue, Enhance the Thermal Stability and Glass Forming Ability of Al-based Metallic Glass by Ca Minor-Alloying, Intermetallics, 2012, 19, p 35-40
L.G. Zhang, P.J. Masset, X.M. Tao, G.X. Huang, H.T. Luo, L.B. Liu, and Z.P. Jin, Thermodynamic Description of the Al-Cu-Y Ternary System, CALPHAD, 2011, 35, p 574-579
J.P. Huang, B. Yang, H.M. Chen, and H. Wang, Computational Study of Mobilities and Diffusion in Ti-Sn Alloy, J. Phase Equilib. Diffus., 2015, doi:10.1007/s11669-015-0390-6
L.G. Zhang, H.Q. Dong, J.F. Nie, F.G. Meng, J. Shan, L.B. Liu, and Z.P. Jin, Thermodynamic Analysis of the La-Mg-Ni Bulk Metallic Glass System, J. Alloy Compd., 2010, 491, p 123-130
N. Dupin, I. Ansara, and B. Sundman, Thermodynamic Re-assessment of the Ternary System Al-Cr-Ni, CALPHAD, 2001, 25, p 279-298
S.L. Shang, Z.J. Liu, and Z.K. Liu, Thermodynamic MODELING Of The Ba–Ni–Ti System, J. Alloy Compd., 2007, 430, p 188-193
E. Alberti, Investigation of the Aluminum-Barium System, Z. Metallkd., 1934, 26, p 6-9
E.M. Flanigen, Unpublished thesis, Syracuse University, Syacuse, NY (1952)
M. Iida, Nippon Kinzoku Gakkaishi, 1953, 17, p 632-634
R.P. Elliott and F.A. Shunk, The Al-Ba (Aluminum-Barium) System, Bull. Alloy Phase Diagr., 1981, 2, p 351-353
G. Bruzzone and F. Merlo, The Strontium-Aluminum and Barium-Aluminum Systems, J. Less Common Met., 1975, 39, p 1-6
M.L. Fornasini, Ba3Al5, A Simple Atomic Arrangement Also Present in More Complex Structures, Acta Crystallogr. C, 1988, 44, p 1355-1357
M. Notin, B. Djamshidi, and J.C. Gachon, Calorimetric Measurement of the Enthalpy of Formation of Some Al-Ba Alloys, Thermochim. Acta, 1982, 57, p 57-66
S. Srikanth and K.T. Jacob, Thermodynamics of Aluminum-Barium Alloys, Metall. Trans. B, 1991, 22, p 607-616
K. Turban and H. Schafer, Zur kenntnis des BaFe2Al9-strukturtyps: Ternäre aluminide at2Al9 MIT A = Ba, Sr und T = Fe, Co, Ni, J. Less-Common Met., 1975, 40, p 91-96
F.A. Ainutdinov, S.K.H. Khairidinov, and A.V. Vakhobov, The Phase Diagram of the Al-Ba-Ni System, Dokl. Akad. Nauk Tadzh. SSR, 1987, 30, p 169-172
X. Wang, L.B. Liu, M.F. Wang, X. Shi, G.X. Huang, and L.G. Zhang, Computational Modeling of Elastic Constants as a Function of Temperature and Composition in Zr–Nb Alloys, CALPHAD, 2015, 48, p 89-94
A.T. Dinsdale, SGTE Data for Pure Elements, CALPHAD, 1991, 15, p 317-425
B. Sundman, B. Jansson, J.O. Andersson, The Thermo-Calc Databank System. CALPHAD (1985) 153–190
A. Prince, Aluminium-Calcium-Nickel. Ternary Alloys, Vol 3, VCH Verlagsgesellschaft, Weinheim, 1990, p 618-619
A. Prince, Aluminium-Nickel-Strontium. Ternary Alloys, Vol 7, VCH Verlagsgesellschaft, Weinheim, 1993, p 481-482
Acknowledgments
The authors would like to express gratitude to the financially support by the National Natural Science Foundation of China (Grant Nos. 51371200 and 51501229), National Basic Research Program of China (2014CB644000), Fundamental Research Funds for the Central Universities, Central South University (502044009). This work is also sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, X., Liu, L., Jiang, Y. et al. Thermodynamic Assessment of the Al-Ba System. J. Phase Equilib. Diffus. 37, 345–349 (2016). https://doi.org/10.1007/s11669-016-0466-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-016-0466-y