Abstract
The solid-solid interfacial reaction in the Mg-Cu binary diffusion couples was studied at temperatures of 673, 703 and 733 K, with reaction time ranging from 24 to 72 h. MgCu2 and Mg2Cu were formed between Mg and Cu matrixes at the studied temperatures. The growths of MgCu2 and Mg2Cu followed the parabolic kinetics, which suggests that the growth of the two phases is controlled by bulk diffusion mechanisms. The activation energies for growths of MgCu2 and Mg2Cu were determined to be 147.57 ± 1.49 and 139.12 ± 1.30 kJ/mol, respectively. The interdiffusion coefficients were evaluated as functions of compositions in MgCu2 and Mg2Cu intermetallic phases at the studied temperatures, which were further utilized for evaluating the activation energies and frequency factors for interdiffusion in each phase. The Mg impurity diffusion coefficient in Cu was higher than the Cu impurity diffusion coefficient in Mg. The activation energy and pre-exponential factor for diffusion of Mg impurity in Cu were determined to be 139.38 ± 0.65 kJ/mol and 1.02(±0.26) × 10−4 m2/s, respectively, while those for diffusion of Cu impurity in Mg were determined to be 164.04 ± 7.18 kJ/mol and 3.10(±0.31) × 10−3 m2/s, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium (Mg) alloys with low density, high specific strength, good machinability and recyclability, are regarded as promising structural metallic materials and are increasingly used in aerospace, automotive and electrical industries (Ref 1-3). Mg is alloyed with Cu to enhance its high temperature strength (Ref 4). Moreover, Mg-Cu alloy exhibits extraordinary hydrogen storage capacities (Ref 5,6 ). In order to understand and predict the complex chemical reactions in Mg-Cu binary system, diffusion kinetics knowledge of Mg-Cu alloys is indispensable.
Recently, the formation and diffusion kinetics of intermetallic phases are investigated between Mg and Al, Zn, rare-earth elements through solid-solid diffusion couple experiments (Ref 7-10). Twenty years ago, the experimental investigation of reaction diffusion in Mg-Cu system was reported (Ref 11). Mg2Cu and MgCu2 in the Mg/Cu diffusion couples were observed in the temperature ranging from 683 to 748 K. The growth rate of the Mg2Cu layer obeyed a parabolic law while that of MgCu2 layer could not be evaluated. The experiment of reaction diffusion between Mg and Cu thin films determined that Mg2Cu was the first phase to form in the Mg-Cu system and Cu was the dominant diffusing species in Mg2Cu (Ref 12). However, no relevant diffusion data regarding interdiffusion coefficients of intermetallic phases and impurity diffusion coefficients in Mg-Cu system could be found in the literature.
The present work involves a detailed study into the diffusion reaction between pure Mg and pure Cu at temperatures 673, 703 and 733 K. The morphology and composition of the formed intermetallic phases are characterized. The interdiffusion coefficients and the phase growth kinetics are evaluated for the two binary intermetallic phases, Mg2Cu and MgCu2. Impurity diffusion coefficients are determined simultaneously.
Experimental
Pure Mg (99.98 wt.%), pure Cu (99.98 wt.%) were used. Mg/Cu diffusion couples were made by embedded technology, shown schematically in Fig. 1. A 2.5 mm diameter hole was drilled in the middle of a copper bar with a size of 6 mm in diameter and 6 mm in length to encase the magnesium. A magnesium bar with 2.55 mm diameter was filed to a taper pin in accordance with the size of the hole in the copper bar. The bar was sanded smooth, and then fitted into the hole tightly to form a diffusion couple. Due to the higher thermal expansion coefficient of Mg compared to that of Cu, an intimate contact between the Mg bar and Cu matrix was formed during annealing. The samples were encapsulated in quartz tubes at ambient pressure, and put into a furnace to react. The heat treated temperatures were at 673, 703 and 733 K, and the heat treated time of each temperature for 24, 48 and 72 h. After the diffusion anneal, the couples were immediately quenched by breaking the quartz capsule in cold water.
The diffusion couples were sectioned laterally and the surface is polished. The specimens were then metallographically prepared and etched for 5 s in the etchant of 20 mL glycerol, 2 mL hydrochloric acid, 3 mL nitric acid and 5 mL acetic acid, for optical microscopy (OM) and scanning electron microscopy (SEM). Each diffusion couple was examined using OM first to check the quality of the intermetallic layers at the interface. Then the microstructure of the interface was identified using the SEM and the constituent phases were determined using an x-ray diffractometer (XRD). A minimum of 20 random-location measurements radially were made to determine the average thickness of each intermetallic layer from backscatter electron (BSE) micrographs. Electron probe microanalysis (EPMA) was employed to determine the concentration profiles of diffusion couples using a point-to-point scan with a 1 μm step size. An accelerating voltage of 20 kV was used with a 20 nA beam current, a spot size of ~1 μm. The pure metals Mg and Cu were used as the calibration standards. A ZAF (factors of atomic number denoted as Z, absorption as A, and fluorescence correction as F) method correction was employed for converting the x-ray intensity to the concentration.
Results and Discussion
Microstructure and Phase Composition of the Diffusion Reaction Layers
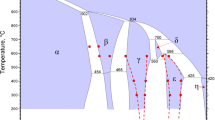
Figure 2 shows BSE images of the Mg/Cu diffusion couples annealed at 673, 703 and 733 K for 24 h. It can be seen that two intermetallic layers are formed between Mg and Cu at all temperatures studied. Figure 2(b) and (c) show a separation and a notable contrast between the Mg and Mg2Cu intermetallic layer. The probable reason is that the samples were etched by etchant. Mg was etched, but Mg2Cu was not etched at the Mg and Mg2Cu interface. Deep Mg2Cu was exposed. Different depth of Mg2Cu leads to the contrast. The corresponding concentration profiles determined by EPMA are presented in Fig. 3. The XRD patterns of the Cu side and the Mg side for Mg/Cu diffusion couple diffused at 733 K for 24 h is shown in Fig. 4. The XRD patterns of Mg2Cu and MgCu2 are in close agreement with JCPDS card number 65-2526 and JCPDS card number 65-9042, respectively. A schematic diagram of the XRD samples preparation procedures is shown in Fig. 4. A long strip is cut from the center of the Mg bar along the longitudinal direction, which contains the diffusion layer. Then the strip is divided into two parts at the center of the diffusion layers along the longitudinal direction. The separated two parts are ground at the diffusion layers until the diffusion layer is so thin that only one kind of the intermetallic layer remains. Then, these two sides were used for diffraction measurement. EPMA and XRD analyses confirmed that these two layers are MgCu2 and Mg2Cu. The MgCu2 layer is close to the Cu side, and the Mg2Cu layer is close to the Mg side. Mg-Cu binary phase diagram (Ref 13) is shown in Fig. 5, in which the dashed lines indicate the tie lines for the three diffusion couple temperatures. The phase diagram suggests that the two intermetallic phases existing between Cu and Mg at 673, 703 and 733 K are MgCu2 and Mg2Cu. Thus, the formation of the two intermetallic phases in the Mg/Cu diffusion couples studied is in accordance with the equilibrium phase diagram. The solubility ranges of Cu in the MgCu2 are shows in Fig. 3, which are 64.51-66.26 at.% Cu at 673 K, 64.92-66.86 at.% Cu at 703 K and 65.42-67.5 at.% Cu at 733 K. The compositions of Cu in MgCu2 intermetallic phase boundary have been obtained by EPMA, as shown in Fig. 5. The present result is in agreement with the phase diagram. A line intermetallic phase for the Mg2Cu is observed in Fig. 3 in accordance with the phase diagram. The Mg2Cu, with almost no solubility, was much thicker than the MgCu2 at all temperatures examined. The position of the Matano interface is shown in Fig. 3. This result agrees well with those reported by Nonaka et al. (Ref 11).
The porosities near Mg matrix of the couples are shown in Fig. 3. They may be shrinkage induced porosities, rather than Kirkendall porosities. Because shrinkage porosity would be of the “worm hole” type that is interconnected (Ref 14), while Kirkendall porosity is normally individual spheres (Ref 15,16).
Growth Kinetics of Intermetallic Phases
Growth constants for both MgCu2 and Mg2Cu phases are determined to find the growth kinetics for the intermediate phases. The diffusion layer thickness against square root of time is plotted in Fig. 6. As it can be seen in the figure, the linear fit of the experimental data for diffusion reaction layers intersects through the origin, which proves that the nucleation time is negligible compared with the annealing time. It is suggested that the layer growth process is diffusion controlled, and the growth of the intermetallic phase layers obeys a parabolic growth law:
where x is the thickness of intermetallic layer, t is the annealing time and k is the growth constant. An Arrhenius relationship can be applied for the growth constant k:
where k 0 is the pre-exponential factor, R is the gas constant, Q is the activation energy and T is the annealing temperature.
The plot of lnk against 1/T are presented in Fig. 7. Table 1 reports the growth constants, pre-exponential factor, and the activation energy for each layer calculated using Eq 1 and 2. MgCu2 layer has higher activation energy than Mg2Cu layer. However, MgCu2 layer has lower pre-exponential factor than Mg2Cu layer. The growth constants of MgCu2 layer are an order of magnitude lower than Mg2Cu layer at the same temperature. The activation energy for the Mg2Cu phase from our study is slightly lower than the result of Nonaka et al. (Ref 11).
Analysis of Interdiffusion Coefficients
The interdiffusion coefficients of intermetallic phases in binary alloys can be calculated using the famous Heumann-Matano method (Ref 17,18). This method is valid for the intermetallic phase that has a linear concentration profile throughout the entire phase:
where \(\tilde{D}_{\text{i}}\) and C i are the interdiffusion coefficient and the concentration of solute of i phase, respectively, x is the distance from the Matano interface, t the diffusion time, w i the layer thickness of i phase, \(\Delta C_{i}\) the concentration difference of solute between both ends of i phase and \(C_{i}^{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-0pt} 2}}}\) is the middle solute composition of the i phase.
The interdiffusion coefficients of MgCu2 and Mg2Cu phases were calculated using Eq 3. The calculated average interdiffusion coefficients, activation energies and frequency factors of MgCu2 and Mg2Cu intermetallic phases are listed in Table 2 and Arrhenius plot shown data points of three different anneal times for each temperature in Fig. 8.
Impurity Diffusion Coefficients
The Hall interdiffusion coefficient at the infinitely dilute composition can be estimated as the impurity diffusion coefficient by extrapolating the diffusion profile to a zero concentration. Thus the concentration versus distance profiles obtained in the terminal phases of a diffusion couple can be used to evaluate impurity diffusion coefficients based upon the method proposed by Hall (Ref 19). According to Hall’s method the relationships are given as:
where C 0 is the initial concentration and h and k are constant parameters, the variable u is a linear function of the Boltzmann parameter \(\lambda = \frac{x}{\sqrt t }\). Thus, the plot of u versus λ is fitted with a straight line whose slope is equal to h and the intercept is k. Once the values of h and k are found, the impurity diffusion coefficients can be obtained by the equation:
Figure 9 shows the concentration profiles of Cu in Mg diffused and Mg in Cu diffused at 673-733 K after 24 h of annealing. Table 3 reports the estimated average impurity diffusion coefficients of Mg in Cu (\({}^{\text{Cu}}D_{\text{Mg}}^{ * }\)) and Cu in Mg (\({}^{\text{Mg}}D_{\text{Cu}}^{ * }\)), and the corresponding activation energies and frequency factors at 673, 703 and 733 K. The estimates \({}^{\text{Cu}}D_{\text{Mg}}^{ * }\), is an order of magnitude faster than \({}^{\text{Mg}}D_{\text{Cu}}^{ * }\), however, the activation energy and frequency factor of Mg in Cu are lower than Cu in Mg.
Impurity diffusion coefficients for various elements in Mg, and the self-diffusion coefficients for Mg and Cu are presented in Fig. 10. It can be seen that diffusion of Zn (Ref 20), Ag (Ref 20), La (Ref 21), and Ce (Ref 21) in Mg is faster than the self-diffusion of Mg (Ref 21), while In (Ref 20), Mn (Ref 22), Be (Ref 23), Fe (Ref 24), Ni (Ref 24), U (Ref 24), Al (Ref 25) and Cu diffuse in Mg slower than Mg itself. Mg self-diffusion is faster than Mg impurity diffusion in Cu, which in turn, is faster than the Cu impurity diffusion Mg, self-diffusion coefficient of Cu is the lowest (Ref 26), \(D_{\text{Mg}}^{ * }>{}^{\text{Cu}}D_{\text{Mg}}^{ * }>{}^{\text{Mg}}D_{\text{Cu}}^{ * }>D_{\text{Cu}}^{ * }\).
Conclusions
Diffusion kinetics in Mg-Cu binary system has been investigated at the temperature 673, 703 and 733 K, with reaction time ranging from 24 to 72 h, respectively. The main conclusions are summarized as follows:
-
1.
MgCu2 and Mg2Cu intermetallic phases were detected in the Mg/Cu diffusion zones in this work. The relationships between thickness of intermetallic phases and reaction times follow the parabolic law, indicating that the growth kinetics of the intermetallic phases is diffusion controlled. The activation energies of MgCu2 and Mg2Cu intermetallic phases for the growth were 147.57 ± 1.49 and 139.12 ± 1.30 kJ/mol, respectively.
-
2.
The interdiffusion coefficients and activation energies for interdiffusion of MgCu2 and Mg2Cu intermetallic phases were evaluated as functions of compositions in the temperature range of 673-733 K. Heumann-Matano method was utilized to evaluate the interdiffusion coefficients as functions of composition in MgCu2 and Mg2Cu intermetallic phases occurred in the diffusion couples. The activation energies for interdiffusion of MgCu2 and Mg2Cu intermetallic phases were 143.52 ± 10.45 and 97.19 ± 6.01 kJ/mol.
-
3.
Impurity diffusion coefficients of \({}^{\text{Cu}}D_{\text{Mg}}^{ * }\) and \({}^{\text{Mg}}D_{\text{Cu}}^{ * }\), and the corresponding activation energy and frequency factor were determined based on Hall’s method. The activation energies of Cu in Mg and Mg in Cu were 164.04 ± 7.18 and 139.38 ± 0.65 kJ/mol, respectively. The frequency factors of Cu in Mg and Mg in Cu were 3.10(±0.31) × 10−3 m2/s and 1.02(±0.26) × 10−4 m2/s, respectively.
References
M. Kiani, I. Gandikota, M. Rais-Rohani, and K. Motoyama, Design of Lightweight Magnesium Car Body Structure Under Crash and Vibration Constraints, J. Magnes. Alloys, 2014, 2(2), p 99-108
A.A. Luo, Magnesium Casting Technology for Structural Applications, J. Magnes. Alloys, 2013, 1(1), p 2-22
B.L. Mordike and T. Ebert, Magnesium: Properties-Applications-Potential, Mater. Sci. Eng., A, 2001, 302(1), p 37-45
M. Mezbahul-Islam, A.O. Mostafa, and M. Medraj, Essential Magnesium Alloys Binary Phase Diagrams and Their Thermochemical Data, J. Mater., 2014, doi:10.1155/2014/704283
J. Lei, H. Huang, X. Dong, J. Sun, B. Lu, M. Lei, Q. Wang, C. Dong, and G. Cao, Formation and Hydrogen Storage Properties of In Situ Prepared Mg-Cu Alloy Nanoparticles by Arc Discharge, Int. J. Hydrogen Energy, 2009, 34(19), p 8127-8134
K. Tanaka, H.T. Takeshita, K. Kurumatani, H. Miyamura, and S. Kikuchi, The Effect of Initial Structures of Mg/Cu Super-Laminates on Hydrogen Absorption/Desorption Properties, J. Alloys Compd., 2013, 580, p S222-S225
S.K. Das and I.-H. Jung, Effect of the Basal Plane Orientation on Al and Zn Diffusion in hcp Mg, Mater. Charact., 2014, 94, p 86-92
S. Brennan, K. Bermudez, N.S. Kulkarni, and Y. Sohn, Interdiffusion in the Mg-Al System and Intrinsic Diffusion in β-Mg2Al3, Metall. Mater. Trans. A, 2012, 43(11), p 4043-4052
A. Mostafa and M. Medraj, On the Atomic Interdiffusion in Mg-{Ce, Nd, Zn} and Zn-{Ce, Nd} Binary Systems, J. Mater. Res., 2014, 29(13), p 1463-1479
J. Dai, B. Jiang, X. Li, Q. Yang, H. Dong, X. Xia, and F. Pan, The Formation of Intermetallic Compounds During Interdiffusion of Mg-Al/Mg-Ce Diffusion Couples, J. Alloys Compd., 2015, 619, p 411-416
K. Nonaka, T. Sakazawa, and H. Nakajima, Reaction diffusion in Mg-Cu system, Mater. Trans., JIM, 1995, 36, p 1463-1466
Q.Z. Hong and F.M. d’Heurle, The Dominant Diffusing Species and Initial Phase Formation in Al-Cu, Mg-Cu, and Mg-Ni Systems, J. Appl. Phys., 1992, 72(9), p 4036
C. Coughanowr, I. Ansara, R. Luoma, M. Hamalainen, and H. Lukas, Assessment of the Cu-Mg System, Z. Metallkd., 1991, 82(7), p 574-581
P.D. Lee and J.D. Hunt, Hydrogen Porosity in Directional Solidified Aluminium-Copper Alloys: In Situ Observation, Acta Mater., 1997, 45(10), p 4155-4169
M.S.A. Karunaratne, P. Carter, and R.C. Reed, On the Diffusion of Aluminium and Titanium in the Ni-Rich Ni-Al-Ti System Between 900 and 1200 Degrees C, Acta Mater., 2001, 49(5), p 861-875
H. Strandlund and H. Larsson, Prediction of Kirkendall Shift and Porosity in Binary and Ternary Diffusion Couples, Acta Mater., 2004, 52(15), p 4695-4703
C. Matano, Phys (Trans), 8, 109 (1933), in Proc. Phys. Math. Soc. Japan, 1933, p 405
T. Heumann, ‘Zur Berechnung von Diffusions Koeffizienten bei Einund Mehrphasiger Diffusion in Festen Legierungen, Z. Physik. Chem., 1952, 201(1), p 168-187, in German
L.D. Hall, An Analytical Method of Calculating Variable Diffusion Coefficients, J. Chem. Phys., 1953, 21(1), p 87-89
K. Lal. Diffusion of Some Elements in Magnesium, CEA Report, 1967, p 54
K. Lal, V. Levy. Study of the Diffusion of Cerium and Lanthanum in Magnesium, Compt. Rend., Ser. C, 1966, 262, p 107
L. Jin, D. Kevorkov, M. Medraj, and P. Chartrand, Al-Mg-RE (RE = La, Ce, Pr, Nd, Sm) Systems: Thermodynamic Evaluations and Optimizations Coupled with Key Experiments and Miedema’s Model Estimations, J. Chem. Thermodyn., 2013, 58, p 166-195
V.F. Yerko, V.F. Zeleniskiy, and V.S. Krasnorutskiy, Difuzija Berillija v Magnii, Fiz. Met. Metalloved., 1966, 22(1), p 112-114, in Slovenian
L.V. Pavlinov, A.M. Gladyshev, and V.N. Bykov, Self-Diffusion in Calcium and Diffusion of Barely Soluble Impurities in Magnesium and Calcium, Phys. Met. Metall., 1968, 26(5), p 53-59
S. Brennan, A.P. Warren, K.R. Coffey, N. Kulkarni, P. Todd, M. Kilmov, and Y. Sohn, Aluminum Impurity Diffusion in Magnesium, J. Phase Equilib. Diffus., 2012, 33(2), p 121-125
K. Maier, Self-Diffusion in Copper at “Low” Temperatures, Phys. Status Solidi A, 1977, 44(2), p 567-576
Acknowledgments
The authors are grateful for the financial supports from Chongqing Science and Technology Commission (CSTC2013jcyjC60001, cstc2012ggB50003, cstc2013jcyjA50020), National Natural Science Foundation of China (51171212, 51474043, 51531002), and The National Science and Technology Program of China (2013DFA71070, 2013CB632200), and the Fundamental Research Funds for the Central Universities (CDJZR13138801, CDJXS12131106), Postdoctoral Science Foundation of China (2015M572452).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, J., Jiang, B., Zhang, J. et al. Diffusion Kinetics in Mg-Cu Binary System. J. Phase Equilib. Diffus. 36, 613–619 (2015). https://doi.org/10.1007/s11669-015-0417-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-015-0417-z