Abstract
Duplex stainless steel (DSS) grades are used in pulp mills for their superior properties and resistance to general corrosion. However, stress corrosion cracking (SCC) of DSS equipment has been experienced in different pulp mills. The susceptibility of DSS grades to SCC can be mainly attributed to the various heating processes involved during the manufacturing of industrial equipments, especially welding. It is generally understood that heating cycles during welding may affect the dual microstructure (ferrite/austenite ratio) of the steel, making it more prone to cracking in aggressive environments such as chlorides and caustics and further exposure to high temperatures. Welded 2205 DSS failed in white liquor (mainly NaOH + Na2S) was examined for SCC crack morphology and microstructure. Heat-treated 2205 DSS samples were tested in simulated white liquor to see the effect of microstructure on SCC susceptibility. Austenite is more susceptible to SCC than ferrite, but the SCC susceptibility primarily depends on the composition of the alloy and the chemistry of the exposure environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duplex stainless steels (DSSs) have a dual microstructure consisting of nearly equal volume fraction of α-ferrite (BCC) and γ-austenite (FCC) phases. The dual microstructure provides unique properties such as high tensile and fatigue strength, good toughness and formability and excellent resistance to general corrosion and stress corrosion cracking (SCC) [1–3]. Due to their superior properties they are increasingly being used in the oil and gas, pulp and paper, petrochemical, and other chemical process industries.
Commonly used DSS grades like 2205 and 2304 have better resistance to corrosion and SCC in high pH environments as compared to carbon steel and austenitic stainless steels. In the pulp and paper industry, carbon steel pulp mill equipment, like digesters, storage vessels, and ancillary equipment, have been found to undergo severe general corrosion and stress corrosion cracking [4–6]. Hence these equipments are being replaced by duplex stainless steels [5]. DSSs provide improved corrosion resistance in sulfide containing caustic solutions (pulping liquor) at high temperatures. Although most of DSS equipments in pulp mills have performed very well, SCC failures have been reported, in welded areas as well as in the base metal [7–9]. This has called for further investigation of the material and the environmental conditions under which different DSS alloys are susceptible to SCC in pulping environments.
Welding is one of the main fabrication processes which may influence the local microstructure of DSS. During welding, if the balance in ferrite–austenite ratio of duplex stainless steel is disrupted, it may become more prone to SCC in the presence of high temperature, tensile stresses, and aggressive environments [10]. During welding process, in the fusion zone and heat-affected zone (HAZ), the base metal may experience temperatures above 1100 °C. At such temperatures, the austenite phase dissolves partially or completely in the ferrite phase, depending upon the chemical composition of the alloy. Further rapid cooling may result in deviation from the balanced microstructure. During cooling, precipitation of brittle intermetallic phases may also take place, making it more susceptible to SCC, depending upon the corrosive environment. Precipitation of phases like nitrides, carbides and σ phase may take place. These phases can cause significant deterioration in fracture toughness and corrosion resistance of duplex stainless steels.
Duplex stainless steel equipments in different pulp mills have been reported to show stress corrosion cracking in the weld and HAZ in pressure vessels and other high stress components. This study was aimed at understanding and analyzing the failure of a white liquor accumulator plate section that failed in field in less than 3 months of service. The effect of welding conditions on the microstructure and phase stability of 2205 DSS and the effect of its microstructure on the stress corrosion cracking susceptibility in sulfide-containing caustic solution (white liquor) were investigated through a series of experiments under controlled environmental conditions.
Experimental Procedure
Analysis of Failed DSS White Liquor Accumulator
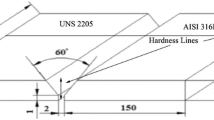
The failed 2205 DSS plate was removed from the hot white liquor accumulator for failure analysis. The vessel typically contained hot sulfide-containing caustic solution (white liquor) at ∼140 °C. White liquor was heated by introducing steam at about ∼240 °C at the top of this vessel. Cracks developed from SCC were visible near circumferential welds, which were field welds. Longitudinal welds were welded in shop. The cracks were parallel to the weld of the shell and were often found to have continued into the base metal. Figure 1 shows a section of the white liquor accumulator shell plate with a large crack in the weld region. The plate section was further sectioned to facilitate metallographic examination using optical and scanning electron microscopes (SEM). Samples were also etched to reveal their microstructure and crack morphology.
Slow Strain Rate Test of Simulated Welded Specimens
To further investigate the effect of welding parameters on SCC of DSS under simulated pulp mill conditions, welded duplex stainless steel bars with different compositions and filler materials were used in the lab study. The welding procedure used to prepare these test samples was done in accordance to standard EN1011-1. Flux cored arc welding (FCAW) and submerged arc welding (SAW) procedures used in this standard were in accordance with the process numbers 136 and 121, respectively. These welding methods were chosen as they are commonly used in manufacturing process of pulping equipments. SAW procedure was chosen to imitate the welding procedure used for the manufacturing of the white liquor accumulator. The chemical composition (in wt%) of the base material and weld consumables of the white liquor accumulator are presented in Table 1.
Tensile test samples for slow strain rate test (SSRT) were machined out of the welded DSS bars. Specimens were made such that the composition of the weld metal and base metal were very similar to that of the white liquor accumulator. The tensile samples had a gage length of 25.4 mm and a gage diameter of ∼3.175 mm. Samples were machined such that at least one edge of the weld was in the middle of the gage (Fig. 2). The welding methods and chemical compositions of the tensile specimens and the specimen codes are presented in Table 2. L and H in the specimen codes represent low chromium and high chromium filler metal, respectively. Whereas h and u denote welding in horizontal and upright positions, respectively.
The effect of different heating and cooling cycles on the phase balance of welded DSS was studied by measuring the Ferrite numbers for each weld, HAZ, and base metal. This was done using Feritscope®. Ferrite numbers gave the volume fraction of ferrite and austenite in each welded specimen. Subsequent to this, small samples of the weld and HAZs were cut from the bars. These samples were mounted, polished, and etched to reveal their microstructure, austenite–ferrite phase distribution and grain size in differently heat-treated parts of welded samples. Image analysis was also used to measure the area fraction of ferrite and austenite in weld and HAZ of each specimen.
After quantifying phase ratios and performing metallography, specimens were prepared for SSRTs to study their SCC susceptibility. The gage length of each tensile specimen was polished to 1000 grit paper finish and cleaned with acetone before using them for SSRT. The specimens were then subjected to an initial strain rate of 2 × 10−6 s−1 in synthetic white liquor (a mixture of 150 g/L NaOH + 50 g/L Na2S) at 170 °C and 200 °C. All tests were carried out in a SSRT rig equipped with an autoclave. Samples were electrically isolated from the test rig by use of ceramic washers. All SSRTs in this study were carried out under open circuit potential. Identical specimens were also subjected to SSRTs in the absence of environment (white liquor) for comparison.
After the test, the fractured samples were mounted, polished, and electroetched in 40% NaOH solution to see the crack morphology and to measure the crack length. Optical microscope was used to study the microstructure and examine for different precipitates formed after welding. The length of the deepest crack in each specimen was measured and was used to calculate the maximum crack velocity in mm/s.
Fractured surfaces were examined using SEM to examine the mode of fracture. Energy-dispersive spectroscopy (EDS) was used to obtain chemical composition of local areas of the fractured surface and phase composition.
Results and Discussion
Analysis of Failed 2205 DSS Plate
White liquor accumulator shell showed weld-related leaks within 3 months of installation. The accumulator vessel had suffered severe cracking, mainly in the circumferential welds of its shell. Figure 1 shows a section of the white liquor accumulator shell plate with a large crack in the weld region. Sections of the plate near crack and away from this crack were prepared for metallography. Relative ferrite to austenite volume fraction was quantified by measuring ferrite number in the weld and HAZ and compared with the ferrite numbers of the lab-welded 2205 DSS bars. The ferrite numbers, listed in Table 3, have an uncertainty of ±16% of the ferrite number measurement. To verify the phase ratio differences, measurements were also done by image analysis, where the ferrite to austenite area ratios is quantified. It is evident from the table that the ferrite/austenite ratio in the weld and HAZ of differently processed laboratory welded bar samples is very similar to that for the equivalent areas of the failed white liquor accumulator plate.
The micrograph of the weld bead and HAZ of the white liquor accumulator is shown in Fig. 3. HAZ, which appears as a clouded area near the weld, contains lesser amount of retransformed austenite. This may be due to the low heat input and faster cooling rate in this region. Hence the resulting microstructure in this region shows mostly ferrite phase due to the dissolution of austenite.
Examination of the failed plate section revealed that the white liquor accumulator failure was due to stress corrosion cracking in hot caustic solution, as suggested by the branched cracks shown in Figs. 4–6. The cracks seemed to have started in the weld region and had propagated through the HAZ into the base metal, as is evident from Fig. 4a–c. It was further evident from the micrographs that in all three regions, that is, the weld region, HAZ, and the base metal, the cracks seem to preferentially follow the austenite phase. This is clearly visible in Fig. 5a,b and Fig. 6a,b, respectively, where the cracks are following the lighter (austenite) phase. This indicates that the austenite phase is more susceptible to stress corrosion cracking and embrittlement in sulfide-containing caustic solutions.
Slow Strain Rate Tests
To understand the effect of weld-related microstructure on stress corrosion cracking susceptibility of 2205 DSS, welded tensile samples were used in SSRTs. Welding condition simulated and composition of materials used are listed in Table 2. Results indicate that most of the welded DSS samples tested in the white liquor fractured at a lower % fracture strain compared to equivalent samples tested in sand at the same test temperature. This is shown in Table 4. Also, the % strain to fracture was higher for specimens tested at 200 °C in white liquor as compared to equivalent specimens tested at 170 °C in the same environment. These results clearly indicate that sulfide-containing caustic solution (white liquor) has an adverse effect on the mechanical properties of the welded duplex stainless steel samples and this effect is more pronounced at higher temperatures (200 °C).
The crack velocity and the region of fracture for weld specimens at 170 °C and 200 °C are summarized in Table 5. As can be seen from the table, at 170 °C, the crack velocity was lower compared to the equivalent test at 200 °C. Also, at both tested temperatures, the submerged arc welded (SAW) specimen, 2205-SAW, showed the highest crack velocity among tested samples, compared to other samples which were welded by FCAW process. Weld metal of 2205-SAW samples had more Mn (which leads to higher volume fraction of austenite after similar heat treatment), as compared to the weld metal of other specimens tested in this study. Differences in the chemical composition of the filler metal may also be responsible for the higher SCC susceptibility of 2205-SAW weld. In all cases, cracks were transgranular in nature, both in the base metal and in the weld metal, as shown in Fig. 7.
All welded DSS samples tested in white liquor had stress corrosion cracks in the base metal S31803. SCC cracks were also found in the weld and HAZ of some of the specimens, as summarized in Table 5. Susceptibility of weld and HAZ to SCC was significantly different for differently welded specimens. Weld and the HAZ region of the specimens (2205-Hh) and (2205-Hu) was found to be more resistant to SCC and had fewer cracks in the weld and HAZ as compared to the base metal. Filler metal used for the 2205-Hh and 2205-Hu samples was richer in chromium and molybdenum, which may have contributed to their resistance to SCC in the weld region of these specimens.
A tensile sample machined out of base metal DSS (without welding) was also tested in white liquor. The 2205 DSS base metal sample was found to be susceptible to stress corrosion cracking in white liquor, with crack velocity of 1.8 × 10–6 mm/s. Hence, comparing results of welded 2205 DSS and unwelded 2205 base metal, it can be said that welded DSS as well as DSS base metal are equally susceptible to SCC in sulfide-containing caustic solution.
Fractography of samples tested in sand revealed microvoid coalescence and ductile mode of failure on the fracture surface (Fig. 8a), whereas specimens tested in white liquor showed stress corrosion cracking fracture and brittle mode of failure on the surface (Fig. 8b).
Examination of tested tensile sample under SEM revealed that crack initiation in the as-received DSS and welded DSS was preferentially in the austenitic phase. Phases were identified by EDS analysis of local areas in the SEM. Figure 9a,b shows crack initiation sites in the as-received DSS and welded specimen 2205-Hu respectively. Composition of the austenite and ferrite phases in the fractured specimens was found out by EDS and is given in Table 6. The EDS results from these samples confirmed that the SCC crack initiation prefers the austenite phase.
2205-Lh sample had the final fracture in the weld region. Micrograph of this specimen in Fig. 10 and EDS of this fracture surface also revealed that the small SCC cracks initiated and propagated in the austenitic phase. Hence in this study, crack initiation both in the weld region and in the base metal was found to be in the austenite phase. Previous study has shown that the austenite phase is in tension whereas the ferrite phase is in compression [8].
Conclusion
-
1.
2205 DSS white liquor accumulator which had been in operation for less than 3 months had suffered severe stress corrosion cracking. Cracks had started in the weld region and continued into the HAZ and base metal. Metallographic examinations showed that cracks were mainly in the austenite phase.
-
2.
Simulated welded 2205 DSS specimens with SAW similar to that of the accumulator and flux core arc welding were tested for SCC resistance in pulp mill environments (sulfide-containing caustic solutions). Results indicated that the submerged arc welded specimens with higher Mn content in the weld metal was most susceptible to stress corrosion cracking with highest crack velocities. Cracks were mostly in the weld region.
-
3.
Flux core arc welds with highly alloyed weld metal (higher chromium) was found to be the most resistant to stress corrosion cracking. These specimens had fewer cracks in the weld and HAZ as compared to the base metal.
-
4.
The severity of stress corrosion cracking and degradation of mechanical properties was found to increase with increase in temperature. This could explain the failure of the accumulator. Since the upper part of the accumulator was exposed to temperatures higher than 140 °C, it had become more susceptible to stress corrosion cracking.
-
5.
Unwelded 2205 DSS as-received specimens were also found to undergo severe stress corrosion cracking in sulfide-containing caustic solutions.
-
6.
SEM and EDS studies of SSRT specimens showed that crack initiation was mostly in the austenite phase. This suggests that the austenite phase in DSS is more susceptible to embrittlement and SCC sulfide-containing caustic solutions.
Summary
Failure analysis of failed DSS white liquor accumulator shell plate revealed that the failure was due to SCC in the weld region. Cracks were also found in the base metal in this section. Our lab results from welded samples tested in white liquor showed that weld region as well as base metal may be susceptible to SCC in sulfide-containing caustic solutions. Austenite phase was found to be more susceptible to cracking than the ferrite phase in all tested samples and the failed accumulator shell plate. However, SCC susceptibility depends upon the composition of alloy used and environmental conditions.
References
Chen, T.H., Yang, J.R.: Microstructural characterization of simulated heat affected zone in a nitrogen-containing 2205 duplex stainless steel. Mater. Sci. Eng. A338, 166–181 (2002)
Muthupandi, V., Bala Srinivasan, P., Seshadri, S.K., Sundaresan, S.: Effect of weld metal chemistry and heat input on the structure and properties of duplex stainless steel welds. Mater. Sci. Eng. A358, 9–16 (2003)
Nelson, D.E., Baeslack, W.A. III, Lippold, J.C.: Characterization of the weld structure in a duplex stainless steel using color metallography. Mater. Char. 39, 467–477 (1997)
Leinonen, H.T.: Corrosion resistance of duplex stainless steel and its welds in modern kraft batch cooking. 11th international symposium on corrosion in the pulp and paper industry, Charleston, SC, USA, 7–11 June 2004, pp. 55–66
Wensley, A.: Corrosion of carbon and stainless steels in kraft digesters. NACE corrosion, paper no. 589, Houston, CA, USA, 2000
Singbeil, D., Garner, A.: Caustic stress corrosion cracking of pressure vessel steels in dilute alkaline-sulfide solutions. NACE corrosion, paper no. 350, Boston, MA, USA, 1985
Gorog, M.: Digester outlet device scraper arm cracking. 2006 engineering, pulping and environmental conference, Atlanta, GA, USA, 5–8 November 2006
Leinonen, H.T., Pohjanne, P.: Stress corrosion cracking susceptibility of duplex stainless steels and their welds in simulated cooking environments. NACE corrosion 2006, paper no. 06244, San Diego, CA, USA, 12–16 March 2006
Reid, C.: Stress corrosion cracking of austenitic and duplex stainless steels in the kraft pulp mill. 1999 TAPPI engineering/process and product quality conference, Anaheim, CA, USA, 12–16 September 1999
Liu, H.Y., Hsieh, R.I., Tsai, W.T.: Microstructure and pitting corrosion in simulated heat-affected zones of duplex stainless steels. Corrosion Sci. 44, 2841–2856 (2002)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharya, A., Singh, P.M. Stress Corrosion Cracking of Welded 2205 Duplex Stainless Steel in Sulfide-containing Caustic Solution. J Fail. Anal. and Preven. 7, 371–377 (2007). https://doi.org/10.1007/s11668-007-9069-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11668-007-9069-6