Abstract
The development of high-strength cold spray deposits using amorphous/nanocrystalline aluminum high-entropy alloy (Al HEA) powder is hindered by the lack of understanding of correlations between powder microstructure and its deformation behavior. In this study, gas-atomized Al HEA powder (Al90.05-Y4.4-Ni4.3-Co0.9-Sc0.35 at.%) is devitrified at 298, 345, 362, and 450 °C to optimize strength and deformation for cold spraying. Devitrification-induced atomic rearrangement developed equiaxed Al grains and Al3Ni and Al3Sc precipitates. The amorphous content, growth of grains, hard precipitates, and reduced dislocation density increased the hardness by 16% to 515 HV at 298 °C and decreased the hardness by 55% to 190 HV at 450 °C. The compressive strength of Al HEA powder increased by 5% to 1559 MPa at 298 °C and decreased by 49% to 760 MPa at 450 °C. To enhance the limited sprayability of Al HEA powder, compressive strength is used to model optimized cold spray process maps. Helium gas with temperatures from 300 to 800 °C and a pressure of 40 bar can produce cold spray deposits with deposition efficiency greater than 70%. The scientific insights acquired from the present study provide a gateway toward developing novel lightweight and high-strength aluminum alloy deposits, thus marking an advancement in cold spray technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold spray (CS) is an innovative thermal spray technique that uses layer-by-layer deposition of feedstock powder to consolidate parts by plastic deformation (Ref 1). It has progressed from repair and coating deposition to additive manufacturing of large, structural freeform parts (Ref 1). Due to the diverse CS applications for repair, coating deposition, and additive manufacturing, various alloys such as aluminum, copper, and titanium have been well studied (Ref 1, 2). CS additive manufacturing (CSAM) has enormous potential to manufacture lightweight, high-strength structural components with aluminum alloys. Commercial polycrystalline aluminum (Al) alloys such as Al 2024 (Ref 3,4,5,6), Al 6061 (Ref 7,8,9,10), and Al 7075 (Ref 11,12,13) are used for developing thick cold sprayed deposits (> 5 mm). The highest strength, 550 MPa, is demonstrated by cold sprayed and heat-treated Al 7075 (Al-Mg-Zn-Cu) due to MgZn2 nanoscale precipitates (Ref 13). Although Al alloys (density 2.7 g cm−3) are most appropriate for making lightweight structures, their strength is limited when compared to other high-density structural materials like titanium (868 MPa, density 4.5 g cm−3) (Ref 14) and steel (723 MPa, density 7.85 g cm−3) (Ref 15). Accordingly, current high-strength Al alloys often cannot meet the increasing demand of many engineering applications requiring low density and high strength. Therefore, it is critical to address the need to develop high-strength Al alloys that can surpass the strength of Al 7075 to spearhead the lightweight applications of structural systems (Ref 16, 17).
To strengthen aluminum alloys beyond the limits of heat treatment, solutionizing, and precipitation hardening, an effective approach involves developing unique powder feedstock with high-entropy alloy compositions (HEAs). Unlike conventional solid solution or precipitation-hardenable alloys, HEAs comprise five or more principal elements, including rare earth elements (La, Ce, Gd, Y, or Sc) and transition metals (Ni, Co, or Fe) as solute atoms. These solute atoms, larger than aluminum, create a significant atomic size disparity (> 12%) and yield a negative enthalpy of mixing, reaching up to − 22 kJ mol−1. This restricts the formation of ordered crystal structures, instead favoring the formation of amorphous, high-entropy, metastable phases (Ref 16). Therefore, high-entropy aluminum alloys (Al HEAs) distinguish themselves from conventional polycrystalline Al alloys by exhibiting greater inherent strength due to their absence of long-range atomic order, the absence of low-energy deformation mechanisms like stacking faults and dislocations, and a high degree of phase stability (Ref 18). To date, a limited number of amorphous Al HEA powders such as Al88Ni6Y4.5Co1La0.5 (Ref 19), Al86Ni7Y5Co1La1 (Ref 20,21,22), Al86Ni8Co1La1Y2Gd2 (Ref 23), Al61Co13Ce26 (Ref 24), Al90.05Ni4.3Y4.4Co0.9Sc0.35 (Ref 25,26,27,28,29) have been employed for cold spray deposition. These studies reveal that Al HEA coatings produced from amorphous precursors have better mechanical properties than polycrystalline Al 7075 with certain limitations. High hardness (300 to 550 HV) and poor ductility of the Al HEA powders due to highly localized shear banding limit the extent of plastic deformation during high strain rate CS (Ref 19,20,21,22,23,24,25,26,27,28,29). This results in poor deposit buildup and a maximum thickness of 0.250 to 1.800 mm, curtailing the ability to manufacture thick parts additively (Ref 19,20,21,22,23,24,25,26,27,28,29). Partial or complete devitrification is a potential approach to tailor the plastic deformation and the strength of amorphous Al HEA powder (Ref 30). Devitrification generates nanoscale and short-range ordered precipitates in the amorphous matrix and, therefore, available dislocations and slip systems for enhancing plastic deformation and strength (Ref 30). In the current state-of-the-art, no comprehensive knowledge exists on the devitrification of amorphous Al HEA powder and its effect on tailoring the powder strength and plastic deformation to improve cold sprayability. This knowledge gap is a significant roadblock that impedes the advancement of large-scale cold spray of lightweight aluminum structures with strength superior to precipitation-hardened Al 7075.
The present investigation aims to establish the fundamental correlations between thermally induced amorphous to semicrystalline devitrification and its effect on microstructure, compressive strength, and optimized cold spray process parameters of amorphous Al HEA powder for advancing CS of thick deposits. Gas-atomized Al HEA powder of composition Al90.05Ni4.3Y4.4Co0.9Sc0.35 (at.%) is heat treated at 298-450 °C for inducing crystallization in the amorphous or nanocrystalline precursor matrix. The effect of controlled crystallization on grain morphology, dislocation density, and precipitate evolution is investigated systematically by microscopy and crystallographic analysis techniques. The role of this microstructural tailoring on the localized and bulk mechanical response is unraveled by multiscale indentions and single-particle compression. The tailored compressive strength is used for developing process maps for manufacturing thick and high-strength Al alloy deposits by cold spray simulation.
Materials and Methods
Aluminum High-Entropy Powder Macro- and Microstructure
The present study used gas-atomized Al HEA powder of composition Al90.05Ni4.3Y4.4Co0.9Sc0.35 (at.%) custom made in a laboratory. The details about the powder preparation can be found in previous studies published by the present authors’ group (Ref 25,26,27,28,29). The powder morphology was observed using a scanning electron microscope (SEM; JEOL, F100). The thermal behavior of the Al HEA powder was evaluated using differential scanning calorimetry (DSC; TA Instruments, Q600) with a heating rate of 5 Kmin−1 in an argon atmosphere for developing heat treatment parameters. The Al HEA powder was heat treated at the established heat treatment temperature and time in the DSC apparatus, followed by cooling to ambient temperature.
The gas-atomized and heat-treated Al HEA powders were dispersed and hot-mounted in a conductive mount for microscopy at 140 °C without affecting the tailored atomic structure. The hot-mounted powders were ground and polished by following standard metallographic procedures. The polished samples were etched by using Keller's reagent for 5 s. The Al HEA powder's microstructure was studied using optical microscopy (Zeiss, Axioscope 5), SEM, and electron backscatter diffraction (EBSD, Oxford, Symmetry S2). SEM microscopy was performed in secondary electron (SE) and backscattered electron (BSE) mode on the polished cross-section to observe the evolution and morphology of precipitates. EBSD analysis was performed in the SEM on samples polished up to 12 h using a vibratory polisher (Vibromet 2, Buehler) using a step size of 0.3 to 0.4 µm and accelerating voltage of 12 kV. The acquisition and post-processing of the EBSD data were carried out using Aztec Crystal software (Oxford Instruments). X-ray diffraction (XRD; Rigaku Smartlab Studio 2) equipped with a monochromatic Cu Kα X-ray source was used to study the phase evolution as a function of heat treatment. The XRD Parameters employed were: λ = 1.541 Å, 40 kV, 40 mA, scan speed:0.50° min-1, step size: 0.01°, scan range: 10 < 2θ < 90. Phase fraction from the XRD data was calculated by Rietveld analysis using Smartlab Studio II software.
Multiscale Mechanical Measurements of Aluminum High-Entropy Powder
The effect of heat treatment and microstructural tailoring on the mechanical behavior was investigated using indentation on the cross section and microparticle compression experiments on the overall bulk of the Al HEA powder. The localized or low load hardness was probed by a nanoindenter (Hysitron Triboindenter, TI- 900) equipped with a Berkovich tip in load-controlled mode consisting of a loading, dwell, and unloading segment. During the nano-indentation, the static force was increased to 5 mN at a load rate of 0.5 mN s−1, held constant for 5 s, followed by unloading at 0.5 mN s−1 to the initial state. The nanohardness and elastic modulus were analyzed by the Oliver-Pharr method from the load-displacement response. The high-load microhardness was performed using a Vicker’s hardness tester (Leco Corporation, LM810AT) at a peak load of 10 gf and a dwell time of 15 s. Both low- and high-load hardness experimental parameters were optimized as discussed in the authors' earlier publication (Ref 31). The representative load-displacement response from the nano-indentation, average nanohardness, nano-indentation derived elastic modulus, and microhardness of the Al HEA powder as a function of heat treatment from at least eight experiments are reported.
To assess the bulk mechanical response of the Al HEA powder, a microparticulate compression tester (Shimadzu MCT-510 Series) with a diamond flat punch indenter was used to compress individual particles that were dispersed and spread on a steel plate. The compression plate was securely fixed onto a horizontal sliding stage with a vice, and the particle size was measured before testing with a 50X objective lens attached to the MCT system. Compression testing was conducted by moving the compression plate under a 50 µm diamond flat punch indenter at a loading rate of 14.8 mN s−1 to a peak load of 1000 mN and then retracting at the same rate without dwelling. The nominal stress-strain response of the powder was calculated from the force-displacement data using Eq 1 & 2 (Ref 32,33,34,35,36).
where σnom and εnom are the nominal stress (MPa) and strain, F and δ are the force (N) and displacement (mm), and d0 is the mean particle diameter (mm). The ultimate tensile strength of the Al HEA powder was calculated from the load-displacement data at a displacement of 50% particulate compression as Eq 3 (Ref 32,33,34,35,36).
where σu is the ultimate tensile strength (MPa), a is 2.48 (Ref 32), F is the force (N) at a displacement of 50% of the diameter of the powder, and d is the particle diameter. Representative load-displacement response, nominal stress-strain response, and average ultimate tensile strength of the Al HEA powder as a function of heat treatment from at least 20 experiments are reported.
Cold Spray Process Map Simulation
Cold spray process parameters for depositing the Al HEA powder were developed using KSS software (Kinetic Spray Solutions) (Ref 31, 37,38,39). In KSS software, the nozzle geometry of the cold spray equipment, size, and mechanical properties of the Al HEA powder can be assigned as a constant input parameter. The process gas parameters such as temperature, pressure, and type can be assigned as variable parameters to generate process maps. A proprietary nozzle geometry was assigned for the present simulation. The Al HEA powder's size and compressive strength established from the present experiments were provided as an input material property to the software. The specific process gas parameters used for the present simulation are shown in Table 1. The output parameters were reported, such as critical velocity, particulate temperature and velocity, velocity ratio, and deposition efficiency.
Results and Discussion
Microstructure of Gas-Atomized Aluminum High-Entropy Alloy Powder
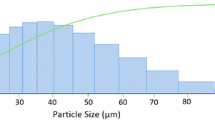
Gas-atomized Al HEA powder has a spherical morphology and Gaussian diameter distribution from 2.5 to 25.0 µm with a mean diameter of 7.8 ± 3.9 µm, as presented in Fig. 1(a) and (b). The XRD powder spectrum in Fig. 1(c) shows a broad hump, typical of amorphous/nanocrystalline materials with narrow face-centered cubic (FCC) Al peaks. To understand the effect of the amorphous and crystalline structure on the microstructure, the Al HEA powder’s cross-sectional view is presented in Fig. 1(d), (e), and (f) by OM and SEM. The microstructure of the powder varies according to the powder size. Therefore, microstructural investigations are performed on multiple powder particles across the range of diameter to gain insight into the representative behavior of Al HEA powder particles. Generally, the microstructure of Al HEA powder is characterized by featureless (white contrast), featured (dark contrast), or a mixture of both, as shown in Fig. 1(d). Fine particles with a diameter less than 10 µm are mostly featureless (Fig. 1e) and the particles greater than 10 µm are both partially and fully crystallized (Fig. 1f). The particles with a diameter greater than 20 µm are mostly crystallized. This difference in the microstructure stems from the rapid gas atomization process (with cooling rates > 105 K s−1) to produce amorphous Al HEA powder. Smaller particles cool fast and retain an amorphous state. However, particles with a size greater than 10 µm crystallize due to the tradeoff between differential cooling and rapid kinetics to crystallization. From the crystalline and amorphous peaks in the XRD spectrum (Fig. 1c), it was estimated that about 56% of the powders are amorphous, while the rest 44% are crystalline.
Bulk and microstructural characteristics of gas-atomized aluminum high-entropy alloy (Al HEA) powder. The bulk structure depicts (a) Spherical morphology, (b) Gaussian powder size distribution, and (c) XRD diffractogram. (d) Optical micrograph of the Al HEA powder, depicting (e) Featureless particles (d < 10 µm), and (f) Featured particles (d > 10 µm). Microstructure further delineated by performing (g) EBSD performed at a region consisting of two particles with diameter 5 and 13 µm, (h) Kikuchi pattern performed on a 5 µm particle at a region, (i) Sharp Kikuchi patterns and equiaxed grain structure of 13 µm particle
The grain morphology of the Al HEA powder is investigated by performing EBSD on particles with a diameter of 5 and 13 µm, marked as Powder A and B, respectively, as shown in Fig. 1(g). Kikuchi patterns obtained from Powder A and Powder B are shown in Fig. 1(h) and (i), respectively. No Kikuchi pattern is resolved for Powder A, indicating that the smaller diameter powder is amorphous or nanocrystalline. In contrast, Powder B exhibited sharp Kikuchi patterns of the FCC Al phase and secondary phases, indicating crystallinity as earlier confirmed from the microstructure and the XRD analysis. However, a few amorphous regions could not be indexed in Powder B. The orientation map of the crystallized powder consists of equiaxed grains oriented randomly. The mean diameter of the grain was evaluated to be ~ 250 nm, as shown in Fig. 1(i). Compared to the commercial gas-atomized Al 7075 powder with a mean grain size of 22 µm (Ref 31), Al HEA powder has a finer grain size in the nano or sub-micron scale. It can be concluded that the powders with a diameter < 10 μm are featureless and amorphous, whereas the larger powders are crystalline with equiaxed grains oriented randomly. Compared to commercial polycrystalline Al alloys, which are solid solution strengthened and precipitation hardened, the current Al powder in this study contains unique alloying elements with atomic radii, such as nickel (1.93 Å), yttrium (3.52 Å), cobalt (2.00 Å), and scandium (2.61 Å), in contrast to aluminum (1.36 Å) (Ref 40). These larger atomic radii lead to a negative enthalpy of mixing and result in an amorphous structure, as confirmed by microstructure, XRD, and EBSD studies detailed earlier, thereby classifying it as a high-entropy alloy.
Mechanical Properties of Gas-Atomized Aluminum High-Entropy Alloy Powder
The nano elastic modulus, nanohardness, and microhardness of the amorphous/ nanocrystalline and crystalline Al HEA powders are probed by using nano- and micro-indentation methods to understand the plastic deformability for the cold spray deposition as illustrated in Fig. 2. The nano-elastic modulus of the Al HEA amorphous/nanocrystalline and crystalline powder is 83.8 ± 4.9 and 96.1 ± 3.9 GPa, which is 29% and 47% more than Al 7075 powder (65.10 ± 4.40 GPa) (Ref 31). Al HEA composition with rare earth (Sc, Y) and transition metals (Ni, Co) increases the inherent elastic modulus of the Al alloys due to their larger atomic sizes. In addition, the nanohardness (4.46 ± 0.8 GPa) and microhardness (4.35 ± 0.2 GPa) of the Al HEA amorphous/nanocrystalline powder are three times higher than Al 7075 (nanohardness 1.50 ± 0.1 GPa and microhardness—1.36 ± 0.02 GPa). The same for Al HEA crystalline powder (nanohardness—3.30 ± 0.42 GPa and microhardness—2.87 ± 0.29 GPa) is two times higher than the Al 7075 powder. Therefore, the Al HEA powder has a higher inherent modulus and hardness than Al 7075, thereby showing enormous potential for manufacturing structural components that can withstand higher force without yielding compared to commercial Al alloys. However, the increase in the hardness can detrimentally affect the plastic deformation behavior during cold spray deposition.
The higher intrinsic hardness of Al HEA powder is advantageous but comes with a tradeoff in the required plastic deformation for cold spray deposition. Different amorphous Al HEA compositions deposited by cold spray are listed in Table 2 in the decreasing order of coating hardness and corresponding thickness achieved. It can be understood that as the hardness of the Al HEA powder increases, consequently, the maximum thickness achieved decreases. For example, our group cold sprayed Al90.05Ni4.3Y4.4Co0.9Sc0.35 (at.%) alloy earlier with a thickness of 250 µm (Ref 9, 25,26,27,28,29). Although the 250 µm coating proved promising for higher hardness, wear resistance, and corrosion resistance, additive manufacturing of thick, complex structures is debatable due to the limited plastic deformation of amorphous Al HEA powders. Heat treatment or devitrification can potentially modify the microstructure and tailor the ductility.
Devitrification of Aluminum High-Entropy Alloy Powder by Thermal Investigations
Unlike conventional polycrystalline Al alloys such as Al 6061 and Al 7075, novel Al HEA compositions do not have any established heat treatment protocol. Heat treatment of these Al HEA powders means devitrifying the disordered amorphous matrix to precipitate ordered crystallized volumes. Therefore, the heat treatment or devitrification protocol is designed by leveraging the thermal evolution from the amorphous to the crystalline structure. The Al HEA powder is subjected to isochronal (heating at a constant rate) heating from RT to 500 °C in the DSC apparatus to design the heat treatment temperature at a heating rate of 5 °C/min. The exothermic heat flow (W g−1) of Al HEA powder as a function of temperature (oC) obtained from DSC is presented in Fig. 3(a). Three regions (I/II/III) are visible from the DSC response. In region I, from RT to 298 °C, the Al HEA powder demonstrated thermal stability of their amorphous or nanocrystalline structure manifested as no discernible exothermic peaks. Therefore, there will be no thermal transformation in the powder upon heat treatment at these temperatures.
Further, in region II, from 298 to 370 °C, the phase transformation is observed as one prominent exothermic crystallization peak initiating at Tx1 = 298 °C with the peak intensity at Tp1 = 325 °C as presented in Fig. 3(a). Apart from this, two small peaks are visible which onsets at 345 (Tx2) and 362 °C (Tx3). Amorphous and crystalline phases coexist in this region, representing a composite microstructure. The onset temperatures of these three peaks are chosen from this region for heat treating the Al HEA powder, namely 298, 345, and 362 °C, to precipitate crystallized volumes with varying volumes. In the third region above 370 °C, the Al HEA powder will be fully crystallized, and 450 °C is selected as the heat-treating temperature. To establish the heat treatment dwell time, the Al HEA powder is subjected to isothermal (heating at constant temperature) holding at 298, 345, 362, and 450 °C to obtain exothermic heat flow (W g−1) as a function of time (minutes), as presented in Fig. 3(b). It is observed that all the exothermic phenomena at the respective temperatures cease in less than 30 min which is selected as the heat treatment dwell time.
As observed in earlier section, Al HEA powder consists of amorphous/nanocrystalline and crystallized powder. The devitrification can only be applied to the amorphous/nanocrystalline powder to study the tailoring of microstructure and mechanical properties. Therefore, from now on, the paper discusses the heat treatment and characterizations performed on the amorphous/nanocrystalline Al HEA powder (powders with diameters less than 10 µm). After heat treatment, there are three different groups of powders, namely amorphous/nanocrystalline (gas atomized), composite (298, 345, and 362 °C treated), and fully crystalline (450 °C treated) powders.
Microstructure of Devitrified Aluminum High-Entropy Alloy Powders
The precipitate evolution, grain morphology, grain orientation, and grain size distribution of the Al HEA powder are investigated, and representative BSE micrographs and EBSD orientation maps are presented in Fig. 4(a) to (e) and (f) to (i), as a function of controlled devitrification. To capture the representative behavior of the Al HEA powder, the study includes the analysis of a minimum of 20 different powder particles to evaluate how devitrification and crystallinity affect the reported microstructure across the spectrum of powder particle sizes. Gas-atomized amorphous/nanocrystalline Al HEA powder initially showed a microstructure devoid of grains, grain boundaries, and precipitate, as shown in Fig. 4(a).
Microstructure of devitrified Al HEA powder at progressively increasing temperatures. BSE micrograph of Al HEA powder, (a) gas atomized and heat treated at (b) 298 °C, (c) 345 °C, (d) 362 °C, (e) 450 °C demonstrating needle-like precipitate evolution. The evolution of grain morphology, grain size distribution, and Kernel average misorientation maps of Al HEA powder heat treated at (f, g) 298 °C and (h, i) 450 °C obtained by EBSD, (i) quantified by geometrically necessary dislocation density, and proportion of low and high angle grain boundaries
The amorphous or nanocrystalline Al HEA powder atoms are in a high-energy or metastable state. As the temperature increases to 298 °C, the atoms within the Al HEA powder undergo structural rearrangement to achieve lower energy states and form a stable and ordered crystalline structure. The atomic rearrangement leads to the formation of grains, grain boundaries, and different phases in the amorphous matrix of the Al HEA powder. This atomic ordering is manifested as a chemically separated fine needle-like precipitate of mean size 0.15 ± 0.06 µm nucleated in the amorphous/nanocrystalline matrix at 298 °C, as presented in Fig. 4(b). In the corresponding EBSD orientation map at 298 °C, equiaxed grains with a non-crystallized amorphous region is observed (white colored) indicating a composite microstructure in Fig. 4(f). The grains close to the amorphous region, i.e., the newly formed grains, are finer with a grain size ranging from 100 to 200 nm and grains toward the periphery are coarser with a diameter ranging from 400 to 600 nm. Overall, the equiaxed grain followed a Gaussian size distribution with a mean of 0.20 ± 0.11 µm, as presented in Fig. 4(g).
As the temperature increases from 298 to 450 °C, the previously nucleated needle precipitates, and equiaxed grains undergo growth. The growth of the nucleated crystals propagates through the material, transforming the remaining amorphous regions in the Al HEA composite powder to crystallized regions as observed from the BSE micrographs Fig. 3(c) to e and EBSD results from Fig. 3(f) to (i). At 450 °C, the EBSD patterns were fully indexed, confirming the powder-free from the amorphous region and fully crystallized, as depicted in Fig. 4(h). The average precipitate size of 450 °C treated powder increased from 0.15 ± 0.06 µm at 298 °C to 1.17 ± 0.50 µm (Fig. 3e). Similarly, the average grain size increased from 0.20 ± 0.11 µm at 298 °C to 0.31 ± 0.14 µm (Fig. 4i). This indicates that heat treatment temperature promotes grain growth due to enhanced diffusion of atoms.
Apart from the evolution of grains and the precipitate size, thermally induced crystallization in the amorphous matrix also alters the dislocation density and misorientation angles that influence the bulk mechanical response of the Al HEA powder deposited by cold spray. The orientation maps reveal that the grains are equiaxed and oriented randomly, as presented in Fig. 4(f) and (h). The low-angle grain boundaries (LAGB, 2° to 15°) and high-angle grain boundaries (HAGB, > 15°), marked in red and black colors, respectively, are quantified to study the misorientation profiles of the powder. The geometrically necessary dislocation (GND) density is obtained by Kernal average misorientation (KAM) maps (Fig. 4j, k). The grain boundary distribution and the GND density are quantified in Fig. 4(l). The fraction of LAGB and HAGB are evaluated as 34.2% and 65.8%, respectively, for powders treated at 298 °C. In contrast, at 450 °C, the fraction of LAGB and HAGB is 21.8% and 78.2%, respectively. The formation of LAGBs is considered an array of dislocation pile-up zones. These dislocations restrict the plastic deformation of the Al HEA powder during cold spray deposition by dislocation strengthening (Ref 42, 43). HAGBs are linked to grain growth in the material. Since the material is fully crystallized at 450 °C, the powder dominated HAGBs with a fraction of about 78.2%. Similarly, the reduction in the LAGB is manifested as a reduction in the GNDs from 13.8 × 1014 m−2 at 298 °C to 10.1 × 1014 m-2 at 450 °C. Therefore, the heat treatment process causes the grains to grow or coarsen, as observed earlier in Fig. 4(f) to (i), which means that the individual grains increase in size. As the grains grow, the low-angle grain boundaries tend to be consumed or transformed into high-angle grain boundaries; correspondingly, the GNDs reduce. Overall, the heat treatment induces structural changes influencing grain size, precipitate formation, and dislocation density in the HEA powder.
Phases in Devitrified Aluminum High-Entropy Alloy Powders
The microstructural analysis showed the formation of equiaxed grains and needle-like precipitates as a function of temperature in the amorphous/nanocrystalline Al HEA powder matrix. XRD analysis was employed to identify the type of intermetallic precipitates and their percentage volume fraction at each heat treatment stage. The XRD powder diffractogram obtained at each heat treatment temperature is presented in Fig. 5(a). The Bragg intensities and structural details of the phases observed in the XRD powder diffractograms can be correlated to the phases present in the alloy by Rietveld analysis. In Rietveld analysis, the XRD intensities are extracted, refined, and converted to the phase fractions by peak fitting. The phase fraction obtained from the Rietveld analysis of Al HEA powder is shown in Fig. 5(b).
The percentage crystallinity introduced in the gas-atomized Al HEA powder as heat treatment temperature increases is calculated using the intensity count of the significant aluminum peak (111) by Eq 4 (Ref 44).
Tn+1 is the higher heat treatment temperature, and Tn is the lower heat treatment temperature. Here, the % crystallinity of the gas-atomized powder is 0%, and 450 °C treated powder is 100%. Using Eq 1, the percentage crystallinity is calculated as 0%, 20%, 40%, 60%, and 100% for gas-atomized Al HEA powder and heat-treated powders at 298, 345, 362, and 450 °C.
As discussed in earlier section, the reference gas-atomized powder consists of both amorphous or nanocrystalline (d < 10 µm) and fully crystallized powder (d > 10 µm). The XRD spectra of the gas-atomized powder are obtained from both powders together. This leads to Al peaks with a face-centered cubic crystal structure with an amorphous hump. Therefore, in the initial stage, the Al HEA powder only consists of aluminum crystallites without any precipitates. At 298 °C (x = 20%), the powder undergoes atomic rearrangement as observed in the microstructure, and the XRD spectra are characterized by aluminum as well as Al3Ni (crystal structure = orthorhombic) peaks as observed in the BSE microstructure in Fig. 5(b).
The first stage of crystallization at 298 °C,
In the second heat treatment temperature, 345 °C (x = 40%), the intensity of the peaks increases, indicating the growth of the Al and Al3Ni, and new precipitates are formed in the Al HEA powder. The new precipitates formed are Al3Y, Al19Ni5Y3, Al9CO2, Al4NiY, and Al3Sc.
In the second stage of crystallization (345 to 450 °C),
In successive heat treatment temperatures such as 362 and 450 °C, the growth of Al and other precipitates occurs as the intensity of the respective peaks increases. These phases obtained in this study conform with the ones obtained in the previous literature (Ref 26,27,28,29). Overall, from the microstructure and the XRD analysis, it can be observed that, as the temperature increases, crystallization and growth of aluminum and precipitates increase, which will tailor the plastic deformation behavior and bulk mechanical response during the cold spray deposition process.
Multiscale Mechanical Response of Devitrified Aluminum High-Entropy Alloy Powder
Mechanical properties of the powder, such as hardness and compressive strength, are crucial for plastic deformation and high strain rate compression during the cold spray process. Therefore, to understand the effect of devitrification on the mechanical performance of the Al HEA powder, the hardness is probed via nano- and micro-indentation, as presented in Fig. 6(a) to (b). The effect on global plasticity is acquired by single-particle compression. The force-displacement response, nominal stress and strain response, and the ultimate tensile strength of the Al HEA powder are presented in Fig. 6(c) to (e). In order to get the representative effect of devitrification and crystallinity on the mechanical properties, the analysis is performed on at least 20 different particles.
Multiscale mechanical response of the Al HEA powder as a function of devitrification from nano-indentation, micro indentation, and single particle compression. (a) nanohardness, (b) microhardness, (c) compressive force-displacement behavior (d) stress-strain plasticity response, (e) ultimate tensile strength
Composite Al HEA Powder
Al HEA powder heat treated at 298 °C enhanced the nano- and microhardness by 4% and 16% to 5.78 ± 0.04 GPa and 515 ± 113 HV compared to the gas-atomized counterpart. This enhancement also translated to a 15% increase in powder compressive yield strength to 565 MPa and a 5% increase in the ultimate tensile strength to 1559 ± 78 MPa. In contrast, all mechanical properties decreased when the Al HEA powders were devitrified at 345 and 362 °C. Specifically, at 362 °C, nano- and microhardness decreased by 11% and 31%, respectively (4.97 ± 0.09 GPa and 308 ± 46 HV). Similarly, compressive yield and ultimate tensile strength decreased by 37% and 36% (311 MPa and 956 ± 89 MPa) compared to the gas-atomized powder. The observed variations in strengthening can be attributed to the presence of deformation carriers, such as amorphous regions, shear bands, equiaxed grains, grain boundaries, and intermetallic precipitates in the composite powder, as observed in the microstructure in Fig. 4 and 5. During compressive deformation, interfaces between amorphous and crystallized regions, precipitates and dislocations, shear bands and dislocations, shear bands, and grain boundaries impede the applied force, resulting in strengthening. The strengthening is caused by nano-precipitates restricting the shear band propagation, Hall-Petch strengthening, and precipitation hardening.
When the compressive load surpasses the elastic limit of the amorphous region in the Al HEA powder, deformation occurs through the nucleation and propagation of shear bands, as illustrated schematically in Fig. 7(a). The interfaces of grain boundaries and nano-precipitates restrict these shear bands. According to the second phase strengthening theory, the resistance caused by the movement of the shear band can be expressed as
where α is the material constant, ε is the coherent strain, G is the shear modulus of the amorphous matrix, f is the volume fraction of the second phase particles, b is the Burgers vector, and λ is the spacing between the second phase particles. As the devitrification temperature increases, the amorphous region is fully transformed into crystallized regions (Fig. 4), resulting in lower strength.
As observed from the EBSD results in Fig. 4, the grain size increases as the devitrification temperature increases, resulting in a decrease in Hall-Petch strengthening. Precipitation strengthening is achieved by restricting the dislocation motion by the nano-precipitates formed in the Al HEA powder. Initially, when the precipitates are small (as in 298 °C devitrified powder), the dislocations will shear the nano-precipitates. When the precipitates coarsen (increase in diameter when temperature increases to 345 and 362 °C), the dislocations will form Orowan loops. In the case of the 298 °C devitrified Al HEA powder, all these strengthening mechanisms are active, resulting in enhanced hardness and compressive strength. However, as the temperature increases to 345 °C and 362 °C, the dominant mechanisms change due to the amorphous to crystalline transition, grain growth, and precipitate coarsening. Consequently, the hardness and compressive strength decreases.
Fully Crystalline Al HEA Powder
The fully crystallized powder exhibits the lowest nano- and microhardness values of 3.64 ± 0.09 GPa and 189 ± 35 HV, respectively. Similarly, the compressive yield and ultimate tensile strength reach their lowest values at 272 MPa and 760 ± 64 MPa, respectively. The absence of amorphous regions eliminates the contribution of amorphous-based strengthening mechanisms. Instead, the strengthening arises solely from the restriction of dislocations by grain boundaries and precipitates, as depicted in Fig. 7(c). This includes the effects of precipitation hardening and Hall-Petch strengthening as any polycrystalline alloy. However, the strength reduces compared to the composite powders because of grain growth and precipitate coarsening, as observed in the microstructure and XRD results presented in Fig. 4 and 5.
The ability to tune the Al HEA powder’s strength and plastic deformation behavior is achieved by manipulating the strengthening mechanisms. In cold spray applications, the devitrified powders at temperatures ranging from 345 to 450 °C exhibit customized strength characteristics that outperform those of commercial Al 7075. This highlights the significant potential of devitrified Al HEA powders for producing high-strength Al deposits. However, achieving the desired properties requires optimizing the cold spray process parameters investigated in the subsequent section.
Cold Spray Process Parameters for Aluminum High-Entropy Alloy Powder
Optimum cold spray gas parameters are simulated for cold spray of Al HEA powder using mechanical properties tailored by devitrification. Cold spray involves several process parameters, including feedstock characteristics (such as powder size and strength), carrier gas parameters (such as type, pressure, and temperature), and equipment configuration (including nozzle parameters), which all play a role in determining the outcome (Ref 45). By utilizing Kinetic Simulation Software (KSS) modeling, we can simultaneously optimize all these parameters to obtain dependable output characteristics, such as velocity ratio (η) and deposition efficiency (DE). The ultimate tensile strength obtained from single-particle compression tests and the gas parameters listed in Table 1 develop the cold spray process maps.
In CSAM, for successful consolidation and layer buildup, the powder velocity (Vp) should exceed critical velocity (Vc). The critical velocity depends upon the strength of the material and is expressed in Eq 8
where UTS (MPa) is the ultimate tensile strength of the powder, ρp is the density of the powder (g cm−1), Cp is the specific heat of the powder (J g−1 k-1), Ts is the softening or melting temperature (°C), Timp is the particle impact temperature (°C). Therefore, the critical velocity required for successful cold spray deposition is directly influenced by the ultimate tensile strength of the powder, as indicated by their relationship in Eq 8. Figure 8(a) presents a representative graph from the KSS simulation carried out with helium gas at 800 °C and 40 bar, depicting the alteration in critical velocity of the Al HEA powder in response to devitrification-induced crystallized volume. For amorphous or nanocrystalline powder, the critical velocity for the given gas parameters is 1313 m s−1. At 298 °C, with an increase in the ultimate tensile strength of the powder (Fig. 6e), the critical velocity experienced a slight increase of 1%. Similarly, in the following heat-treated Al HEA powders from 345, 362, and 450 °C, as the ultimate tensile strength decreases (Fig. 6e), critical velocity also reduced by 13%, 17%, and 27%. Thus, it can be inferred that controlled devitrification aimed at tailoring the microstructure resulted in a 27% enhancement in the critical velocity required for cold spray consolidation.
Cold spray process parameter development for Al HEA powder. (a) tailored critical velocity as a function of controlled devitrification, (b) cold spray process map. Helium gas with temperatures ranging from 300 to 800 °C and a pressure of 40 bar can produce cold spray deposits with deposition efficiency > 70%
The effect of controlled devitrification on coating quality (η) and the deposition efficiency under various gas parameters is correlated by a process map as presented in Fig. 8(b). The coating quality parameter (η) is the ratio of the impact particle velocity and the critical velocity for bond formation which correlates the coating properties and deposition efficiency. When η < 1, no powder adhesion is visible, and poor deposition efficiency is observed. When η > 1, coating built-up and enhancement in deposition efficiency is observed, whereas when η > 2, a decrease in deposition efficiency due to hydrodynamic erosion is observed. In the case of amorphous or nanocrystalline powder, and the composite powders heat treated at 298, 345, and 362 °C, air and nitrogen (at gas temperatures of 100 to 800 °C) and helium (at gas temperatures of 100 to 200 °C) is not a feasible option due to the very high hardness. These parameters yielded a coating quality parameter of less than 0.8 and deposition efficiency of less than 20%; hence are neglected in the process map. Generally, helium with temperatures above 200 °C is optimum for CSAM for these powders, yielding a coating quality parameter greater than 0.8 and deposition efficiency above 20%. For fully crystallized powder (450 °C), the air and nitrogen from 700 to 800 °C and helium with a temperature of 100 to 800 °C yield a coating quality parameter above 0.8 and deposition efficiency from 27 to 98%.
Future Perspective for Cold Spray Deposition of High Strength Aluminum Alloy by Al HEA Powder
The current landscape of state-of-the-art manufacturing of aluminum alloys by cold spray is presented in Fig. 9. The graph illustrates the ultimate tensile strength of different Al alloys deposited by cold spray with respect to different Al alloy compositions. Among various polycrystalline aluminum alloys presented, the direct-aged Al 7075 cold sprayed deposits showed the highest tensile strength of 550 MPa. So far, the development of high-strength polycrystalline aluminum alloys by cold spray has been constrained by this limitation. Despite using amorphous Al HEA powders in some studies, achieving thick deposits has been challenging due to their high inherent hardness.
The present study can overcome this challenge by the engineered Al HEA powders with varying crystallinity. It enables tailoring the powder hardness from 189 to 515 HV, surpassing that of all existing polycrystalline aluminum alloys (Al 7075 ~ 180 HV). These Al HEA powders showed tailored plastic deformation behavior as demonstrated by the single particle compression test compared to their amorphous counterpart. Therefore, with the enhanced plasticity and the optimized cold spray process parameters (using helium at temperatures between 300 and 800 °C and at a pressure of 40 bar), these powders hold promise for producing thick high-strength aluminum deposits. The development of tailored plasticity in Al HEA powders is a significant step toward creating custom alloys that meet specific performance requirements, opening new possibilities for designers and engineers in various industries. This breakthrough is expected to accelerate the adoption of Cold spray of aluminum alloys across various industries due to its potential for producing high-strength aluminum components with superior performance.
Conclusions
The present study established an engineering protocol for tailoring the microstructure of a novel amorphous or nanocrystalline Al HEA powder by intermittent devitrification of crystallized phases, focusing on its suitability for cold spray deposition. The influence of intermittent devitrification on the microstructure, and mechanical properties was analyzed concerning the cold spray process. The key findings from this study are summarized as follows:
-
Gas-atomized Al HEA powder possesses an amorphous/nanocrystalline structure, making it three times harder than polycrystalline Al 7075. To enhance plastic deformation, intermittent devitrification is employed to induce varying amounts of amorphous to crystalline transitions in the powder at 298, 345, and 362 °C to form a composite microstructure and at 450 °C to form a fully crystallized microstructure. The amorphous to crystalline transition in the Al HEA powder caused structural changes by precipitation of FCC Al, Al3Ni, and Al3Sc phases.
-
At 298 °C, the Al HEA powder exhibits a composite microstructure comprising equiaxed grains (0.2 µm), amorphous regions, and precipitates. This composite microstructure contributes to a 16% higher microhardness (515 HV) and a 5% increase in ultimate tensile strength (1559 MPa) compared to the amorphous powder.
-
At further elevated temperatures from 345 to 450 °C, the Al HEA powder underwent amorphous volume reduction and increased crystallized regions, eventually fully crystallizing at 450 °C. Grain size increased from 0.2 to 0.3 µm, accompanied by needle-like precipitate growth. These changes led to a decrease in dominant strengthening mechanisms (shear band restriction, Hall–Petch strengthening, and precipitation hardening), resulting in a tailored reduction in hardness (12-57%) and ultimate tensile strength (31-49%) compared to the amorphous counterpart between 362 and 450 °C.
-
A process map was simulated by combining the experimentally established ultimate tensile strength of Al-HEA powder with cold spray process parameters simulations. The optimal gas parameter for cold spray consolidating the Al HEA powder is helium at temperatures between 300 to 800 °C/40 bar. This parameter results in a deposition efficiency of over 70% in the ideal coating buildup regime.
This study highlights the potential of devitrification to create custom microstructures in amorphous materials, paving the way for developing advanced Al HEA powders suitable for improved cold spray deposition. In addition, the findings suggest that all powders treated at temperatures ranging from 298 to 450 °C have the potential to yield high-strength aluminum deposits, thus warranting further coating deposition investigation in future studies.
References
R.F. Vaz, A. Garfias, V. Albaladejo, J. Sanchez, and I.G. Cano, A Review of Advances in Cold Spray Additive Manufacturing, Coatings, 2023, 13(2), p 267.
P. Vo, D. Goldbaum, W. Wong, E. Irissou, J.G. Legoux, R.R. Chromik, and S. Yue, Cold-spray processing of titanium and titanium alloys, in Titanium Powder Metallurgy: Science, Technology and Applications, Elsevier Inc., 2015, doi:https://doi.org/10.1016/B978-0-12-800054-0.00022-8.
D. Cruz, M.Á. Garrido, Á. Rico, C.J. Múnez, and P. Poza, Wear Resistance of Cold Sprayed Al Alloys for Aeronautical Repairs, Surf. Eng., 2019, 35(4), p 295-303.
P. Sirvent, M.A. Garrido, C.J. Múnez, P. Poza, and S. Vezzù, Effect of Higher Deposition Temperatures on the Microstructure and Mechanical Properties of Al 2024 Cold Sprayed Coatings, Surf. Coat. Technol., 2018, 337(January 2018), p 461-470. https://doi.org/10.1016/j.surfcoat.2018.01.055
B.C. White, W.A. Story, L.N. Brewer, and J.B. Jordon, Fatigue Behavior of Freestanding AA2024 and AAA7075 Cold Spray Deposits, Int. J. Fatigue, 2018, 112(March), p 355-360. https://doi.org/10.1016/j.ijfatigue.2018.03.007
W.A. Story and L.N. Brewer, Heat Treatment of Gas-Atomized Powders for Cold Spray Deposition, Metall. Mater. Trans. A Phys. Metall. Mater. Sci., 2018, 49(2), p 446-449. https://doi.org/10.1007/s11661-017-4428-8
P. Nautiyal, C. Zhang, V.K. Champagne, B. Boesl, and A. Agarwal, In-Situ Mechanical Investigation of the Deformation of Splat Interfaces in Cold-Sprayed Aluminum Alloy, Mater. Sci. Eng. A, 2018, 737(July), p 297-309. https://doi.org/10.1016/j.msea.2018.09.065
M.R. Rokni, C.A. Widener, O.C. Ozdemir, and G.A. Crawford, Microstructure and Mechanical Properties of Cold Sprayed 6061 Al in As-Sprayed and Heat Treated Condition, Surf. Coatings Technol., 2017, 309, p 641-650. https://doi.org/10.1016/j.surfcoat.2016.12.035
P. Nautiyal, C. Zhang, V. Champagne, B. Boesl, and A. Agarwal, In-Situ Creep Deformation of Cold-Sprayed Aluminum Splats at Elevated Temperatures, Surf. Coatings Technol., 2019, 372(May), p 353-360. https://doi.org/10.1016/j.surfcoat.2019.05.045
Y.K. Wei, X.T. Luo, X. Chu, G.S. Huang, and C.J. Li, Solid-State Additive Manufacturing High-Performance Aluminum Alloy 6061 Enabled by an in-Situ Micro-Forging Assisted Cold Spray, Mater. Sci. Eng. A, 2020, 776(November 2019), p 139024. https://doi.org/10.1016/j.msea.2020.139024
M.R. Rokni, C.A. Widener, V.K. Champagne, and G.A. Crawford, Microstructure and Mechanical Properties of Cold Sprayed 7075 Deposition during Non-Isothermal Annealing, Surf. Coat. Technol., 2015, 276, p 305-315. https://doi.org/10.1016/j.surfcoat.2015.07.016
M.R. Rokni, C.A. Widener, and G.A. Crawford, Microstructural Evolution of 7075 Al Gas Atomized Powder and High-Pressure Cold Sprayed Deposition, Surf. Coat. Technol., 2014, 251, p 254-263. https://doi.org/10.1016/j.surfcoat.2014.04.035
M.R. Rokni, C.A. Widener, V.K. Champagne, G.A. Crawford, and S.R. Nutt, The Effects of Heat Treatment on 7075 Al Cold Spray Deposits, Surf. Coatings Technol., 2017, 310, p 278-285. https://doi.org/10.1016/j.surfcoat.2016.10.064
D. Boruah, X. Zhang, P. McNutt, R. Khan, and H. Begg, Effect of Post-Deposition Thermal Treatments on Tensile Properties of Cold Sprayed Ti6Al4V, Metals (Basel), 2022, 12(11), p 1908.
S. Bagherifard, J. Kondas, S. Monti, J. Cizek, F. Perego, O. Kovarik, F. Lukac, F. Gaertner, and M. Guagliano, Tailoring Cold Spray Additive Manufacturing of Steel 316 L for Static and Cyclic Load-Bearing Applications, Mater. Des., 2021, 203, p 109575. https://doi.org/10.1016/j.matdes.2021.109575
D. John, T. Paul, S.M.A.K. Mohammed, G. Seisdedos, B. Boesl, and A. Agarwal, Profilometry-Based Indentation Plastometry for Evaluating Bulk Tensile Properties of Aluminum-Silicon Carbide Composites, Adv. Eng. Mater., 2023, 25(14), p 1-12.
A.E. Tallman, T. Paul, D. John, and A. Agarwal, Uncertainty Quanti Fi Cation of a High-Throughput Pro Fi Lometry-Based Indentation Plasticity Test of Al 7075 T6 Alloy, Front. Mater., 2022, 9(July), p 1-14.
Y. Wu, D. Cao, Y. Yao, G. Zhang, J. Wang, L. Liu, F. Li, H. Fan, X. Liu, H. Wang, X. Wang, H. Zhu, S. Jiang, P. Kontis, D. Raabe, B. Gault, and Z. Lu, Substantially Enhanced Plasticity of Bulk Metallic Glasses by Densifying Local Atomic Packing, Nat. Commun., 2021, 12(1), p 1-9.
J. Henao, A. Concustell, I.G. Cano, S. Dosta, N. Cinca, J.M. Guilemany, and T. Suhonen, Novel Al-Based Metallic Glass Coatings by Cold Gas Spray, Mater. Des., 2016, 94, p 253-261. https://doi.org/10.1016/j.matdes.2016.01.040
C. Sun, X. Zhou, and C. Xie, Effect of Processing Conditions on Al-based Amorphous/Nanocrystalline Coating by Cold-Spraying, Surf. Coatings Technol., 2019, 362(January), p 97-104. https://doi.org/10.1016/j.surfcoat.2019.01.096
C. Sun, X. Zhou, C. Xie, L. Xu, R. Li, and B. Liu, Formation of Al-based Amorphous/Nanocrystalline Coatings by Cold Spraying, Surf. Coatings Technol., 2020, 389(March), p 125644. https://doi.org/10.1016/j.surfcoat.2020.125644
C. Sun, X. Zhou, J. Lu, L. Xie, R. Li, Y. Wu, X. Dan, and M. Zhang, Formation of Al-Based Metallic Glasses Composites Prepared by Cold Spraying, J. Therm. Spray Technol., 2022, 31(6), p 1844-1859. https://doi.org/10.1007/s11666-022-01406-z
L. Jin, L. Zhang, K. Liu, Z. Che, K. Li, M. Zhang, and B. Zhang, Preparation of Al-Based Amorphous Coatings and Their Properties, J. Rare Earths, 2021, 39(3), p 340-347. https://doi.org/10.1016/j.jre.2020.04.018
E. Sansoucy, G.E. Kim, A.L. Moran, and B. Jodoin, Mechanical Characteristics of Al-Co-Ce Coatings Produced by the Cold Spray Process, J. Therm. Spray Technol., 2007, 16(5-6), p 651-660.
D. Lahiri, P.K. Gill, S. Scudino, C. Zhang, V. Singh, J. Karthikeyan, N. Munroe, S. Seal, and A. Agarwal, Cold Sprayed Aluminum Based Glassy Coating: Synthesis, Wear and Corrosion Properties, Surf. Coatings Technol., 2013, 232, p 33-40. https://doi.org/10.1016/j.surfcoat.2013.04.049
P.S. Babu, L. Venkatesh, A. Jyothirmayi, K. Suresh, L.R. Krishna, A. Agarwal, and D.S. Rao, Salt Spray (Fog) Corrosion Behavior of Cold-Sprayed Aluminum Amorphous/Nanocrystalline Alloy Coating, J. Therm. Spray Technol., 2022, 31(4), p 1173-1183. https://doi.org/10.1007/s11666-021-01309-5
S.B. Pitchuka, D. Lahiri, G. Sundararajan, and A. Agarwal, Scratch-Induced Deformation Behavior of Cold-Sprayed Aluminum Amorphous/Nanocrystalline Coatings at Multiple Load Scales, J. Therm. Spray Technol., 2014, 23(3), p 502-513.
P.S. Babu, R. Jha, M. Guzman, G. Sundararajan, and A. Agarwal, Indentation Creep Behavior of Cold Sprayed Aluminum Amorphous/Nano-Crystalline Coatings, Mater. Sci. Eng. A, 2016, 658, p 415-421. https://doi.org/10.1016/j.msea.2016.02.030
S.B. Pitchuka, B. Boesl, C. Zhang, D. Lahiri, A. Nieto, G. Sundararajan, and A. Agarwal, Dry Sliding Wear Behavior of Cold Sprayed Aluminum Amorphous/Nanocrystalline Alloy Coatings, Surf. Coatings Technol., 2014, 238, p 118-125.
S.Y. Kim, G.Y. Lee, G.H. Park, H.A. Kim, A.Y. Lee, S. Scudino, K.G. Prashanth, D.H. Kim, J. Eckert, and M.H. Lee, High Strength Nanostructured Al-Based Alloys through Optimized Processing of Rapidly Quenched Amorphous Precursors, Sci. Rep., 2018, 8(1), p 1-12.
D. John, T. Paul, K. Orikasa, C. Zhang, B. Boesl, and A. Agarwal, Engineered Aluminum Powder Microstructure and Mechanical Properties by Heat Treatment for Optimized Cold Spray Deposition of High-Strength Coatings, J. Therm. Spray Technol., 2022, 31(8), p 2537-2559. https://doi.org/10.1007/s11666-022-01455-4
K. Tsaknopoulos, J. Grubbs, B.C. Sousa, M. Siopis, A. Nardi, and D.L. Cote, Evaluation of a Laser Powder Bed Fusion Designer Al-Mg-Zr-Si Alloy for Cold Spray Additive Manufacturing, Mater. Des., 2022, 222, p 111105. https://doi.org/10.1016/j.matdes.2022.111105
H. Assadi, I. Irkhin, H. Gutzmann, F. Gärtner, M. Schulze, M. Villa Vidaller, and T. Klassen, Determination of Plastic Constitutive Properties of Microparticles through Single Particle Compression, Adv. Powder Technol., 2015, 26(6), p 1544-1554. https://doi.org/10.1016/j.apt.2015.08.013
C. Huang, A. List, J. Shen, B. Fu, S. Yin, T. Chen, B. Klusemann, F. Gärtner, and T. Klassen, Tailoring Powder Strengths for Enhanced Quality of Cold Sprayed Al6061 Deposits, Mater. Des., 2022, 215, p 110494. https://doi.org/10.1016/j.matdes.2022.110494
H. Assadi and F. Gärtner, Particle Compression Test: A Key Step towards Tailoring of Feedstock Powder for Cold Spraying, Coatings, 2020, 10(5), p 458.
M.A. Gleason, B.C. Sousa, K. Tsaknopoulos, J.A. Grubbs, J. Hay, A. Nardi, C.A. Brown, and D.L. Cote, Application of Mass Finishing for Surface Modification of Copper Cold Sprayed Material Consolidations, Materials (Basel), 2022, 15(6), p 1-16.
A. List, F. Gärtner, T. Mori, M. Schulze, H. Assadi, S. Kuroda, and T. Klassen, Cold Spraying of Amorphous Cu50Zr50 Alloys, J. Therm. Spray Technol., 2014, 24(1-2), p 108-118.
T. Schmidt, H. Assadi, F. Gärtner, H. Richter, T. Stoltenhoff, H. Kreye, and T. Klassen, From Particle Acceleration to Impact and Bonding in Cold Spraying, J. Therm. Spray Technol., 2009, 18(5-6), p 794-808.
H. Assadi, T. Schmidt, H. Richter, J.O. Kliemann, K. Binder, F. Gärtner, T. Klassen, and H. Kreye, On Parameter Selection in Cold Spraying, J. Therm. Spray Technol., 2011, 20(6), p 1161-1176.
D.C. Ghosh and R. Biswas, Theoretical Calculation of Absolute Radii of Atoms and Ions. Part 1. Theatomic Radii, Int. J. Mol. Sci., 2002, 3(2), p 87-113.
P.K. Koh, P. Cheang, K. Loke, S.C.M. Yu, and S.M. Ang, Deposition of amorphous aluminium powder using cold spray, in Proceedings of the International Thermal Spray Conference, 2012, p 249-253.
S.M.A.K. Mohammed, A.A. Aleman, D. John, T. Paul, and A. Agarwal, Exploring the Potential of Wire Fed Direct Energy Deposition of Aluminum-Boron Nitride Nanotube Composite: Microstructural Evolution and Mechanical Properties, Adv. Eng. Mater., 2023, 25(20), p 1-7.
S.M.A.K. Mohammed, T. Paul, D. John, C. Zhang, and A. Agarwal, Understanding the Role of Ultrasonic Cavitation Assisted Casting of Boron Nitride Nanotube-Reinforced Aluminum Matrix Composite, J. Mater. Process. Technol., 2023, 25, p 2405-2418. https://doi.org/10.1016/j.jmrt.2023.06.111
A.N. Jinoop, J. Denny, C.P. Paul, J. Ganesh Kumar, and K.S. Bindra, Effect of Post Heat-Treatment on the Microstructure and Mechanical Properties of Hastelloy-X Structures Manufactured by Laser Based Directed Energy Deposition, J. Alloys Compd., 2019, 797, p 399-412. https://doi.org/10.1016/j.jallcom.2019.05.050
R. Banerjee, D. John, C. Zhang, A. Agarwal, and P. Raj, Cold-sprayed aluminum capacitors on leadframes for 3D power packaging, in 2023 4th International Symposium on 3D Power Electronics Integration and Manufacturing PEIM 2023, IEEE, 2023, p 1-4.
M.R. Rokni, A.T. Nardi, V.K. Champagne, and S.R. Nutt, Effects of Preprocessing on Multi-Direction Properties of Aluminum Alloy Cold-Spray Deposits, J. Therm. Spray Technol., 2018, 27(5), p 818-826. https://doi.org/10.1007/s11666-018-0723-1
J.K. Bi, Z.C.K. Loke, C.K.R. Lim, K.H.T. Teng, and P.K. Koh, Mechanical Properties of Cold Sprayed Aluminium 2024 and 7075 Coatings for Repairs, Aerospace, 2022, 9(2), p 1-17.
W. Wang, P. Han, Y. Wang, T. Zhang, P. Peng, K. Qiao, Z. Wang, Z. Liu, and K. Wang, High-Performance Bulk Pure Al Prepared through Cold Spray-Friction Stir Processing Composite Additive Manufacturing, J. Mater. Res. Technol., 2020, 9(4), p 9073-9079. https://doi.org/10.1016/j.jmrt.2020.06.034
Acknowledgments
The authors acknowledge the financial support of DEVCOM—Army Research Laboratory (ARL) grant W911NF2020256. The Advanced Materials Engineering Research Institute (AMERI) at FIU is greatly acknowledged for the characterization facilities used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
John, D., Sousa, B.C., Paul, T. et al. Devitrification-Induced Tailoring of Microstructure and Strength in Aluminum High-Entropy Alloy Powder for Cold Spray Deposition. J Therm Spray Tech 33, 1348–1364 (2024). https://doi.org/10.1007/s11666-024-01787-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-024-01787-3