Abstract
In most applications, the performance of thermally sprayed Cr3C2-NiCr cermet coatings is known to be adversely affected by the presence of the NiCr binder phase. A processing technique for the rapid synthesis of Cr3C2 on industrial-scale components could improve the functionality of these coatings by eliminating the metallic binder phase. To form a thick, continuous surface layer of adherent, binder-free Cr3C2, the reduction of plasma-sprayed Cr2O3 with methane-containing gas was investigated. Conversion of the plasma-sprayed Cr2O3 to carbide resulted in a significant increase in coating porosity, yielding a highly microporous Cr3C2 surface layer. The physical characteristics of the reduction process appear to be dependent on the coating defect structure at the reduction temperature. Phase morphology and porosity evolution throughout the reduction process were qualitatively examined using x-ray diffraction and scanning electron microscopy. The utility of the resultant Cr3C2 coating is discussed with respect to these microstructural characterizations and microindentation hardness measurements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reaction mechanisms and the kinetics of the reduction of chromium oxide (Cr2O3) powder and pressed pellets with methane-containing gas have been studied extensively, and the body of related work is a testament to the industrial importance of this process (Ref 1-5). Although the details of the proposed reaction mechanisms describing reduction vary, one central notion prevails—namely, a particular phase of chromium carbide, Cr3C2, is the end product of complete conversion. Also, there is an agreement on the pronounced effect that methane concentration has on the rate of reduction—above a critical methane concentration, the deposition of carbon can strongly retard Cr3C2 formation. In addition, the relatively low temperatures and short reaction times observed by many authors for complete conversion to Cr3C2 compared to solid-state carbothermal reduction processing (Ref 6, 7) highlight the usefulness of exploiting a vapor-phase reducing agent. Beyond the production of Cr3C2, this material plays a central role as a component in feedstock powder for cermet thermal spray coatings.
In order to facilitate bonding between the feedstock powder and the substrate during spraying, a metallic binder phase (e.g., NiCr) is used for the deposition of Cr3C2 via thermal spray techniques such as high-velocity oxy-fuel (HVOF). Significant effort examining the behavior of HVOF Cr3C2-NiCr cermet coatings in diverse end-use conditions has shown that the functionalities of these coatings are heavily influenced by the presence of the NiCr binder phase (Ref 8-10). For example, for erosion performance, one of two erosion mechanisms predominately affected the NiCr binder phase, degrading coating integrity in turn (Ref 8). Similarly, hot corrosion in molten oxide salt environments was limited by oxidation of the NiCr binder phase (Ref 9). For tribology applications, the size and the distribution of Cr3C2 particles within the NiCr binder phase affected the abrasive wear resistance, suggesting that variations in cohesion between these differing materials contributed to the measured wear rate fluctuations (Ref 10). These characteristics of HVOF Cr3C2-NiCr cermet coatings and the deposition requirements for Cr3C2 indicate that a carbide coating formation process that takes advantage of thermal spray technologies without requiring the use of an NiCr binder phase could produce coatings with improved performance for a range of applications.
Even though there are many studies concerning the reduction of Cr2O3 powder and pressed pellets with methane-containing gas, the synthesis of binder-free Cr3C2 coatings via reduction of thermally sprayed Cr2O3 appears to be relatively unexplored. In this work, the reduction of atmospheric-plasma-sprayed (APS) Cr2O3 with methane-containing gas has been investigated. Plasma-sprayed Cr2O3 coatings were exposed to a flowing, methane-containing atmosphere at 1000 °C to isothermally convert the as-deposited oxide to Cr3C2. Physical mechanisms of reduction as well as the minimum reaction times required for complete conversion to Cr3C2 were investigated using microstructural characterization throughout the reduction process. Measurements were carried out using x-ray diffraction (XRD) and scanning electron microscopy (SEM) to qualitatively examine phase morphology and porosity evolution in the coating. Resultant carbide coating hardness was also determined via microindentation methods for comparison to values reported in the literature for thermally sprayed Cr3C2-NiCr cermets.
Experimental
Substrate Materials and Preparation
Due to the temperature range of interest for Cr2O3 reduction experiments that exploit methane-containing gas as a reducing agent (800-1200 °C) (Ref 1-5), substrate materials are generally limited to those capable of undergoing short-term, high-temperature exposure without incurring considerable microstructural re-arrangement. These materials include advanced ceramics (e.g., carbon fiber-reinforced carbon) and Ni-based alloys commonly used in high-temperature applications. Since carbon-based materials could act as a supplementary solid-state source of carbon for Cr2O3 reduction, an Ni-based alloy substrate was selected for this study—Haynes 230 (H230) (Haynes International, Windsor, Connecticut 06095, USA). The nominal chemical composition of this alloy is shown in Table 1 (Ref 11). Coupons measuring 76.2 × 25.4 × 3.175 mm were machined from a H230 sheet (per AMS 5878C) using waterjet cutting. Surface preparations for plasma spraying involved grit blasting with 36 grit Al2O3, followed by cleaning with isopropyl alcohol and compressed air in an effort to remove adherent oxide particles.
Cr2O3 Coating Deposition
Owing to the congruent nature by which Cr2O3 melts, deposition by means of APS does not require the use of a metallic binder phase (as is the case of incongruently melting Cr3C2). However, a bond layer is typically applied between ceramic deposits and metallic substrates to reduce thermal-mismatch stresses that can occur in high-temperature applications. In this work, an NiCr-Al bond layer between the Cr2O3 top coat and the H230 substrate was used to moderate cracking and spallation of the oxide coating during heating to the reduction temperature (1000 °C). SEM micrographs of the feedstock powders used in this work are shown in Fig. 1 and their characteristics are provided in Table 2 (Metco, Westbury, NY 11590, USA). An APS system consisting of a 9 MB plasma gun (Metco, Westbury, NY 11590, USA), Model 1200 powder feeder (Bay State Surface Technologies, Auburn, MA 01501, USA), and HP20 robot movement (Yaskawa Motoman, Miamisburg, OH 45342, USA) was used for the deposition of both the NiCr-Al bond layer and the Cr2O3 top coat. APS deposition conditions in Ar/H2 plasma are reported in Table 3.
Reduction of Plasma-Sprayed Cr2O3 with Methane-Containing Gas
Plasma-sprayed Cr2O3 coatings were exposed to a flowing, methane-containing atmosphere (80 vol.% Ar with 20 vol.% CH4; 250 SCCM) in a horizontal quartz tube (60 × 64 × 1300 mm) furnace for 0.1-0.4 h at 1000 °C. The system was purged for 3 h with 400 SCCM Ar prior to the initiation of the thermal cycle. Heating and cooling ramps were carried out at a rate of 400 and 200 °C/h, respectively, with an atmosphere of 200 SCCM Ar. Ultra-high-purity gas sources were used in conjunction with an in-line Nanochem Purifilter (Matheson, Basking Ridge, NJ 07920, USA) to further reduce O2 and H2O impurity levels (<0.1 ppb O2 and H2O). The gas mixture composition was regulated through the use of Series MC mass flow controllers (Alicat Scientific, Tucson, AZ 85743, USA) (Ref 12).
Microstructural Characterization
Phase Identification
The coating phase evolution throughout the reduction process was determined using x-ray diffraction with an X’Pert Pro Materials Research Diffractometer (Philips, Andover, MA 01810, USA) equipped with a Cu-Kα source. A programmable divergence slit was used to hold the irradiated length constant (8 mm) throughout the scan range, thereby increasing the signal-to-noise ratio for high-angle diffraction peaks.
Metallographic Sample Preparation and SEM
Phase morphology and porosity evolution were qualitatively examined in coating cross sections for various reduction times (0.1-0.4 h) with a TM3000 tabletop SEM (Hitachi, Schaumburg, IL 60173, USA). Samples were initially vacuum-impregnated with EpoThin low-viscosity epoxy (Buehler, Lake Bluff, Illinois 60044, USA) at a pressure of 33.6 kPa. Sectioning, grinding, and polishing were then performed using the recommended techniques for the metallographic preparation of thermally sprayed ceramics (ASTM E1920-03). An IsoMet 1000 low-speed precision diamond saw (Buehler, Lake Bluff, Illinois 60044, USA) and an EcoMet 3000/AutoMet 2000 semi-automatic grinder/polisher (Buehler, Lake Bluff, Illinois 60044, USA) were used for sectioning and grinding/polishing, respectively.
Metallographic Sample Preparation and Microindentation Hardness Testing
Samples for microindentation hardness testing were cross-sectioned in free-standing form and subsequently encapsulated in EpoCure 2 epoxy (Buehler, Lake Bluff, Illinois 60044, USA) without vacuum impregnation. Encapsulation of samples with a relatively high-viscosity epoxy at atmospheric pressure inhibits the impregnation of mounting media into the coating pore structure. Therefore, the measured values reflect the true hardness of the porous coating under investigation. Grinding and polishing procedures followed those implemented in the preparation of coating cross sections for SEM. A series of 10 indentations were made on coating cross sections with an LM100 microindenter (LECO, St. Joseph, MI 49085) and a Vickers indentation tip. Test force (100 gf), indentation spacing (0.635 mm) and dwell time (15 s) were chosen in accordance to the standard test method for Vickers indentation hardness of advanced ceramics (ASTM C1327-08).
Results
The aspects of the Cr2O3 reduction process are directly related to the characteristics of the as-deposited coating microstructure. XRD measurements of the Cr2O3 feedstock powder and the resulting plasma-sprayed coating (Fig. 3) indicate no appreciable compositional changes as a result of the deposition process. Although thicker Cr2O3 coatings were employed for reduction experiments, the SEM cross-sectional micrograph shown in Fig. 2 is representative of the plasma-sprayed Cr2O3 used in this work. The as-deposited coating microstructure contains globular voids and interlamellar porosity, which are characteristic of a wide range of thermally sprayed materials (Ref 13), as well as the intralamellar microcracks that occur in brittle ceramics (Ref 14).
Despite uncertainty regarding the reaction mechanisms of reduction of Cr2O3 powder and pressed pellets with methane-containing gas, there is an agreement that the conversion to Cr3C2 largely proceeds by the following overall chemical equation (Ref 1-5):
In this work, it was found that the complete conversion of plasma-sprayed Cr2O3 to Cr3C2 occurred over timescales similar to those reported in related work which exploited vapor-phase reducing agents (Ref 1-5), but differed significantly from those reported for solid-state carbothermal reduction investigations (Ref 6, 7). The XRD patterns in Fig. 3 show the coating phase evolution for reduction times of 0.1-0.4 h at 1000 °C.
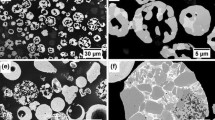
For a reduction time of 0.1 h, the onset of Cr3C2 formation is observable while Cr2O3 clearly remains the major phase in the coating. With increased reduction time (0.2 h), a substantial volume fraction of the Cr2O3 coating is converted to Cr3C2. After a reduction time of 0.3 h, the conversion to Cr3C2 is complete (to the penetration depth of the x-ray beam), and an XRD pattern with relative intensities closely matching randomly oriented, polycrystalline Cr3C2 is achieved (Ref 15). An extended reduction time of 0.4 h however produces no appreciable compositional change in the carbide coating. These measurements demonstrate the ability to form binder-free Cr3C2 coatings via reduction of plasma-sprayed Cr2O3 with methane-containing gas—this is one of the principal results of this work. Previous investigation has shown that the initial porosity in sprayed Cr coatings affects the carburization process (Ref 16), and it is conceivable that defects in plasma-sprayed Cr2O3 could serve a similar role in achieving through-thickness conversion. The SEM micrographs in Fig. 4 show the coating phase morphology and porosity evolution throughout the reduction process, and illustrate characteristics of the physical mechanism of reduction with methane-containing gas when employing a plasma-sprayed coating precursor.
At the earliest reduction time (0.1 h), the onset of Cr3C2 formation (visible in areas of increased image brightness relative to the surrounding unconverted Cr2O3) appears to be limited to the near-surface region of the coating and to areas containing sizeable microcracks oriented normal to the plane of the coating (Fig. 4a). Considering that the out-of-plane dimensions of these microcracks far exceed splat thicknesses and also that they were not present in the as-deposited Cr2O3 coating microstructure (Fig. 2), it is likely that they result from thermal-mismatch stresses generated by heating to the reduction temperature (1000 °C) (Ref 13, 14). Even though the NiCr-Al bond layer did not prevent microcracking, the enhanced rate of reduction in the vicinity of microcracks indicates that defects in plasma-sprayed Cr2O3 facilitate through-thickness conversion. Inspection of Fig. 4(b) for a reduction time of 0.2 h further suggests that multiple pathways for reduction are acting. While reduction seems to proceed in plane by means of thermal-stress-generated microcracks, carbide formation also occurs progressively from the surface to underlying unconverted Cr2O3 in other areas of the coating microstructure. Although the imaging results for a reduction time of 0.3 h (Fig. 4c) agree with the XRD measurements presented in Fig. 3 (the Cr3C2 surface layer thickness exceeds the x-ray beam penetration depth), an underlying portion of oxide remains unconverted in regions of increased Cr2O3 thickness. In areas of the microstructure containing thermal-stress-generated microcracks, carbide formation has progressed through the coating thickness. Closer examination of the interface between the Cr3C2 surface layer and the underlying unconverted Cr2O3 in Fig. 4(c) reveals a significant difference in porosity between the two phases, as well as preferential carbide formation along Cr2O3 grain boundaries (Fig. 5). Void formation of similar character has been documented in previous work involving the conversion of Cr2O3 powder to Cr3C2 via reduction with methane-containing gas (Ref 1); however, in this particular instance it is probable that the newly formed microporous Cr3C2 is a combined result of the constraint on coating dimensions imposed by the substrate and the specific volume decrease associated with Eq 1 (38.1%). After 0.4 h of reduction (Fig. 4d), carbide formation has advanced through the coating thickness along the entirety of the cross section under observation. Irrespective of the coating location under consideration, a thick, continuous surface layer of adherent, binder-free Cr3C2 is formed after a reduction time of 0.3 h.
Microindentation hardness testing has been performed on the binder-free Cr3C2 coating cross section resulting from 0.4 h of reduction, and the results are summarized in Table 4. The measured average hardness for indentations that passed the acceptability criteria outlined in ASTM C1327-08 (344 HV100) is well below what has been reported for conventional and nanostructured Cr3C2-NiCr cermet coatings (Ref 17), though this result likely reflects the diminished hardness of a highly microporous, binder-free Cr3C2 coating relative to the fully dense, pure carbide. Indentations with unacceptable deviations in measured diagonal lengths arose from intersection of the indentation tip and the coating defect structure. Based on these observations, improvements to the reduction process should focus on manipulation of the as-deposited Cr2O3 coating microstructure to yield nanoporous, binder-free Cr3C2 coatings. Unlike the coatings reported in this work, nanoporous carbide coatings could provide near-theoretical hardness.
Discussion
Although thermogravimetric or on-line off-gas mass spectroscopy techniques will be needed to quantify the reaction kinetics of the reduction of plasma-sprayed Cr2O3 with methane-containing gas (Ref 1, 2, 5), a qualitative understanding can be gained from the XRD and SEM results presented in Fig. 3 and 4. Since non-catalytic gas-solid reactions are inherently multi-step phenomena, both chemical and physical (diffusional) processes must be considered when identifying the rate-limiting step in the overall reaction (Ref 18, 19). The lack of diffraction peaks belonging to carbon in the XRD patterns for all of the reduction times in this work (Fig. 3) suggests the newly formed microporous Cr3C2 is a non-catalytic surface for methane decomposition. Also, this lack of carbon deposition indicates that the diffusion of reducing agents rather than their chemical reactivity is probably the rate-limiting step in the overall reaction. This notion contrasts with previous modeling involving the reaction kinetics of the reduction of pressed Cr2O3 pellets with methane-containing gas (Ref 2), which operated under the assumption that chemical reactivity was the rate-limiting step. However, the low-density pressed pellets in preceding studies were formed from an initially porous Cr2O3 powder which likely resulted in a physical system that supported such a model. Regardless of the rate-limiting step under consideration, the progression of conversion over time (Fig. 4) seems to indicate that the overall reaction extent is non-linear in nature when employing a plasma-sprayed Cr2O3 coating precursor. This is in agreement with previous work using Cr2O3 powder and pressed pellets, which quantified the extent of overall reactions under isothermal conditions (Ref 1, 5). In addition, the timescale over which complete conversion occurs (less than 0.5 h) compares favorably with these previous reports—this was unexpected considering the large differences in density between the powder, pressed pellet, and plasma-sprayed Cr2O3 preforms.
Also of significance is the disparity between the timescales required for the complete conversion of plasma-sprayed Cr and Cr2O3 to Cr3C2 under identical conditions. In this work, the complete conversion of plasma-sprayed Cr2O3 to Cr3C2 is realized in less than 0.5 h, whereas previous investigation involving the carburization of plasma-sprayed Cr suggested that complete conversion required at least several hours (Ref 16). A fundamental difference in the physical mechanism of conversion is probably responsible for this discrepancy. While microcracking in the Cr2O3 coating defect structure facilitates rapid through-thickness reducing agent penetration, subsequent transport is likely occurring by means of methane gas diffusion through microporosity in the newly formed Cr3C2 in order to reach unconverted oxide. Specific models will be needed to confirm a self-assisted physical conversion mechanism of this nature.
Defects such as microcracks and microporosity are ordinarily considered to be detrimental microstructural features in industrially important carbide coating applications—including those that require wear-corrosion resistance. However, the defect-ridden Cr3C2 coatings produced in this work could serve as refractory scaffoldings for the formation of multi-functional coatings. Owing to the high level of microporosity, as well as the relatively uniform pore size and spatial distribution, the pore structure of such refractory-carbide scaffoldings could be filled with materials possessing desirable application-oriented properties. Furthermore, refractory carbides are well known to exhibit low chemical reactivity at high temperatures. As a result of these characteristics, refractory-carbide scaffoldings could be impregnated with a range of materials, providing a variety of potential multi-functional, impermeable protective coatings. For example, pores could be filled with various polymers to yield wear-resistant, sealed coatings for low-temperature applications, while liquid metal infiltration could produce coatings for use at significantly higher temperatures (Ref 20).
The expeditious conversion of plasma-sprayed Cr2O3 to Cr3C2 may point toward directions for the rapid synthesis of other binder-free refractory carbide coatings of industrial importance, such as tungsten carbide (WC). The conversion of tungsten oxide (WO3) powder to WC via reduction with methane-containing gas has been shown to result in an increase in particle porosity (Ref 21); therefore, it is likely that plasma-sprayed WO3 would follow a physical reduction mechanism similar to what has been observed in the instance of plasma-sprayed Cr2O3. Future efforts should involve investigating the ability to convert the remaining group IV-VI refractory oxides to binder-free carbide in order to further substantiate the significance of this processing technique.
Conclusion
In this work, a method has been demonstrated for the synthesis of binder-free Cr3C2 coatings on nickel-based alloys via the reduction of plasma-sprayed Cr2O3 with methane-containing gas. A thick, continuous surface layer of adherent, binder-free Cr3C2 has been achieved, with the physical mechanism of reduction being strongly influenced by the coating defect structure at the reduction temperature. Despite large differences in density between oxide preforms, the conversion of plasma-sprayed Cr2O3 to Cr3C2 reported here occurs over timescales similar to those in related work that used Cr2O3 power and pressed pellets. This might be due in part to the formation of microporosity in the newly formed Cr3C2, which appears to expedite the diffusion of methane gas to unconverted Cr2O3. Beyond playing a significant role in the physical mechanism of reduction, microporous Cr3C2 could serve as a refractory scaffolding for use in the development of multi-functional coatings.
References
N. Anacleto and O. Ostrovski, Solid-State Reduction of Chromium Oxide by Methane-Containing Gas, Metall. Mater. Trans. B, 2004, 35B(4), p 609-615
P.J. Read, D.A. Reeve, J.H. Walsh, and J.E. Rehder, Reduction of Chromites in Methane-Hydrogen Mixtures-Chromium Sesquioxide, Can. Metall. Q., 1974, 13(4), p 587-595
M.A. Qayyum and D.A. Reeve, Reduction of Chromites to Sponge Ferrochromium in Methane-Hydrogen Mixtures, Can. Metall. Q., 1976, 15(3), p 193-200
R. Ebrahimi-Kahrizsangi, H. Monajati Zadeh, and V. Nemati, Synthesis of Chromium Carbide by Reduction of Chromium Oxide with Methane, Int. J. Refract. Met. Hard Mater., 2010, 28(3), p 412-415
B. Khoshandam, R.V. Kumar, and E. Jamshidi, Producing Chromium Carbide Using Reduction of Chromium Oxide with Methane, Am. Inst. Chem. Eng. J., 2006, 52(3), p 1094-1102
L.-M. Berger, S. Stolle, W. Gruner, and K. Wetzig, Investigation of the Carbothermal Reduction Process of Chromium Oxide by Micro- and Lab-Scale Methods, Int. J. Refract. Met. Hard Mater., 2001, 19(2), p 109-121
W. Gruner, S. Stolle, and K. Wetzig, Formation of COx Species During the Carbothermal Reduction of Oxides of Zr, Si, Ti, Cr, W, and Mo, Int. J. Refract. Met. Hard. Mater., 2000, 18(2-3), p 137-145
G.-C. Ji, C.-J. Li, Y.-Y. Wang, and W.-Y. Li, Erosion Performance of HVOF-Sprayed Cr3C2 NiCr Coatings, J. Therm. Spray Technol., 2007, 16(4), p 557-565
T.S. Sidhu, S. Prakash, and R.D. Agrawal, Characterizations and Hot Corrosion Resistance of Cr3C2-NiCr Coating on Ni-base Superalloys in an Aggressive Environment, J. Therm. Spray Technol., 2006, 15(4), p 811-816
G. Matthäus, J.A. Picas, and A. Forn, Effect of Feedstock Powder Size on the Sliding Wear Behavior of Thermal Sprayed HVOF Cr3C2-NiCr Coatings. Thermal Spray 2004: Advances in Technology and Application, Proceedings of the International Thermal Spray Conference, 2004. ASM International, May 10-12, 2004 (Osaka, Japan), ASM International, 2004, p 529-533
M.C. Brupbacher, D. Zhang, W.M. Buchta, J.B. Spicer, and D.C. Nagle, Formation of Molten Fluoride Salt Corrosion Resistant Coatings on Nickel-Based Alloys, ANS Transactions, Vol. 111, (Anaheim, CA), ANS, 2014, p 584-587
C.-J. Li and A. Ohmori, Relationship Between the Microstructure and Properties of Thermally Sprayed Deposits, J. Therm. Spray Technol., 2002, 11(3), p 365-374
S. Kuroda and T.W. Clyne, The Quenching Stress in Thermally Sprayed Coatings, Thin Solid Films, 1991, 200(1), p 49-66
S. Rundqvist and G. Runnsjö, Crystal Structure Refinement of Cr3C2, Acta Chem. Scand., 1969, 23(4), p 1191-1199
M.C. Brupbacher, D. Zhang, W.M. Buchta, M.L. Graybeal, Y.-R. Rhim, D.C. Nagle, and J.B. Spicer, Synthesis and Characterization of Binder-Free Cr3C2 Coatings on Nickel-Based Alloys for Molten Fluoride Salt Corrosion Resistance, J. Nucl. Mater., 2015, 461, p 215-220
J. He, M. Ice, and E.J. Lavernia, Synthesis of Nanostructured Cr3C2-25(Ni20Cr) Coatings, Metall. Mater. Trans. A, 2000, 31A(2), p 555-564
J.J. Carberry, Fluid-Solid Noncatalytic Reactions, Chemical and Catalytic Reaction Engineering, General Publishing Company, Ltd, Toronto, Ontario, 2001, p 310-356
P.A. Ramachandran and L.K. Doraiswamy, Modeling of Noncatalytic Gas-Solid Reactions, Am. Inst. Chem. Eng. J., 1982, 28(6), p 881-900
J. Knuuttila, P. Sorsa, and T. Mäntylä, Sealing of Thermal Spray Coatings by Impregnation, J. Therm. Spray Technol., 1999, 8(2), p 249-257
F.F.P. Medeiros, S.A. De Oliveira, C.P. De Souza, A.G.P. Da Silva, U.U. Gomes, and J.F. De Souza, Synthesis of Tungsten Carbide through Gas-Solid Reaction at Low Temperatures, Mater. Sci. Eng. A, 2001, 315(1-2), p 58-62
Acknowledgment
The authors gratefully acknowledge the support of the U.S. Department of Energy (DOE) through the Nuclear Energy University Program (NEUP) Contract No. 101630.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brupbacher, M.C., Zhang, D., Buchta, W.M. et al. Post-treatment of Plasma-Sprayed Cr2O3 with Methane-Containing Gas for Conversion to Binder-Free Cr3C2 . J Therm Spray Tech 24, 1513–1519 (2015). https://doi.org/10.1007/s11666-015-0348-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11666-015-0348-6