Abstract
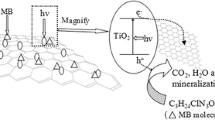
The TiO2-graphene (TiO2-GR) composites have been successfully synthesized through the hydrothermal reaction. Different structures of TiO2-GR composites were modified using graphene oxide (GO) and different titanium sources in hydrothermal conditions. The structure and properties of the photocatalysts have been characterized by field emission scanning electron microscope (FESEM), x-ray diffraction (XRD), Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR), x-ray photoelectron spectroscopy (XPS), photoluminescence spectra (PL), UV–vis diffuse reflectance spectra (DRS), and Brunauer–Emmett–Teller (BET). The results showed that due to the larger interfacial contact between TiO2 and graphene, and its greater surface area, the poriferous TiO2-GR composite exhibited the best photocatalytic properties and adsorption performance compared with the other nanocomposites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With regard to increasing public concern over organic dyes in wastewater and strict regulations on the environment, the photocatalytic process is now widely used to treat organic pollutants. Its advantages are low cost, high purification efficiency, and products that are not secondary pollutants (Ref 1,2,3). In particular, titanium dioxide (TiO2) has been researched, as a promising photocatalyst, due to its outstanding photocatalytic activity (Ref 4, 5). TiO2 is also an excellent semiconducting metal oxide with many advantages such as stability, nontoxicity, commercial availability, and a strong oxidation capacity. Of the three crystal forms of TiO2, anatase is considered to have the best photocatalytic activity (Ref 6). When ultraviolet (UV) light irradiates anatase, excited electrons transfer from the valence band to the conduction band, creating electron-hole pairs. This is the main reason for its photocatalytic properties. However, the photo-generated electron-hole pairs are only generated when the wavelength is less than 387 nm because of a large band gap of 3.2 eV, which only uses 3-5% of the solar energy (Ref 7). In addition, the photo-generated electron-hole pairs have a flash recombination time in the order of 10−9 s, while the chemical interaction time of TiO2 with the adsorbed pollutant is in the range of 10−8-10−3 s, which greatly limits on its application (Ref 8). To solve these problems, many methods have been investigated, such as doping, i.e., combination with metal oxides, semiconductors, and carbon materials (Ref 9,10,11,12). In particular, TiO2 and graphene composites have been proposed as appropriate additives to achieve better photocatalytic properties.
Graphene (GR), as a two-dimensional carbonaceous material, has attracted considerable attention since it was discovered in 2004. Due to its economic cost and superior properties, individual graphene has been prepared using different strategies, such as mechanical methods, sol-gels, chemical vapor deposition, and hydrothermal method. In particular, the hydrothermal method has become a common and well-known technique because of its simplicity. Graphene has several excellent properties such as high electron mobility (200,000 cm2/V s), thermal conductivity (5000 W/m K), and large specific surface area (2630 m2/g) (Ref 13, 14). Due to these superior properties, graphene is regarded as a good option as a catalyst carrier and photoelectron acceptor compared with noble metals and metal oxides. For one thing, graphene can improve the absorption of organics because of its large specific surface area (Ref 15). For another, it promotes charge separation, hinders electron-hole pair recombination, and stabilizes TiO2 owing to its superior electron mobility and high chemical stability, which overcomes the defects in TiO2. Therefore, TiO2-GR composites must surely be able to improve the photocatalytic properties of TiO2. Many papers on graphene/TiO2 composite for the improvements in photocatalytic activity have been published (Ref 16,17,18,19,20,21,22,23). Zhang and co-workers reported that TiO2 nanoparticles on graphene synthesized by the hydrothermal method showed higher photocatalytic performance than pure TiO2 nanoparticles (Ref 24). Liu et al. reported on TiO2 nanorods on graphene oxide sheets at the water/toluene interface and observed an improved photocatalytic activity for the degradation of methylene blue in aqueous solution (Ref 25). Liu et al. synthesized nanocrystalline TiO2 with exposed (001) facets on high-quality graphene sheets, which showed an enhancement in photocatalytic properties (Ref 7). Thus, compared with pure TiO2, these composites result in higher photocatalytic activity. There are some excellent papers reporting the various morphologies of TiO2-GR (Ref 20, 26,27,28). However, the influence of TiO2 morphology on TiO2-GR composites has not been interpreted clearly. In this work, to determine how different morphologies and sizes of TiO2 affect photocatalytic properties, a new poriferous TiO2-GR composite was prepared and characterized using various instruments. We found that poriferous TiO2-GR nanocomposites exhibited higher photocatalytic activity than other photocatalysts.
Experimental Section
Preparation of Graphite Oxide
Graphite oxide (GO) was synthesized using the modified Hummers method (Ref 29). Briefly, 1 g of natural graphite powder, 1 g of NaNO3, and 6 g of KMnO4 were successively added in 55 mL of concentrated H2SO4 under the condition of ice bath for 2 h. This suspension was transferred to a 35 °C water bath and magnetically stirred for 4 h. Then, the dark green colored suspension was diluted with the slow addition of 100 mL of deionized water and allowed to stir for a further 30 min under the oil bath condition at the temperature of 90 °C. After that, 200 mL of deionized water and 30 mL of 30% H2O2 solution were slowly added in the above mixture. The obtained blended solution was heated to 98 °C and kept stirring for 30 min. After placed in fume hood for 12 h, the resultant product was separately washed with 10% HCl solution and deionized water until the pH value of the filtrate reached at near 6. Finally, the product was dried at 60 °C for 24 h.
Synthesis of TiO2-Graphene Composites
The TiO2-graphene composite (denoted as solid TiO2-GR) was synthesized using the hydrothermal reaction (Ref 30). In a typical preparation procedure, 50 mL of concentrated GO solution was firstly dissolved into 500 mL deionized water and then treated under ultrasonic dispersion for about 5 h. To anchor TiO2 particles onto the surfaces of GO, 100 mL of the diluted solution was taken out and vigorously stirred, and then 0.5 g of TiO2 powder was gradually added to the solution. It can be clearly seen that the color changed from brown into gray yellow. Subsequently, 3 g of sodium hydroxide was added to the solution. After 10 h of vigorously stirring, the solution was transferred into Teflon container with a stainless steel reaction kettle and kept under 120 °C for 24 h. The color of the GO and TiO2 mixed dispersion turned into dark gray after the hydrothermal treatment. The product was then centrifuged and washed with deionized water. Finally, the solid TiO2-GR composite was successfully synthesized after dried under 60 °C for 24 h.
The other TiO2-graphene composites were prepared through a hydrothermal method similarly. In details, 30 and 50 mg of GO (denoted as hollow and poriferous TiO2-GR) were taken out and dissolved into 10 mL of ethanol, respectively. Then, 5 mL of isopropanol was added to the suspension solution and vigorously stirred for 60 min after sonication for 30 min. Subsequently, 25 mL of the mixture of ammonium hexafluorotitanate (0.05 mol/L) and boric acid was added to the suspension, and then, the solution was stirred for 60 min. Successively, the solution was transferred into Teflon container with a stainless steel reaction kettle and kept under 140 °C for 20 h and 140 °C for 16 h, respectively. The product was then centrifuged and washed with deionized water. Finally, the hollow and poriferous TiO2-GR composites were successfully synthesized after dried under 60 °C for 24 h.
Characterization
The morphologies of the obtained materials were observed using field emission scanning electron microscope (FESEM, JEOL JSM-7800F) and transmission electron microscopy (TEM, JEM-2100F). The phase composition of samples was characterized using powder x-ray diffraction (XRD, Rigaku D/Max-1200 x-ray diffractometer with Cu Kα radiation). Raman spectroscopy was recorded using Lab RAM HR Evolution with 532 nm as an excitation wave number. Fourier transform infrared spectra (FTIR) were performed using Nicolet iS5 FTIR. X-ray photoelectron spectroscopy (XPS, ESCALAB 250) was acquired using an Al Kα irradiation. The chamber pressure was 2 × 10−10 mbar under testing conditions. UV–vis diffuse reflectance spectra (DRS, Cintra 40) were obtained with BaSO4. The photoluminescence spectra (PL) were recorded using Cary eclipse. Brunauer-Emmett-Teller (BET) was measured using Quadrasorb 2MP for calculating the specific surface area.
Performance Testing
The photocatalytic activity of samples was evaluated by the photodegradation of Rh-B (10 mg/L). The degradation progress of Rh-B was completed in self-reactor. In details, 10 mg of TiO2-GR was dispersed in 100 mL of Rh-B solution with ultrasonic for 1 min. Then, the suspension was placed in a dark box with stirred for 30 min to achieve the adsorption-desorption equilibrium of Rh-B dye. A 300-W UV lamp with a wavelength centered at 365 nm was employed as the illumination source and placed 30 cm away from the suspension. Then, the suspension was irradiated for 30 min, and 6 mL of suspension was taken out and centrifuged at a 5-min interval. Finally, the concentration of Rh-B was measured by a UV–vis spectrophotometer.
Results and Discussion
FESEM and TEM are the imaging techniques necessary for the identification of nanomaterial morphology. Figure 1 shows the morphology and size of the composites studied. Different morphologies and sizes of TiO2 nanoparticles, anchored onto the surface of graphene, can be clearly observed in the FESEM images. Due to different hydrothermal conditions and titanium sources, three types of TiO2 morphology were generated on the graphene sheets. The size of the solid TiO2-GR nanoparticles was 20-30 nm. The size of the hollow TiO2-GR and poriferous TiO2-GR was approximately 100 nm. The structure of TiO2 and graphene can also be seen in the TEM images, which confirms the presence of graphene in the TiO2-GR composites. The high-resolution transmission electron microscopy (HRTEM) image (Fig. 1g) clearly shows the lattice spacing of TiO2 at 0.35 nm, corresponding to the (101) planes of anatase.
XRD is a critical technique for characterizing of the degree of crystallization of the composites. Figure 2 shows the XRD patterns of different catalysts. Notably, the (002) peak of GO disappears completely after hydrothermal reaction, suggesting that the GO in all the TiO2-GR composites was reduced to graphene. Meanwhile, it is hard to distinguish the diffraction peak of graphene in the XRD patterns because the anatase (101) and graphene (002) peaks are located relatively close to each other at 25.28° and ~26°, respectively. These TiO2-GR composites show characteristic peaks at 25.28°, 37.80°, 48.05°, 53.89°, 55.06°, 62.69°, 68.76°, 70.31°, 75.01°, and 82.66° corresponding to the (101), (004), (200), (105), (211), (204), (116), (220), (215), and (224) phases of the anatase crystal (Ref 31), which is consistent with pure TiO2 (Fig. 2a).
Raman spectroscopy was performed to further confirm the formation of the TiO2-GR composites. Raman spectroscopy is a super-effective technique that can characterize the crystal form and microstructure of TiO2 and the crystalline quality of carbon. Figure 3 shows the Raman spectrographs of GO, TiO2, and the TiO2-GR composites. It was found that the peaks at 399 cm−1 (B1g), 513 cm−1 (A1g), and 638 cm−1 (Eg) were indexed to the anatase crystal phase (Ref 30). This further demonstrates the synthesis of anatase and was consistent with the XRD results. The peaks of 1347 and 1598 cm−1 of GO and TiO2-GR can also be clearly seen, corresponding to the D and G bands of graphene. The D and G bands were ascribed to a common feature of disordered sp3 defects in carbon and the in-plane vibration of sp2-bonded carbon, respectively. The intensity ratio of the D to the G band usually reflects the order of defects in GO and GR (Ref 32). Compared with the D/G intensity ratio of GO (I D/I G = 1.06), the D/G intensity ratio of poriferous TiO2-GR (I D/I G = 1.10) was slightly increased. This indicates that a decrease in the average size of the in-plane sp2 dominated during the reduction of GO, which provides a further evidence of the existence of graphene sheets in the TiO2-GR composites (Ref 33). In addition, it was interesting to notice a blueshift in the G band of the TiO2-GR composites compared with GO. This change can be attributed to the conversion of graphite to graphene or the resonance of isolated double bonds at higher frequencies (Ref 34).
The FTIR spectrum is an appropriate tool for observation and characterization of the functional groups in the catalysts. Figure 4 shows the FTIR patterns of GO and the TiO2-GR composites. The representative absorption peaks of GO (Fig. 4a) are obvious. The peaks at 3423, 1728, 1628, 1385, 1228, and 1077 cm−1 are attributed to the O-H stretching vibration in H2O, C=O stretching in the COOH group, stretching of aromatic C=C, C-OH stretching in the alkoxy group, C-O-C stretching in the epoxide groups, and C-O stretching in carboxylates, respectively. In contrast, the absorption peaks of C=O, C-OH, C-O-C, and C-O decrease and almost disappear in the TiO2-GR composites (Fig. 4b-d). The disappearance of these oxygen-containing functional groups demonstrates the conversion of graphite oxide to graphene and also matches the Raman spectroscopy data. Notably, compared with GO, the broad absorption band in the low-frequency region (600-1000) in the TiO2-GR composites can be considered a combination of Ti-O-Ti vibration and Ti-O-C vibrations. This result confirms that the TiO2-GR composite combined by chemical rather than physical interaction (Ref 24).
XPS is routinely used to estimate the elemental composition of catalysts and provides extremely valuable information on the oxidation state of constituent elements. The full XPS spectra of the TiO2-GR composite with C1s, and O1s core electrons, and Ti 2s, 2p, 3s, and 3p electrons are shown in Fig. 5(a), confirming the existence of graphene. The XPS peaks at about 459.3 and 465 eV (as shown in Fig. 5b) are attributed to the Ti 2p3/2 and 2p1/2 spin orbital components in TiO2. The C1s spectrum of the TiO2-GR composite (Fig. 5c) can be devolved into three peaks at about 284.3, 285.4, and 287.9 eV, corresponding to C-C, C-O, and C=O. Additionally, it should be noted that the deconvoluted peaks for C=O were slight, indicating GO conversion to graphene.
Figure 6 shows the UV–vis absorption spectra of GO and poriferous TiO2-GR. It shows an absorption peak at about 235 nm and a shoulder at 282-337 nm in the GO spectra, due to π → π* transition of the C=C bonds and n → π* transition of the C=O bonds, respectively. Figure 6(b) shows that after hydrothermal reduction, the adsorption peak of GO at 235 nm redshifted to 325 nm and the shoulder also disappeared, which indicates the reduction of GO to GR.
The PL spectroscopy is an effective technique for investigating the electronic structure and optical properties of semiconductor nanocomposites. It can reveal photo-induced carrier transport, and the capture and recombination behavior of photocatalysts. Figure 7 shows the PL spectra of TiO2 and the TiO2-GR composites. The excitation wavelength for the PL spectra was 240 nm. It was found that the PL intensity of TiO2 was higher than that of the TiO2-GR composites, which shows that the electron-hole recombination rate of the former was higher than that of the later composites. This indirectly indicates the existence of graphene sheets that inhibited the electron-hole recombination of TiO2. It is worth noting that the size of the poriferous TiO2-GR composite was larger than the solid TiO2-GR and that the emission intensity was lowest for TiO2-GR composites. This demonstrates that poriferous TiO2-GR composites can transfer excited electrons from the valance band to the conduction band. This may be due to sufficient close interfacial contact between TiO2 and GR (Ref 35).
DRS analysis is an essential measurement method for determining the optical properties of photocatalysts. Figure 8 shows the UV–vis diffuse reflectance spectra of TiO2 and the TiO2-GR composites. It was found that the presence of graphene obviously enhanced the light absorption of the TiO2-GR composites. Meanwhile, an obvious redshift in the absorption edge of the TiO2-GR composites can be observed, which is ascribed to a narrowing of the band gap in TiO2. Therefore, compared with TiO2, the TiO2-GR composites showed greater visible-light absorption.
Table 1 shows the BET surface area of the different photocatalysts. The specific surface area of poriferous TiO2-GR (163.6 m2/g) is higher than pure TiO2 (50.0 m2/g) due to the high surface area of GR and poriferous structure of TiO2. The degradation of Rh-B under UV light irradiation is usually used to measure photocatalytic activity. The photodegradation activity of the different photocatalysts is curved, as shown in Fig. 9(a) and (b). The adsorption performance of the catalysts is shown in Fig. 9(a). In the adsorption phase in a dark box, the TiO2-GR composites, with different weight addition ratios (10, 20, and 30%) of graphene, exhibited relatively high adsorption performance (10-20%) compared with pure TiO2, which indicates that the existence of graphene sheets can improve adsorption performance due to its large specific surface area (201.3 m2/g). Furthermore, compared with the solid TiO2-GR composite, the adsorption ability of the poriferous TiO2-GR composite was improved because of its porous structure and the weight addition ratios of graphene. As for the photocatalytic activity, it was found that all the TiO2-GR composites performed greater photodegradation than pure TiO2. The photocatalytic efficiency under the UV light followed the order: poriferous TiO2-30% GR > solid TiO2-10% GR > hollow TiO2-20% GR > TiO2. On the one hand, due to the existence of graphene, the photo-generated electrons on the CB of TiO2 were transferred to the surface of graphene and effectively hindered the recombination of the photo-generated electron-hole pairs. This induced more reduction and oxidation reactions, thereby enhancing the photodegradation efficiency. On the other hand, the difference in size and morphology led to changes in photocatalytic performance. As shown in Fig. 9(b) and (c), the solid TiO2-GR composite exhibited a higher photocatalytic efficiency than the hollow TiO2-GR because of its smaller size and larger specific surface area. However, poriferous TiO2-GR also exhibited excellent photocatalytic efficiency although the size of TiO2 at 100 nm was larger than solid TiO2-GR. The morphology of poriferous TiO2-GR may result in more interfacial contact with the graphene surface. Meanwhile, the photocatalytic stability of poriferous TiO2-GR was also tested. After three cycles, the photocatalytic efficiency of the poriferous TiO2-GR composite still achieved 95% under the same conditions, indicating that it can be reused. The photodegradation kinetics of solid TiO2-GR, poriferous TiO2-GR, and pure TiO2 were calculated using a pseudo-first-order reaction, \( \ln (C_{t} /C_{0} ) = kt \), where k, C t , and C 0 are the apparent rate constant, initial concentration, and concentration for a time t of Rh-B, respectively (Fig. 9b). The rate constants for solid TiO2-GR and poriferous TiO2-GR were 0.136 and 0.149 min−1, respectively, and were both higher than pure TiO2 (0.101 min−1).
(a) Adsorption performance in a dark box for (a) TiO2, (b) hollow TiO2-GR, (c) solid TiO2-GR, and (d) poriferous TiO2-GR. (b) Photodegradation of Rh-B under UV–vis for (a) TiO2, (b) hollow TiO2-GR, (c) solid TiO2-GR, and (d) poriferous TiO2-GR. (c) Plot of photocatalytic degradation rate constants (k) of Rh-B for the photocatalysts
Conclusion
In this study, the TiO2-GR nanocomposites with different morphologies and sizes were obtained using the hydrothermal method. A new poriferous TiO2 nanoparticle was crystallized and anchored to graphene by chemical interaction. Due to the large specific surface area and the high electron mobility of graphene, TiO2-GR composites exhibited excellent photocatalytic activity and adsorption performance toward the degradation of Rh-B compared with pure TiO2. Remarkably, compared with the other nanocomposites, the poriferous composite exhibited the best photocatalytic properties because of more interfacial contact between TiO2 and graphene, and its larger surface area. This photocatalyst may become a promising candidate for dealing with wastewater.
References
G.M. Wang, H.Y. Wang, Y.C. Ling, Y.C. Tang, X.Y. Yang, R.C. Fitzmorris, C.C. Wang, J.Z. Zhang, and Y. Li, Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting, Nano Lett., 2011, 11(7), p 3026–3033
R. Leary and A. Westwood, Carbonaceous Nanomaterials for the Enhancement of TiO2 Photocatalysis, Carbon, 2011, 49(3), p 741–772
Q.L. Jin, M. Fujishima, and H. Tada, Visible-Light-Active Iron Oxide-Modified Anatase Titanium(IV) Dioxide, J. Phys. Chem. C, 2011, 115(14), p 6478–6483
S. Chusaksri, J. Lomda, T. Saleepochn, and P. Sutthivaiyakit, Photocatalytic Degradation of 3,4-Dichlorophenylurea in Aqueous Gold Nanoparticles-Modified Titanium Dioxide Suspension Under Simulated Solar Light, J. Hazard. Mater., 2011, 190(1–3), p 930–937
S. Ahmed, M.G. Rasul, R. Brown, and M.A. Hashib, Influence of Parameters on the Heterogeneous Photocatalytic Degradation of Pesticides and Phenolic Contaminants in Wastewater: A Short Review, J. Environ. Manag., 2011, 92(3), p 311–330
J. Jiang, D.R. Chen, and P. Biswas, Synthesis of Nanoparticles in a Flame Aerosol Reactor with Independent and Strict Control of Their Size, Crystal Phase and Morphology, Nanotechnology, 2007, 18(28)
B. Liu, Y. Huang, Y. Wen, L. Du, W. Zeng, Y. Shi, F. Zhang, G. Zhu, X. Xu, and Y. Wang, Highly Dispersive 001 Facets-Exposed Nanocrystalline Tio2 on High Quality Graphene as a High Performance Photocatalyst, J. Mater. Chem., 2012, 22(15), p 7484–7491
K.F. Zhou, Y.H. Zhu, X.L. Yang, X. Jiang, and C.Z. Li, Preparation of Graphene-TiO2 Composites with Enhanced Photocatalytic Activity, New J. Chem., 2011, 35(2), p 353–359
V. Subramanian, E. Wolf, and P.V. Kamat, Semiconductor-Metal Composite Nanostructures. To What Extent Do Metal Nanoparticles Improve the Photocatalytic Activity of TiO2 Films?, J. Phys. Chem. B, 2001, 105(46), p 11439–11446
C. Ratanatawanate, A. Chyao, and K.J. Balkus, S-Nitrosocysteine-Decorated PbS QDs/TiO2 Nanotubes for Enhanced Production of Singlet Oxygen, J. Am. Chem. Soc., 2011, 133(10), p 3492–3497
T. Hirakawa and P.V. Kamat, Charge Separation and Catalytic Activity of Ag@TiO2 Core-Shell Composite Clusters Under UV-Irradiation, J. Am. Chem. Soc., 2005, 127(11), p 3928–3934
R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki, and Y. Taga, Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides, Science, 2001, 293(5528), p 269–271
S. Park and R.S. Ruoff, Chemical Methods for the Production of Graphenes, Nat. Nanotechnol., 2009, 4(4), p 217–224
A.K. Geim, Graphene: Status and Prospects, Science, 2009, 324(5934), p 1530–1534
P.V. Kamat, Graphene-Based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Carbon Support, J. Phys. Chem. Lett., 2009, 1(2), p 520–527
H. Zhang, P. Xu, G. Du, Z. Chen, K. Oh, D. Pan, and Z. Jiao, A Facile One-Step Synthesis of TiO2/Graphene Composites for Photodegradation of Methyl Orange, Nano Res., 2010, 4(3), p 274–283
S. Morales-Torres, L.M. Pastrana-Martínez, J.L. Figueiredo, J.L. Faria, and A.M.T. Silva, Graphene Oxide-P25 Photocatalysts for Degradation of Diphenhydramine Pharmaceutical and Methyl Orange Dye, Appl. Surf. Sci., 2013, 275, p 361–368
Q. Zhang, Y. He, X. Chen, D. Hu, L. Li, T. Yin, and L. Ji, Structure and Photocatalytic Properties of TiO2-Graphene Oxide Intercalated Composite, Chin. Sci. Bull., 2011, 56(3), p 331–339
T.-D. Nguyen-Phan, V.H. Pham, E.W. Shin, H.-D. Pham, S. Kim, J.S. Chung, E.J. Kim, and S.H. Hur, The Role of Graphene Oxide Content on the Adsorption-Enhanced Photocatalysis of Titanium Dioxide/Graphene Oxide Composites, Chem. Eng. J., 2011, 170(1), p 226–232
Y. Zhou, Y. Huang, D. Li, and W. He, Three-Dimensional Sea-Urchin-Like Hierarchical TiO2 Microspheres Synthesized by a One-Pot Hydrothermal Method and Their Enhanced Photocatalytic Activity, Mater. Res. Bull., 2013, 48(7), p 2420–2425
N. Zhang, M.Q. Yang, S. Liu, Y. Sun, and Y.J. Xu, Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts, Chem. Rev., 2015, 115(18), p 10307–10377
N. Zhang and Y.-J. Xu, The Endeavour to Advance Graphene–Semiconductor Composite-Based Photocatalysis, Cryst. Eng. Commun., 2016, 18(1), p 24–37
C. Han, N. Zhang, and Y.-J. Xu, Structural Diversity of Graphene Materials and Their Multifarious Roles in Heterogeneous Photocatalysis, Nano Today, 2016, 11(3), p 351–372
H. Zhang, X.J. Lv, Y.M. Li, Y. Wang, and J.H. Li, P25-Graphene Composite as a High Performance Photocatalyst, ACS Nano, 2010, 4(1), p 380–386
J.C. Liu, H.W. Bai, Y.J. Wang, Z.Y. Liu, X.W. Zhang, and D.D. Sun, Self-Assembling TiO2 Nanorods on Large Graphene Oxide Sheets at a Two-Phase Interface and Their Anti-Recombination in Photocatalytic Applications, Adv. Funct. Mater., 2010, 20(23), p 4175–4181
H. Wu, J. Fan, Y. Yang, E. Liu, X. Hu, Y. Ma, X. Fan, and C. Tang, Hydrothermal Synthesis of Graphene-TiO2 Nanowire with An Enhanced Photocatalytic Activity, Russ. J. Phys. Chem. A, 2015, 89(7), p 1189–1194
A. Peter, L. Mihaly-Cozmuta, A. Mihaly-Cozmuta, C. Nicula, A. Jastrzębska, P. Kurtycz, and A. Olszyna, Morphology, Structure, and Photoactivity of Two Types of Graphene Oxide–TiO2 Composites, Chem. Pap., 2015, 69(6), p 839–855
Z. Qianqian, B. Tang, and H. Guoxin, High Photoactive and Visible-Light Responsive Graphene/Titanate Nanotubes Photocatalysts: Preparation and Characterization, J. Hazard. Mater., 2011, 198, p 78–86
J.F. Shen, M. Shi, N. Li, B. Yan, H.W. Ma, Y.Z. Hu, and M.X. Ye, Facile Synthesis and Application of Ag-Chemically Converted Graphene Nanocomposite, Nano Res., 2010, 3(5), p 339–349
S.D. Perera, R.G. Mariano, K. Vu, N. Nour, O. Seitz, Y. Chabal, and K.J. Balkus, Hydrothermal Synthesis of Graphene-TiO2 Nanotube Composites with Enhanced Photocatalytic Activity, Acs Catal., 2012, 2(6), p 949–956
S.W. Liu, C. Liu, W.G. Wang, B. Cheng, and J.G. Yu, Unique Photocatalytic Oxidation Reactivity and Selectivity of TiO2-Graphene Nanocomposites, Nanoscale, 2012, 4(10), p 3193–3200
K.N. Kudin, B. Ozbas, H.C. Schniepp, R.K. Prud’homme, I.A. Aksay, and R. Car, Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets, Nano Lett., 2008, 8(1), p 36–41
S. Stankovich, D.A. Dikin, R.D. Piner, K.A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S.T. Nguyen, and R.S. Ruoff, Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide, Carbon, 2007, 45(7), p 1558–1565
I. Yoon, C.D. Kim, B.K. Min, Y.K. Kim, B. Kim, and W.S. Jung, Characterization of Graphene Sheets Formed by the Reaction of Carbon Monoxide with Aluminum Sulfide, B Korean Chem Soc, 2009, 30(12), p 3045–3048
Y. Zhang, Z.-R. Tang, X. Fu, and Y.-J. Xu, Engineering the Unique 2D Mat of Graphene to Achieve Graphene-TiO2 Nanocomposite for Photocatalytic Selective Transformation: What Advantage Does Graphene Have Over Its Forebear Carbon Nanotube?, ACS Nano, 2011, 5(9), p 7426–7435
Acknowledgments
The author further acknowledges the financially supported by the Natural Science Funds of China (Nos. 51204220, 51274263), Chongqing Natural Science Foundation (cstc2013jjB0035), and Key Technology Innovation Projects of Key Industries in Chongqing (cstc2016zdcy-ztzx0020-03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, G., Yang, J., Zhao, D. et al. Research on Photocatalytic Properties of TiO2-Graphene Composites with Different Morphologies. J. of Materi Eng and Perform 26, 3263–3270 (2017). https://doi.org/10.1007/s11665-017-2776-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11665-017-2776-6