Abstract
In the temperature range of 1773 K to 1873 K, nitrogen solubility and TiN formation in molten nickel were studied using melt-gas and melt-gas-nitride equilibrium at atmospheric nitrogen pressure. The nitrogen solubility increased with the increase of Ti content in molten nickel. TiN was formed when titanium and nitrogen in molten nickel reach critical contents. The critical titanium and nitrogen contents for TiN formation were significantly increased with the increase of melt temperature. Thermodynamic analysis of the experimental results was carried out in the form of Wagner interaction parameter. The standard Gibbs free energy change of nitrogen dissolution reaction in molten nickel was obtained as \(\Delta G_{{\text{N}}}^{0} = 53803 + 27.76T{\text{ (J/mol)}}\). Based on the study of nitrogen solubility in molten Ni-Ti-N alloys, the temperature dependence of interaction parameters between N and Ti can be expressed as \(e_{{\text{N}}}^{{{\text{Ti}}}} = - 800/T + 0.25\) and \(e_{{{\text{Ti}}}}^{{\text{N}}} = - 2728/T + 0.85\). According to the thermodynamic study of TiN formation in molten Ni-Ti-N alloys, the standard Gibbs free energy change of TiN formation was \(\Delta G_{{{\text{TiN}}}}^{0} = - 203553 + 65.29T \, ({\text{J/mol}})\), and the interaction parameter of Ti on itself was obtained as \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}} = 0.046 \, (1773{\text{ to }}1873{\text{ K}})\).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nickel-base superalloys have excellent high-temperature corrosion resistance and high-temperature mechanical properties, so they are widely used in aerospace engines, industrial gas turbines, nuclear energy, petrochemical industry, etc.[1,2,3] Titanium up to 5.0 mass pct is added to nickel-base cast superalloys as a main forming element of γ′ precipitation strengthening phase which is beneficial to the improvement of high-temperature mechanical properties.[4,5,6] However, titanium is a strong nitride-forming element to form TiN inclusions in nickel.[7] TiN inclusions and TiN clusters, with coarse brittle microstructure characteristics, are sensitive locations of crack initiation under cyclic stress, which promote the generation and propagation of cracks.[8,9,10,11]
Therefore, it is desirable to control nitrogen dissolution and TiN formation in molten nickel alloys before casting. At present, however, thermodynamic data for nitrogen solubility and TiN formation in molten nickel alloys are very limited compared to molten ferroalloys.[12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] The effects of various alloy elements on the formation of TiN in molten ferroalloys at different temperatures have been studied extensively.[33,34,35,36,37,38,39] However, \(\Delta G_{{\text{N}}}^{0}\) in molten nickel has not been measured directly up to now. Also, thermodynamic property of Ti such as the value of \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) in molten nickel is not well known. The thermodynamic parameters of many alloying elements in nickel-base alloys have not been measured, and there are some cases where thermodynamic parameters of iron-base alloys were cited for thermodynamic calculation for nickel-base alloys.[29]
In the present study, as a basis for nickel-base alloys, thermodynamics of nitrogen dissolution and TiN formation in molten Ni-Ti-N alloys were studied by melt-gas and melt-gas-nitride equilibrium experiments at the temperature range of 1773 K to 1873 K and under atmospheric nitrogen pressure, respectively. Through thermodynamic analysis, the important thermodynamic parameters logKN, \(\log K_{{{\text{TiN}}}}\), \(e_{{\text{N}}}^{{{\text{Ti}}}}\), \(e_{{{\text{Ti}}}}^{{\text{N}}}\), and \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) in molten nickel were determined.
Experimental Procedure
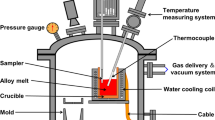
The equilibrium experiments of melt-gas and melt-gas-nitride were carried out at 101.33 kPa nitrogen pressure and 1773 K to 1873 K to obtain the thermodynamic data of nitrogen dissolution and TiN formation in molten Ni-Ti-N alloy. As shown in Figure 1, nitrogen solubility and TiN formation equilibrium in molten Ni-Ti-N alloy system were measured using a well-sealed tubular resistance furnace. About 800 g of nickel bars (99.96 mass pct purity) was put in an alumina crucible for melting, and a graphite crucible was sheathed outside the alumina crucible to prevent breakout during melting. Argon (99.999 vol pct purity) was blown from the bottom of the tubular resistance furnace at a rate of 2 L/min to provide a protective atmosphere during heating and melting of nickel. The material was located in the constant temperature zone of the furnace that was separately determined prior to experiment. By using Pt-30%Rh/Pt-6%Rh thermocouple, the temperature in the constant temperature zone was continuously measured, and the temperature was controlled within the target value of ± 5 K. When the temperature reached the set value, argon from the bottom of the furnace was switched to nitrogen (99.999 vol pct purity) at the same rate of 2 L/min. At the same time, a four-hole alumina capillary tube was inserted into molten nickel and nitrogen was blown at the rate of 0.2 L/min.
During nitrogen dissolution experiment, molten nickel samples (about 30 g each time) were extracted with time by quartz tube and quenched rapidly in ice water. The nitrogen content in nickel sample was analyzed on site, and the change of nitrogen content in molten nickel was checked during the experiment to confirm the attainment of nitrogen solubility. Then a predetermined amount of titanium particles (99.99 mass pct purity) were added into molten nickel through a quartz tube under the same nitrogen blowing condition. Titanium addition was repeated after confirming a new equilibrium for nitrogen solubility in the molten alloy. After titanium content reached the critical value, TiN would form in the molten Ni-Ti-N alloys.[33]
Under the experimental condition of nitrogen blowing into molten Ni-Ti-N alloys, gas stirring would help a fast attainment of metal-nitride-gas equilibrium, but it would also retard the floatation of TiN inclusions to the melt surface. This situation would lead to a large error in measuring the nitrogen content of the sample obtained by sampling technique. Therefore, after 60 minutes of nitrogen blowing into molten Ni-Ti-N alloys, the alumina capillary tube was taken out of the melt and kept above the melt surface to allow the floatation of TiN inclusions. It can be expected that any TiN inclusions formed in the melt can easily float to the surface due to their smaller density compared to that of molten nickel alloy. Figure 2 shows the distribution of TiN inclusions in molten alloy at different nitrogen blowing conditions.
Three specimens were cut from each metal sample obtained by suction and carefully ground with sandpaper for nitrogen and oxygen analysis. The nitrogen and oxygen detector (TC-500, LECO Corporation) using inert gas melting-infrared absorption measurement technology was used to measure the nitrogen and oxygen content in the specimen, and its accuracy was ± 1 mass ppm. The surface of the metal sample was mechanically ground to remove the oxide scale on the surface, and then the samples for titanium analysis were made by lathe, and the metal chips were cleaned by ultrasonic cleaner to avoid pollution. And titanium contents in metal chips were detected using an inductively coupled plasma optical emission spectrometer (Avio 500, PerkinElmer Corporation). The samples including the upper surface of the alloy ingot obtained by cutting the alloy ingot were mechanically ground and polished. Then, Ultra Plus field emission scanning electron microscope (EVO18, Carl-Zeiss Corporation) equipped with energy-dispersive spectroscopy (EDS)[40] was used to characterize and analyze TiN inclusions and layer formed in the molten Ni-Ti-N alloys.
Results and Discussion
The main concern is how to determine whether the equilibrium nitrogen content in molten Ni-Ti-N alloy measured by sampling technique is the soluble nitrogen content in molten alloy when TiN is formed. For this reason, additional experiments were carried out to check whether nitrogen blowing condition would affect the determination of soluble nitrogen content by sampling technique.
The change of nitrogen content with experimental time in molten Ni-1.0 mass pct Ti and Ni-3.0 mass pct Ti alloys at 1823 K and 101.33 kPa nitrogen pressure is shown in Figure 3. The initial alloy compositions of 1.0 and 3.0 mass pct Ti were controlled by adding titanium particles in molten nickel under argon atmosphere. Then argon blown into the furnace was converted into nitrogen, and nitrogen was also blown into molten nickel alloy using an alumina capillary tube. Samplings were carried out at an interval of 15 minutes. After 60 minutes, the alumina capillary tube was taken out from the melt and kept above the melt surface to allow the floatation of TiN inclusions and metal samples were taken at an interval of 30 minutes.
In case of molten Ni-1.0 mass pct Ti alloy, the nitrogen content increased to a constant value within 30 minutes and remained unchanged regardless of nitrogen blowing conditions. In case of molten Ni-3.0 mass pct Ti alloy, however, the nitrogen content continuously increased during nitrogen blowing into the melt for 60 minutes and then decreased to a steady value with time after taking out the alumina capillary tube from the melt. After the experiment, the crucible containing molten Ni-Ti-N alloy was taken out, and helium was blown onto the melt for rapid quenching. The alloy ingot in crucible and quenched samples were cut longitudinally and transversely, respectively, to examine the existence of TiN by SEM and EDS analysis.
Figures 4(a) and (b) show the SEM morphology and EDS analysis of the upper surfaces of Ni-1.0 mass pct Ti and Ni-3.0 mass pct Ti alloy ingots after the nitrogen dissolution equilibrium. No TiN layer or TiN inclusion was observed in Ni-1.0 mass pct Ti alloy, as shown in Figure 4(a). On the other hand, as shown in Figure 4(b), a thick TiN layer was detected on the surface of Ni-3.0 mass pct Ti alloy ingot by the floatation of TiN inclusions formed in the melt during nitrogen blowing. As shown in Figure 4(c), some small size TiN precipitates (about 1 μm or less) were observed in the quenched metal samples. These small size TiN precipitates can be considered as the secondary inclusions formed during solidification in sampling process,[41] and they are not primary inclusions formed in the melt during nitrogen blowing.
Therefore, the data obtained from the nitrogen dissolution equilibrium (about 60 minutes) of the Ni-1.0 mass pct Ti alloy melt in Figure 3 are the nitrogen solubility without TiN saturation at 1823 K and 101.33 kPa nitrogen pressure. On the other hand, the data obtained from the nitrogen dissolution equilibrium (about 90 minutes) of the Ni-3.0 mass pct Ti alloy melt are the equilibrium nitrogen content for TiN precipitation.
Table I summarizes the equilibrium nitrogen content in molten Ni-Ti-N alloys with different Ti contents under 101.33 kPa nitrogen pressure at 1773 K, 1823 K, and 1873 K. The oxygen content in the molten alloys was less than 25 mass ppm during the whole experiments. Figure 5 shows the nitrogen solubility and equilibrium nitrogen content of TiN formation in molten Ni-Ti-N alloys with different Ti contents under 101.33 kPa nitrogen pressure at 1773 K, 1823 K, and 1873 K. It can be found that nitrogen solubility increases with the temperature and the addition of Ti content, and the influence of titanium content is remarkable. When the titanium content exceeds a critical value, the relation of Ti content and N content changes due to the formation of TiN. The increase of melt temperature increases significantly the critical Ti content and N content of TiN formation.
Nitrogen Solubility in Molten Nickel
The nitrogen dissolution reaction of molten nickel and its equilibrium constant are expressed as follows:
where fN represents Henrian activity coefficient of N, taking infinite dilute solution as the reference state, i.e., when [pct N] approaches 0, fN approaches 1; [pct N] represents nitrogen solubility of molten nickel, mass pct; \(P_{{{\text{N}}_{2} }}\) and \(P^{0}\) represent the nitrogen pressure and the standard atmospheric pressure, kPa over the melt surface; R represents the ideal gas constant, and its value is 8.314 J mol−1 K−1; and T represents thermodynamic temperature, K.
In the present study, the nitrogen solubility of molten nickel was determined as 9.3, 10.3, and 11.3 mass ppm at 1773 K, 1823 K, and 1873 K, respectively, under 101.33 kPa nitrogen pressure. Table II compares the available data for nitrogen solubility in molten nickel under 101.33 kPa nitrogen pressure at 1873 K.[17,30,31,42] Under the nitrogen pressure of 101.33 kPa, Figure 6 compares the nitrogen solubility in molten nickel obtained in the present study with the curves of nitrogen solubility with temperature obtained in other literatures.[17,30,31,42] The nitrogen solubility increases with melt temperature, but the effect is not significant. It can be found that the measured nitrogen solubility is very close to the results reported in various literatures,[17,30,31,42] which shows that the experimental device and method in the present study are reliable.
Sieverts’ method and sampling technique are two commonly used methods to measure nitrogen solubility in molten metal. The former method measures the volume of nitrogen that saturates a given mass of melt at given temperature and pressure. The sampling technique is to quickly quench and analyze the samples taken from the melt when the melt and nitrogen atmosphere reach equilibrium. Recently, the latter is more common method with progress in experimental method and analytical devices.[17]
Stomakhin et al.[31] and Fedorchenko et al.[30] used constant pressure Sieverts’ method to measure the nitrogen solubility in molten nickel, and their values are higher than those determined by sampling technique.[17,42] As pointed out in the related research in molten iron system, the Sieverts’ method may have inherent errors due to the reaction between nitrogen and the condensed melt components in the cooler part of the experimental device.[43,44] It was also pointed out by Kim et al. that Sieverts’ method would yield a highly accurate value when the gas volume change was moderately high, but the reverse would be true when the gas volume change was small.[42]
The current results are very consistent with the data obtained by sampling technique.[17,42] Kim et al.[42] determined the nitrogen solubility in molten nickel alloys using an induction furnace at 101.33 kPa nitrogen pressure and temperatures ranging from 1773 K to 1973 K. They reported that using an induction furnace and the suction pin sampling technique resulted in highly accurate measurements of nitrogen solubility in liquid nickel.[42] Qian et al.[17] also used an induction melting furnace to measure the nitrogen solubility under various nitrogen pressures up to 101.33 kPa in molten nickel alloys. They determined that the nitrogen solubility in molten nickel was 10.0 mass ppm at 101.33 kPa and 1873 K, which is slightly lower than the value in the present study. In their study, the sample melt was casted into a water-cooled copper mold in furnace chamber. It is important to note that any delay in the solidification process could result in a decrease in the nitrogen content of the sample, as some of the nitrogen may escape during this stage.
The thermodynamic data of Sigworth et al.[22] are well recognized and used widely by many researchers. However, Sigworth et al.’s recommended thermodynamic value of nitrogen dissolution reaction in molten nickel is a result of statistical data analysis based on previous experimental work.[45,46,47] It can be found that the recommended by Sigworth et al. is slightly higher than that of the present study. The present result is in excellent agreement with Kim et al.’s work.[42] As the solubility of nitrogen in liquid nickel is very low compared to liquid iron, a small difference in nitrogen solubility data by the different experimental techniques such as sampling method and constant pressure Sieverts’ method would give a great influence on the thermodynamic value of nitrogen dissolution reaction in liquid nickel (such as standard Gibbs free energy), which is also a worthy of more research in future.
As a basis for further research, the equilibrium constant and the standard Gibbs free energy change of nitrogen dissolution reaction in molten nickel were obtained from the nitrogen solubility data. From Eq. [2],
where fN approaches to 1 since nitrogen solubility of molten nickel is very low.
Therefore, log KN can be plotted as a function of temperature as shown in Figure 7. log KN can be expressed as a linear relationship with 1/T:
\(\Delta G_{{\text{N}}}^{0}\) in molten nickel can be derived from Eq. [2] as:
Thermodynamic of Nitrogen Solubility in Molten Ni-Ti-N Alloys
The nitrogen solubility in molten nickel increases significantly with Ti content as shown in Table I and Figure 5. \(\log K_{{\text{N}}}\) in Eq. [3] can be expressed in the form of the Wagner interaction parameter as follows:
where \(e_{{\text{N}}}^{{{\text{Ti}}}}\) and \(e_{{\text{N}}}^{{\text{N}}}\) represent the first-order interaction parameters of Ti and N on N in molten nickel, respectively; \(e_{{\text{N}}}^{{\text{N}}}\) can be considered as 0 because the nitrogen dissolution in nickel-base alloys followed the Sieverts’ law at nitrogen pressures up to 101.33 kPa.[14,17]
From Eq. [6], \(\log f_{{\text{N}}}^{{{\text{Ti}}}}\) can be expressed as:
where \(f_{{\text{N}}}^{{{\text{Ti}}}}\) represents the Henrian activity coefficient of Ti on N in molten nickel.
Therefore, \(e_{{\text{N}}}^{{{\text{Ti}}}}\) in Eq. [7] can be determined according to the functional relationship between nitrogen solubility and Ti content in molten Ni-Ti-N alloys. At various temperatures, Figure 8 shows that there is a good linear relationship between \(\log f_{{\text{N}}}^{{{\text{Ti}}}}\) and Ti content in molten nickel. By linear regression analysis, it is determined that \(e_{{\text{N}}}^{{{\text{Ti}}}}\) is − 0.198, − 0.188, and − 0.174 at 1773 K, 1823 K, and 1873 K, respectively. The functional relationship between \(e_{{\text{N}}}^{{{\text{Ti}}}}\) and temperature can be expressed as \(e_{{\text{N}}}^{{{\text{Ti}}}} = - 800/T + 0.25\). According to Wagner's reciprocity relation,[32] \(e_{{{\text{Ti}}}}^{{\text{N}}}\) at different temperatures can be expressed as \(e_{{{\text{Ti}}}}^{{\text{N}}} = - 2728/T + 0.85\). In The thermodynamics of dilute liquid nickel alloys edited by Sigworth et al.,[22] the reported values of \(e_{{\text{N}}}^{{{\text{Ti}}}}\) and \(e_{{{\text{Ti}}}}^{{\text{N}}}\) in Ni-Ti-N system are − 0.2 and − 0.67 in 1823 K to 1873 K, respectively. Their interaction parameters are very consistent with those determined by the melt-gas equilibrium technique in the present study.
Thermodynamics of TiN Formation in Molten Ni-Ti-N Alloys
The reaction and equilibrium constant of TiN formation in molten Ni-Ti-N alloys can be expressed as:
where \(K_{{{\text{TiN}}}}\) represents the equilibrium constant for Eq. [8]; \(a_{{{\text{TiN}}}}\) represents the activity of solid TiN, and \(a_{{{\text{TiN}}}} = 1\); \(f_{{{\text{Ti}}}}\) and \(f_{{\text{N}}}\) represent the activity coefficients of Ti and N, respectively; and [pct Ti] represents the Ti content in mass pct in molten nickel.
\(\log K_{{{\text{TiN}}}}\) can then be expressed in the form of the Wagner interaction parameter as follows:
where \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) represents the first-order self-interaction parameter of Ti in molten nickel.
Eq. [10] can be rewritten as follows in order to determine \(\log K_{{{\text{TiN}}}}\) and \({\text{e}}_{{{\text{Ti}}}}^{{{\text{Ti}}}}\):
Figure 9 plots the relationship between \(\log K_{{{\text{TiN}}}} + e_{{{\text{Ti}}}}^{{{\text{Ti}}}} \left[ {\text{pct Ti}} \right]\) and Ti content using the solubility product data of N and Ti contents determined in the present study. The data measured at various temperatures show that there is a good linear relationship between \(\log K_{{{\text{TiN}}}} + e_{{{\text{Ti}}}}^{{{\text{Ti}}}} \left[ {\text{pct Ti}} \right]\) and Ti content. By extrapolating the fitting line to 0 mass pct Ti content, the \(\log K_{{{\text{TiN}}}}\) at 1773 K, 1823 K, and 1873 K is determined to be 2.586, 2.414, and 2.266, respectively. The value of \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) in molten nickel is equal to the slope of fitting line, which is constant from 1773 K to 1873 K, and \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}} = 0.046\).
As shown in Figure 10, there is a clear linear correlation between \(\log K_{{{\text{TiN}}}}\) and 1/T, and the functional relationship between them is \(\log K_{{{\text{TiN}}}} = 10631/T - 3.41\). Based on the determination of \(\log K_{{{\text{TiN}}}}\), \(\Delta G_{{{\text{TiN}}}}^{0}\) in molten nickel can be obtained according to Eq. [2].
Aleksandrov et al.[32] studied the thermodynamics of Ti-O deoxidation equilibrium in molten Fe-Ni alloys over the whole composition range at 1873 K by thermodynamic calculation using available thermodynamic parameters for Fe-Ti-O[48,49,50] and Ni-Ti-O systems.[22,50] They estimated the value of \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) as 0.08 in nickel at 1873 K from the formula of \(\varepsilon_{{{\text{Ti}}}}^{{{\text{Ti}}}} = - 2\Delta \overline{H}_{i} /RT\) and the enthalpy change of Ti dissolution in molten nickel, \(\Delta \overline{H}_{{\text{Ti(Ni)}}} = - 118486{\text{ (J/mol)}}\) reported by Sigworth et al.[22]
Duan et al.[26] established the thermodynamic model of liquid Ni-Ti alloy by using the theory of atomic and molecular coexistence to study the thermodynamic properties and calculated the activities of structural units of Ni, Ti, Ni3Ti, and NiTi in Ni-Ti binary phase diagram. From standard Gibbs free energy changes of Ni3Ti and NiTi complex molecules formed in liquid Ni-Ti binary alloy, they calculated the activities of Ni and Ti relative to pure liquid as standard state. Based on the thermodynamic model, the activity coefficient of Ti (\(\gamma_{{{\text{Ti}}}}\)) in the range of 0 to 0.01 mole fraction of Ti was calculated. Then, according to Wagner’s relation (\(\ln \gamma_{i} = \ln \gamma_{i}^{{0}} + \varepsilon_{i}^{i} x_{i}\)), they determined the activity coefficient (\(\gamma_{{{\text{Ti}}}}^{{0}}\)) and self-interaction parameter (\(\varepsilon_{{{\text{Ti}}}}^{{{\text{Ti}}}}\)) of Ti at infinite dilution. They obtained the values of \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) in molten nickel as 0.0284 and 0.0286 at 1773 K and 1873 K.
As discussed above, the value of \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) in molten nickel has been only obtained by the estimation or the model calculation.[26,32] The important thermodynamic parameters logKN, \(\log K_{{{\text{TiN}}}}\), \(e_{{\text{N}}}^{{{\text{Ti}}}}\), and \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}}\) in the present study were directly determined by consistent experimental devices and processes. Careful attention to experimentation and chemical analysis improved the accuracy of measuring nitrogen solubility and equilibrium nitrogen content for TiN formation in molten Ni-Ti-N system. Based on the obtained thermodynamic parameters, nitrogen solubility of Eq. [1] and equilibrium nitrogen content of Eq. [8] in molten Ni-Ti-N alloy can be accurately predicted, as shown by the dotted lines in Figure 5.
Conclusions
The melt-gas and melt-gas-nitride equilibrium experiments utilizing a well-sealed tubular resistance furnace and sampling technique provided the guarantee for accurate measurement of nitrogen solubility and equilibrium nitrogen content of TiN formation in molten Ni-Ti-N alloys at atmospheric nitrogen pressure and 1773 K to 1873 K. The following conclusions can be drawn:
-
1.
The standard Gibbs free energy change of nitrogen dissolution reaction in molten nickel was determined as \(\Delta G_{{\text{N}}}^{0} = 53803 + 27.76T{\text{ (J/mol)}}\).
-
2.
The nitrogen solubility increased with the increase of Ti content in molten nickel. In the temperature range of 1773 K to 1873 K, the temperature dependence of interaction parameters between N and Ti can be expressed as \(e_{{\text{N}}}^{{{\text{Ti}}}} = - 800/T + 0.25\) and \(e_{{{\text{Ti}}}}^{{\text{N}}} = - 2728/T + 0.85\).
-
3.
TiN was formed when Ti and N in molten nickel reach critical contents in molten nickel. According to the thermodynamic study of TiN formation in molten Ni-Ti-N alloys, the standard Gibbs free energy change of TiN formation and the interaction parameter of Ti on itself were obtained as \(\Delta G_{{{\text{TiN}}}}^{0} = - 203553 + 65.29T{\text{ (J/mol)}}\) and \(e_{{{\text{Ti}}}}^{{{\text{Ti}}}} = 0.046 \, (1773{\text{ to }}1873{\text{ K}})\).
References
R. Darolia: Int. Mater. Rev., 2019, vol. 64, pp. 355–80.
R.C. Reed and C.M.F. Rae: Physical Metallurgy, 5th ed. Elsevier, Oxford, 2014, pp. 2215–90.
N.E. Prasad and R.J.H. Wanhill: Aerospace Materials and Material Technologies, Springer, Singapore, 2017, pp. 29–52.
A. Pineau and S.D. Antolovich: Eng. Fail. Anal., 2009, vol. 16, pp. 2668–97.
E.O. Ezugwu, Z.M. Wang, and A.R. Machado: J. Mater. Process. Technol., 1999, vol. 86, pp. 1–6.
P. Shi, W. Xu, B. Fu, J. Liu, J. Zhang, K. Wang, and X. Liu: Prog. Nat. Sci.: Mater. Int., 2022, vol. 32, pp. 456–62.
A. Shi, Z. Wang, C. Shi, L. Guo, C. Guo, and Z. Guo: ISIJ Int., 2020, vol. 60, pp. 205–11.
J. Tan, X. Wu, E.H. Han, W. Ke, X. Liu, F. Meng, and X. Xu: Corros. Sci., 2014, vol. 88, pp. 349–59.
D. Texier, J.C. Stinville, M.P. Echlin, S. Pierret, P. Villechaise, T.M. Pollock, and J. Cormier: Acta Mater., 2019, vol. 165, pp. 241–58.
D. Texier, A.C. Gómez, S. Pierret, J.M. Franchet, T.M. Pollock, P. Villechaise, and J. Cormier: Metall. Mater. Trans. A, 2016, vol. 47A, pp. 1096–09.
D. Texier, J. Cormier, P. Villechaise, J.C. Stinville, C.J. Torbet, S. Pierret, and T.M. Pollock: Mater. Sci. Eng. A, 2016, vol. 678A, pp. 122–36.
J. Siwka: International Conference on High Nitrogen Steels, Metallurgy Industry Press, Sichuan, 2006.
M. Herrera-Trejo and D. Ablitzer: Can. Metall. Q., 1997, vol. 36, pp. 341–45.
K. Qian, B. Chen, P. Zhao, M. Zhang, and K. Liu: ISIJ Int., 2019, vol. 59, pp. 2220–27.
R.F. Abdulrahman and A. Hendry: Metall. Mater. Trans. B, 2001, vol. 32B, pp. 1103–12.
R.F. Abdulrahman and A. Hendry: Metall. Mater. Trans. B, 2001, vol. 32B, pp. 1095–1101.
K. Qian, B. Chen, L. Shu, and K. Liu: Metals, 2019, vol. 9, p. 1184.
C. Kowanda and M.O. Speidel: Scripta Mater., 2003, vol. 48, pp. 1073–78.
H. Feng, H.B. Li, J. Dai, Y. Han, J.D. Qu, Z.H. Jiang, Y. Zhao, and T. Zhang: Corros. Sci., 2022, vol. 204, p. 110396.
C. Kowanda and M.O. Speidel: Steel Res., 2000, vol. 71, pp. 423–27.
X.Z. Li, H.B. Li, H. Feng, S.X. Yang, S.C. Zhang, H.C. Zhu, and Z.H. Jiang: Metall. Mater. Trans. B, 2023, vol. 54B, pp. 203–12.
G.K. Sigworth, J.F. Elliott, G. Vaughn, and G.H. Geiger: Can. Metall. Q., 1977, vol. 16, pp. 104–10.
T. Miki: Treatise on Process Metallurgy, Elsevier, Amsterdam, 2014, pp. 557–85.
Y. Haruna: Removal of Inclusions from Cast Superalloy Revert, University of British Columbia, Vancouver, 1994.
F. Yang, W. Zhao, Y. Hou, X. Guo, Q. Li, X. Li, and Z. Ren: ISIJ Int., 2021, vol. 61, pp. 229–38.
S.C. Duan, X. Shi, M.C. Zhang, B. Li, G.X. Dou, H.J. Guo, and J. Guo: J. Mol. Liq., 2019, vol. 294, p. 111462.
S.F. Yang, S.L. Yang, J.L. Qu, J.H. Du, Y. Gu, P. Zhao, and N. Wang: J. Iron. Steel Res. Int., 2021, vol. 28, pp. 921–37.
F. Yang, J. Cao, L. Shi, J. Yu, K. Deng, and Z. Ren: ISIJ Int., 2023, vol. 63, pp. 436–47.
J. Wang, L. Wang, J. Li, C. Chen, S. Yang, and X. Li: J. Alloys Compd., 2022, vol. 906, p. 164281.
V.I. Fedorchenko, V.V. Averin, and A.M. Samarin: Dokl. Akad. Nauk SSSR, 1968, vol. 183, pp. 894–96.
A.Y. Stomakhin, P. Bayer, and A.Y. Polyakov: Metals, 1965, vol. 4, pp. 37–45.
A.A. Aleksandrov, V.Y. Dashevskii, M.A. Makarov, and G.S. Sprygin: Russ. Metall., 2008, vol. 2008, pp. 271–80.
W.Y. Kim, J.O. Jo, T.I. Chung, D.S. Kim, and J.J. Pak: ISIJ Int., 2007, vol. 47, pp. 1082–89.
W.Y. Kim, J.O. Jo, C.O. Lee, D.S. Kim, and J.J. Pak: ISIJ Int., 2008, vol. 48, pp. 17–22.
J.O. Jo, W.Y. Kim, C.O. Lee, and J.J. Pak: ISIJ Int., 2010, vol. 50, pp. 1373–79.
H. Wada and R.D. Pehlke: Metall. Trans. B, 1977, vol. 8B, pp. 443–50.
T.I. Chung, J.B. Lee, J.G. Kang, J.O. Jo, B.H. Kim, and J.J. Pak: Mater. Trans., 2008, vol. 49, pp. 854–59.
Y. Liu, L. Zhang, H. Duan, Y. Zhang, Y. Luo, and A.N. Conejo: Metall. Mater. Trans. A, 2016, vol. 47A, pp. 3015–25.
Q. Tian, G. Wang, Y. Zhao, J. Li, and Q. Wang: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 1149–64.
S. Zhang, J. Yu, H. Li, Z. Jiang, Y. Geng, H. Feng, B. Zhang, and H. Zhu: J. Mater. Sci. Technol., 2022, vol. 102, pp. 105–14.
J. Fu, W. Qiu, Q. Nie, and Y. Wu: J. Alloys Compd., 2017, vol. 699, pp. 938–46.
C.K. Kim, A. McLean, F. Ishii, and S. Ban-Ya: ISS Trans., 1987, vol. 8, pp. 29–35.
R.D. Pehlke and J.F. Elliot: Trans. Met. Soc. AIME, 1960, vol. 218, pp. 1088–1101.
D.W. Gomersall: Solubility of Nitrogen in Liquid Iron Alloys, McMaster University, Hamilton, 1967.
K.W. Lange and H. Schenck: Metall. Trans., 1970, vol. 1, pp. 2036–38.
H. Wada, K. Gunji, and T. Wada: Trans. Iron Steel Inst. Jpn., 1968, vol. 8, pp. 329–36.
H. Schenck, M.G. Frohberg, and H. Graf: Archiv für das Eisenhüttenwesen, 1959, vol. 30, pp. 533–37.
J.J. Pak, J.O. Jo, S.I. Kim, W.Y. Kim, T.I. Chung, S.M. Seo, J.H. Park, and D.S. Kim: ISIJ Int., 2007, vol. 47, pp. 16–24.
The Japan Society for the Promotion of Science: Steelmaking Data Sourcebook, The 19th Committee on Steelmaking, ed., Gordon and Breach Science Publisher, New York, 1988, pp. 45–53.
T. Chiang and Y.A. Chang: Metall. Mater. Trans. B, 1976, vol. 7B, pp. 453–67.
Acknowledgment
This research was sponsored by the National Natural Science Foundation of China [Grant Nos. 52325406/52004060/U1960203], Science Fund for Distinguished Young Scholars of Liaoning Province [Grant No. 2023JH6/100500008], and Program of Introducing Talents of Discipline to Universities [Grant No. B21001].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, XZ., Feng, H., Li, HB. et al. Thermodynamic Study on Nitrogen Solubility and TiN Formation in Molten Ni-Ti-N Alloys. Metall Mater Trans B 55, 3653–3662 (2024). https://doi.org/10.1007/s11663-024-03208-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-024-03208-w