Abstract
The interaction between the minerals in a goethitic iron ore (goethite, quartz, hydrohematite, quartz-dispersed hydrohematite, and quartz-dispersed goethite) and pure flux materials (CaO, MgO, SiO2, and Al2O3) was studied by heating ore–flux interaction couples to different temperatures under 0.5 kPa O2 partial pressure when the samples were loaded into the hot zone of a furnace preheated to 1300 °C. The results show that CaO was the most effective flux for liquid-phase generation during sintering. MgO diffused into ore grains by solid-state diffusion and formed solid solution (Fe, Mg)O·Fe2O3, but did not generate liquid. Al2O3 and SiO2 in the interaction couples were relatively inert during heating. The goethite matrix, as the major mineral phase of the ore, played a key role in the interaction with CaO. At 1200 °C, no apparent liquid formation was observed. CaO diffused into the goethite matrix, and first reacted with SiO2 to form CaO·(SiO2)x. Then CaO combined with Fe2O3 and formed CaO·(Fe2O3)x. When heated to 1225 °C, CaO·(Fe2O3)x and CaO·(Fe2O3)x ·(SiO2)y phases formed by solid-state reaction started to melt. With increasing temperature, more liquid phase was generated. Whole ore particles were almost completely assimilated, with only a few dense ore fragments being observed in the samples heated to 1300 °C. Other mineral phases in the goethitic ore were less reactive due to their dense structure. On heating during sintering, dehydration of goethite generated a porous structure and cracks in the ore body, facilitating the diffusion of flux and penetration of flux-containing liquid phase. The core particles in the ore body, including hydrohematite minerals, remained relatively dense and so their assimilation during sintering is slow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lower grade ores such as goethite have become increasingly used in iron ore sintering worldwide.[1,2] From a mineralogical perspective, the texture of goethitic ores is typically composed of spheroidal grains and porous, with varying amounts of coarser, goethitic fossil wood in a goethite matrix.[1,3,4] From a geometallurgical perspective, goethitic ores are classified into brown, ochreous, and vitreous goethite on the basis of their physical properties (color, luster, streak, hardness, and fracture).[5] However, these classifications cannot reflect the properties and performance of mineral phases in sintering and other ironmaking processes. No specific classification on the characteristic phases has been found in the literature. Furthermore, no information on the sintering performance of each phase or combined minerals has been reported. Therefore, an appropriate assessment, or classification, of goethitic ores from a metallurgical perspective should be developed.
Normally, bench- and/or pilot-scale sintering tests are undertaken prior to a new blend being used in the sinter plant. Previous research shows that goethitic ores have high assimilation with CaO during sintering, forming liquid phase with low-melting point and high fluidity; although the bonding phases present high SFCA content, the sinter strength was low due to the excessively porous structure formed.[6,7,8,9,10,11,12,13,14,15,16] However, these investigations focused on the properties of final sinter product, and the examination of individual mineral phases in the iron ores and their pairwise interaction with fluxing materials were rarely considered.
Previous research investigated the interaction between mineral phases in hematite iron ore and fluxing materials during sintering under representative conditions, which revealed the performance of fluxing materials in hematite iron ore and the initial liquid-phase formation and evolution during the high-temperature sintering.[17,18,19] In this further study, the novel experimental design was used wherein tablets comprised of cube-shaped samples of goethitic iron ore surrounded by fluxing material (CaO, MgO, SiO2 and Al2O3) were first sintered at 1200 °C to 1300 °C, followed by a detailed examination of the reaction interfaces between different minerals in the ore and flux materials under mineral optical microscopy and SEM-EDS. This investigation fills a knowledge gap concerning the interaction between the minerals of representative goethite ores and flux materials (CaO, MgO, Al2O3, and SiO2). A better understanding of the performance of ore minerals is developed, which is helpful for overall improvement of blending and sinter plant operations in general.
Experimental
Full details of the experimental approach and setup are provided elsewhere.[17] The experimental techniques used in the investigation are briefly described below.
Materials
Table I shows the chemical composition of a goethite ore (Ore C). The major impurities of the ore are silica and alumina. Its phosphorus and sulfur contents are very low, so the behaviors of phosphate and sulfide impurities are ignored in this investigation. To keep the representative of the data, the ore sample was collected from different sites of the ore stockpile and crushed for the XRF examination by the current standard ISO for iron ore sampling preparation and measurement, so the data in Table I were an average in chemistry.
Sample Preparation and Sintering
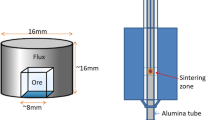
The ore–flux pair tablets were prepared using cube-shaped samples of iron ore and surrounding these with a single pure fluxing material to examine the interaction between the single mineral phase of iron ore and the single flux. A lump of iron ore was cut and polished to form a cube (~ 8 mm sides). The cube was placed at the bottom center of a die of 16 mm in diameter, which was filled with flux powder and then pressed under 25 MPa into a tablet of 16 mm in diameter and ~ 16 mm high.
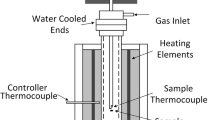
These tablets were sintered and used to study the interaction of individual mineral phases with fluxes at the interface (Figure 1(a) in Reference 17). The sintering was conducted in a vertical electric heating tube furnace (Figure 1(b) in Reference 17) with 1 L/min gas flow, under 0.5 kPa oxygen partial pressure. A tablet was loaded into a stainless steel wire basket and located in the furnace hot zone. After the set sintering time was reached, the sample was taken out of the furnace tube and cooled in air. The sintering time needed to heat a sample to a desired temperature was determined according to the temperature profile curve of tablets, determined in advance (see Figure 2 in Reference 17). In the interaction experiments, the samples were removed from the furnace at the time when it reached the expected temperatures.
Characterization Methods
The sintered tablets were mounted in epoxy resin in preparation for optical and SEM-EDS analyses. After curing, the surface was ground and washed with anhydrous ethanol, and then polished to a 1 μm finish for optical microscopic observation (Leica DM6000 Optical Microscope). The polished samples were platinum coated and analyzed by scanning electron microscopy (JEOL JSM-6490LV) operated at 20 kV.
Results and Discussion
Characterization of Goethitic Ore C
Compared with conventional hematite ores, Ore C is more heterogeneous. The classification of the mineral phases is complex and hence, in this section, a summary of the characterization results is provided.
In general, the relatively pure mineral phases in Ore C include goethite (G), hydrohematite (HH), and quartz (Q). Quartz presents as either separate particles or smaller particles enclosed in goethite and hydrohematite matrices, and has a wide size distribution. The enclosed particles are commonly referred to as cores in mineralogy references.[1,3,4] The dispersion of quartz in goethite and hydrohematite forms new minerals, quartz-dispersed hydrohematite (HHQ) and quartz-dispersed goethite (GQ). As a result, the major minerals include goethite, hydrohematite, quartz, quartz-dispersed hydrohematite, and quartz-dispersed goethite. Quartz-dispersed hydrohematite differs from quartz-dispersed goethite in that it is present only in the form of enclosed cores, while quartz-dispersed goethite can be in the form of enclosed cores and lumps.
Figure 1 shows photos and optical images of a typical lump of Ore C. Porous goethite forms the matrix, showing as dark brown in photography (Figure 1(a)) and gray under optical microscopy (Figure 1(b)), respectively. Many cores are enclosed in the goethite matrix. The matrix represents the largest volume of Ore C, accounting for up to 75 pct of the ore volume.[1,20] By EDS analysis, Al and Si were detected in the goethite phase as impurities, accounting for, on average, ~ 0.6 wt pct and 1.4 wt pct, respectively. It should be noted that goethite has a layered structure surrounding cores at higher magnification (Figures 1(c) and (e)), naturally formed by the sedimentation of iron-rich silt/mud in riverbeds.[4] Therefore, the chemical composition of each layer may vary.
Quartz cores have dense structure and appear milky white in photography (Figure 1(d)), but show dark gray under optical microscopy (Figure 1(e)). EDS analysis showed no other impurities in these cores.
Hydrohematite is a dense, defect solid solution where OH− ions replace oxygen.[21] It is shown black in the photograph (Figure 1(a)), but white under optical microscopy. Sometimes, small quartz particles are observed within hydrohematite.
When quartz content and size increase in the hydrohematite matrix, the cores of quartz-dispersed hydrohematite change in color from black to red (Figure 1(a)), and correspondingly, white to dark gray under optical microscopy. The differences in the color of two quartz-dispersed hydrohematite cores marked in Figure 2 reflect different quartz contents in the cores. Figure 2 shows the surface morphology of two quartz-dispersed hydrohematite-containing cores, via the photo and optical images of their polished cross sections, and detailed Si distribution within the quartz-dispersed hydrohematite cores by EDS mapping. In Figure 2(a), the quartz content and grain size in particle 1 are less than those in particle 2, and the distribution of quartz in particle 2 is less homogeneous.
Quartz-dispersed goethite shows ochreous color in photography (Figure 1(a)) and dark brown color under optical microscopy (Figure 1(b)), respectively, and the distribution of quartz is non-homogeneous. Figure 3 shows the photomicrographs and BSE images, and EDS mapping of Si and Fe. The chemical compositions of the ore at selected sites marked in Figure 3 are presented in Table II. Quartz presents in two different states in the quartz-dispersed goethite mineral. Coarse quartz particles segregate from goethite grains and are quite pure (Sites S1 to S3), presenting in a dendritic structure when observed at higher magnification in Figure 3. Very fine quartz particles distribute in the goethite grains as indicated by the chemical compositions at Sites G1 and G2.
Figure 4 presents the morphology of a typical goethite matrix around cores. All cores in Ore C were cemented by a porous colloform goethite matrix.[20] There is a thin layer (ca. 50 μm) of higher porosity goethite surrounding each core. Hence, Ore C has a highly heterogeneous structure consisting of relatively dense cores, a porous goethite matrix, and an even more porous goethite surrounding the cores as intermediate layer.
During sintering, dehydration of the goethite and hydrohematite minerals first takes place, followed by sintering of the mineral phases, causing non-uniform shrinkage of different minerals in the same ore particle. After calcination at 1200 °C, cracks appeared on the surface of the ore lumps. The ore formed an internal porous structure, affecting the interaction between the mineral phases and fluxing materials.
Interaction Between Mineral Phases in Ore C and CaO
Goethite matrix vs CaO
Goethite matrix is the dominant mineral in Ore C, and CaO is the most reactive fluxing material.[17] The interaction between the ore and CaO flux started from the interface between goethite and CaO. Thus, the interaction between goethite mineral and CaO is the focus of this investigation.
Figure 5 compares the optical microscopic images of representative Ore C samples after heating with CaO to different temperatures. Some gaps were observed between the ore and CaO on some sides. This may be caused by materials shrink during sintering and CaO powder loss at the contact interface during sample preparation (mainly the grinding and washing process with anhydrous ethanol). However, this did not affect the interaction between the iron ore phases with fluxing material in supporting/contact areas. At 1200 °C, the most apparent change was the dehydration of goethite matrix, which resulted in large cracks. The enclosed cores remained almost unaffected. Only a very thin layer near the top edge of the sample shows some solid-state reaction in Figure 5(a). The major part of this image shows that the sample was not affected by CaO. This part of the unchanged sample can be used as a reference in the examination of the effects of fluxes. At 1225 °C, the layer of solid-state reaction is thicker around the sample. It is clearer from the higher magnification image (Figure 6(b)) that a liquid phase, generated on the right-hand side of the sample, has penetrated the ore particle, bypassing some cores. With increasing temperature and the diffusion of Ca2+, more liquid phase was generated at the interface and penetrated into the ore body via the porous goethite matrix and capillary action.[22,23] At ~ 1275 °C, most of the goethite matrices had transformed into liquid phase, but the outline of the original ore remained. At ~ 1300 °C, the whole particle has melted except a few cores which were difficult to melt due to their relatively dense structure. These results correspond well with those observed in Loo’s and Carpenter’s investigations [20,24]
BSE and EDS mapping of areas (a), (b), (b’), and (b’’) in Fig. 5(b). ((b’): the rectangle area in (b); (b’’): the rectangle area in (b’))
Figure 6 shows more detailed information of areas (a) and (b) in Figure 5(b) of a sample heated up to 1225 °C, which reveals the process of solid-state diffusion of CaO into the goethite matrix and the generation of an initial liquid phase (ILP). Figure 6(a) shows the diffusion of CaO into the goethite matrix from the left side where the CaO flux was lost during grinding and polishing in sample preparation for analysis. Prior to the reaction, Ore C contained negligible CaO. From the EDS mapping of area (a) after the reaction, two CaO-rich channels were detected, separated by a denser, purer layer of iron oxide with a lower silica content. The two channels could be identified easily by the EDS mapping of Ca, and the Sites A1 and A3 located on the two channels, respectively. From the EDS mapping of Si, both channels contain higher silica content. The channel close to the core presents a more porous structure, as illustrated in Figure 4, which allowed solid diffusion to take place via the solid surface of the porous structure. Many pores formed in the other channel during sintering due to dehydration of the goethite, which assisted CaO transport.
Figure 7 presents the elemental distributions along the line marked in Figure 6(a). The two Ca diffusion channels with significantly higher Ca and Si concentrations can be easily identified: from 0 to 16 μm, and from ~ 90 to 112 μm, in which the blue triangles were marked as peaks of the two Ca diffusion channels. The Ca and Si contents in minerals were highly correlated due to the high affinity between SiO2 and CaO. Beyond 112 μm, there was another thin layer of dense iron oxide mineral where the average Si content was ~ 3 at.pct, but Ca content was moderate (~ 2 at.pct). This layer is considered to have been fine quartz-dispersed hydrohematite (HHQ), and next to it, coarse quartz-dispersed hydrohematite before interaction took place. The chemical compositions at Sites A1 to A4 in Figure 6(a) by EDS are presented in Table III, in which parameters R and C(Fe2O3) are defined in Eqs. [1] and [2]. The data of Sites A1 and A3 presented Ca2+ diffusion along the two channels, while Site A2 showed tiny amount of Ca2+ diffusion into the relatively dense ore phases. Site A4 was the original ore core part enveloped by the goethite matrix, in which negligible Ca was detected.
Distributions of O, Fe, Si, and Ca along the line marked in Fig. 6(a)
where n(i) denotes the molar fraction of species i. R values present Ca2+ diffusion index/extent into the ore body. R takes a value of 0.5, 1, and 2 for CaO·(Fe2O3)2, CaO·Fe2O3, and (CaO)2·Fe2O3, respectively. C(Fe2O3) is the molar fraction of Fe2O3 which helps identify the position of components in a Fe2O3-CaO phase diagram.
The data in Figure 7 show that the Ca content reached 4 to 5 at. pct in the CaO diffusion channels, and more than 2 at.pct in the denser layer of the goethite mineral. Less than 2 at. pct of CaO diffused into the quartz-dispersed hydrohematite layer due to its dense structure.
According to the CaO-Fe2O3 phase diagram,[25] the system should form a liquid phase above 1208 °C. However, since CaO and hematite have very low solubility in each other below this temperature, and it was very close to the end of the heating cycle (~ 6 seconds from the termination of heating according to the temperature profile (Figure 2 in Reference 17), no noticeable amount of liquid phase was formed in area (a) at 1225 °C.
The porous goethite matrix, as well as the more porous structure surrounding the cores in the matrix and the cracks formed after dehydration, means that Ore C is conducive to solid-state reaction and liquid penetration. From a thermodynamic perspective, dehydrated goethite and hydrohematite phases would likely be transformed to hematite under the testing atmosphere and temperature range of these experiments, and CaO would not form a solid solution with hematite. This means that the diffusion of Ca2+ in the hematite lattice is difficult and slow. Diffusion of CaO into the ore lumps is through surface diffusion. The porous structure of goethitic ore formed by dehydration favors this diffusion mechanism.[22,23]
Figure 6(b) shows the BSE image and elemental distributions of Ca, Fe, and Si in area (b) in Figure 5(b). It is in the same ore cube as area (a) characterized in detail in Figure 6(a) which was heated to 1225 °C. The core was a dense particle which was hardly affected by the interaction. As presented in Table III, the Ca contents at the Sites B1 and B2 were negligible, although the Si content at Site B1 was as high as 14.1 at.pct. In contrast, the whole goethite matrix in the area was affected by the interaction. A few narrow bands rich in Ca were identified, such as at Sites B4 and B5. Similar bands were present on the surface of cracks. The Ca content decreased with distance from these bands. As shown in Table III, the Ca contents at Sites B4 and B5 were high (> 10 at.pct). The composition of the goethite matrix away from the bands, such as at Site B3, was significantly different, i.e., Ca and Si contents were much lower. The area was examined in detail at higher magnifications, by selecting area (b’) from area (b) and further (b”) from area (b’). The elemental composition of Sites selected from the sample was typical and representative along with the penetration of fluxing materials (or ions) into iron ore body, so the elemental composition of the selected sites with concentration gradient would show the trend of the ions’ diffusion and present the fluxing materials diffusion channels or directions.
In area (b’), the Ca-rich band inside the goethite matrix and on the surface of a crack became clearer. Overall, the Ca and Si contents in the band were much higher than in the goethite matrix (Sites B’3, B’4, and B’5 vs B’1 and B’2), and the composition in the band is not uniform. It is clear from the morphology and EDS mapping and point analysis of area (b”) that it contained two phases. The dark areas (Sites B”1 and B”2) contained similar fractions of Ca, Si, and Fe, while the gray areas (Sites B”3 to B”5) were dominated by iron oxide and contained 3 to 7 at.pct of Ca, with little Si. From the appearance of the phases and the CaO-FeOn-SiO2 ternary phase diagram,[25] it is understood that the Ca-rich bands were melted at the final heating temperature (1225 °C) in this interaction experiment, and the gray area represents the mineral which was recrystallized from the liquid phase during cooling, leaving a glass phase rich in Ca and Si (black areas within the bands). The distributions of Fe, Si, and Ca along the line scan marked in Figure 6(b’) are presented in Figure 8, in which the Ca-rich band from ~ 175 to ~ 195 μm indicated the main path of penetration of Ca2+, and the relatively stable elemental concentrations in the band with Ca2+ penetration mean the formation of initial liquid phase (CFx precipitated) in the zone. As shown in the CaO-Fe2O3 phase diagram,[17] the CaO-Fe2O3 system has a eutectic temperature of 1208 °C. However, a liquid phase was not identified in area (a) of the same heated Ore C cube due to the very short time the sample remained above this temperature. However, the melting temperature can be lower when low contents of Si and Al are dispersed in the goethite phase. The initial liquid phase formed in area (b) of the sample heated to 1225 °C rapidly spread on the solid surface and penetrated into the pores of the goethite matrix. This strongly enhanced CaO diffusion and the interaction between the goethite and CaO flux.
Distributions of O, Fe, Si, and Ca along the line marked in Fig. 6(b’)
Based on the above analysis, the penetration of Ca2+ in iron ore body depends on the two factors: porous structure and fine SiO2 distribution. The inherent porous morphology around the cores, shown in Figure 4, and the formed porous structure after dehydration of goethite and hydrohematite provided favorable paths for Ca2+ transport. In addition, the dispersed fine quartz (rather than all quartz phases) in the mineral phases would provide powerful driving force for Ca2+ diffusion due to the high affinity between SiO2 and CaO. In the Ore C, the fine quartz dispersed in the goethite matrix during the natural formation by the sedimentation of iron-rich silt/mud in riverbeds. Thus, the goethite matrix with dispersed fine quartz and porous structure during sintering would be one of the most reactive mineral phases in Ore C during sintering.
Quartz vs CaO
Figure 9 shows the BSE images and EDS mapping of quartz cores after reacting with CaO by heating to 1250 °C. The quartz cores appear to have remained in intimate contact with CaO (Figure 9(a)), but at a higher magnification (Figure 9(b)), a thin reaction layer of ~ 20 µm was observed between quartz and CaO. The chemical compositions at the sites marked in Figure 9 are listed in Table IV. Site 5 showed pure quartz phase with very little content of other elements. At Sites 1 and 2 beyond the thin layer, the composition was close to CaFe2O4 with very a low fraction of Si and Al impurities, typical of reacted goethite matrix. Two mineral phases were present in the thin reaction layer. The major phase was that represented by Sites 3 and 4, showing a composition similar to Ca2SiO4. The other represented by Sites 6 and 7 contained mainly Fe, Ca, and Si.
According to the CaO-SiO2 phase diagram,[25] solid-state reaction between SiO2 and CaO takes place below 1000 °C, but no liquid phase is generated until 1420 °C. Apparently, some liquid was formed by the reaction of goethite surrounding the quartz cores with CaO, which penetrated to form a solid solution of CaO and SiO2 and generate a liquid phase of Fe2O3-CaO-SiO2. The latter liquid brought CaO in contact with the unreacted quartz, degrading the quartz cores further very slowly due to the dense structure. At the sintering temperatures, it was difficult to completely melt the quartz cores due to their dense structure. Some quartz cores could still be observed in samples heated to 1300 °C (Figure 5).
Hydrohematite and quartz-dispersed hydrohematite vs CaO
Figure 10 shows a representative optical microscopic image of the Ore C sample after interaction with CaO by heating to 1300 °C. The sample contained hydrohematite and quartz-dispersed hydrohematite cores which could be identified from the reacted residues included in the well-melted matrix. A white core on the right-hand side in the optical image was an unmelted hydrohematite core. Areas (a) and (b) were further examined in detail by BSE imaging and EDS mapping.
In Figure 10(a), the hydrohematite core was penetrated by the liquid phase, with only a nucleus of ~ 200 µm unreacted. This was verified by the elemental analysis of Sites A1 and A2 in Table V. At Site A1 in the unreacted core, the Ca content was very low. In the melted area, such as Site A2, the Ca content was close to 5 at.pct. At higher magnification of the edge of the unreacted nucleus in Figure 10(a’), Ca was diffusing into the nucleus as shown by the gradual change of the Ca content. Local liquid phase started to form, such as at Sites A’1 to A’3, where the Ca content was in the range of 10 to 25 at.pct.
Figure 11 shows the distribution of Ca, Fe, and Si along the line marked in Figure 10(a’). Within the range of 0 to 70 µm, the grain size of Fe-rich phase was larger compared with that beyond this range. However, pore rounding cannot be observed. Although the generation of a liquid phase enhanced the mass transfer of Ca into the core, significant melting of iron oxides in the area had not yet occurred at this temperature. Beyond 70 µm, Ca diffusion was very low and the morphology was relatively unaffected.
Distributions of Ca, Si, and Fe along the line marked in Fig. 10(a’)
Figure 10(b) shows a partially melted quartz-dispersed hydrohematite core which cracked due to the stress generated by non-uniform shrinkage of the surrounding matrix. The generated liquid phase surrounded and penetrated into the core until area (b’) marked in Figure 10(b). At higher magnification, the BSE image and elemental distributions show that the lower left area in area (b’) had limited liquid phase (Sites B’1 and B’2) penetration which enhanced the growth of iron oxide grains (Sites B’3 and B’4), forming denser morphology in the area. In contrast, the upper right area cracks were not filled with glass (i.e., no Ca and Si present in cracks) and the Fe-rich phase was more porous. More glass phase was generated in the liquid penetrated area due to a higher content of dispersed quartz in this type of core. Quartz-dispersed hydrohematite cores have dense structure similar to hydrohematite, and hence their melting was very slow and required a higher temperature during the interaction with CaO.
Quartz-dispersed goethite vs CaO
Figure 12 shows the optical image of a representative quartz-dispersed goethite sample heated with CaO to 1300 °C, and the BSE images and EDS mapping of local areas (a) and (b). Unlike previous goethite samples, the quartz-dispersed goethite samples were still relatively dense after dehydration. Opaque quartz grains (both fine and coarser grains dispersed in hematite matrix originated from goethite) were observed in the sample. The sample retained its original shape after interaction; only a small amount of liquid phase formed on the edge and penetrated into the sample along cracks.
The mineral phases in the sample after interaction with CaO could be better identified in the EDS image and elemental mapping in area (a) and the high magnification observation of area (b) selected from area (a). The dark band in the upper part of the optical image corresponds to the Ca- and Si- rich band in area (a), which contained mainly Ca and Si with a small fraction of Fe (Site 1 in Table VI). This band represents the liquid phase formed due to the interaction between the ore and CaO. Only a small fraction of the liquid penetrated into the crack of the ore lump (Sites 2 and 3), because a gap formed between the liquid and CaO layer, as observed by a dark band in the BSE image and gray band of resin in the optical image. The dense structure of the ore sample and high viscosity of the liquid phase due to its high Si content restricted the penetration. A quartz band was present in the sample in area (a) (Site 4); however, it was not affected by the interaction with CaO even though SiO2 has high affinity with Ca. This is likely a result of the low diffusion of CaO in the dense solid quartz phase.
In area (b), the liquid on the surface of the ore sample transformed into two phases after cooling: secondary skeletal hematite (Site 6) and glass phase (Site 5). Although the ore–CaO pair was heated to a temperature of 1300 °C, the dense structure of the particle prevented the liquid phase from penetrating into the quartz-dispersed goethite structure, in which Site 7 (iron oxide grain) and Site 8 (quartz grain) were both unaffected by the interaction.
In summary, the assimilation of goethitic ore, Ore C, by CaO during interaction experiments can be described via the following stages:
-
(1)
At relatively low temperature (< 1225 °C), dehydration of goethite matrix and hydrohematite takes place. CaO diffuses into the goethite matrix mainly by solid-state diffusion. Within the goethite matrix, CaO preferentially combines with finely distributed SiO2, and then, subsequently, with Fe2O3.
-
(2)
With increasing temperature and CaO diffusion, such as above 1225 °C, calcium ferrite and CaO-Fe2O3-SiO2 liquid solutions are formed. This initial liquid phase penetrates into the pores in the goethite matrix and CaO layer, enhancing the mass transfer of CaO into the goethitic ore particle and further assimilation of the ore.
-
(3)
Along with the progress of the assimilation of the goethite matrix, and further increasing temperature, more liquid is generated. This liquid surrounds the dense core particles of quartz, quartz-dispersed goethite, hydrohematite, and quartz-dispersed hydrohematite, promoting the assimilation of these cores.
Interaction between Mineral Phases in Ore C and MgO, Al2O3, and SiO2
According to the results of the interaction between Ore C phases and CaO, goethite matrix is the most reactive phase. The examination of the interaction between Ore C and MgO, Al2O3, and SiO2 was focused on the goethite mineral.
Similar to the situation of Ore B,[17] the interaction between goethite in Ore C and MgO, Al2O3, and SiO2 was very limited. The results show that MgO diffused into goethite matrix to a depth of 60 µm by solid-state diffusion at 1300 °C and formed a spinel solid solution (Fe, Mg)O·Fe2O3, while Al2O3 and SiO2 were non-reactive with the goethite phase. For other dense phases, MgO, Al2O3, and SiO2 are relatively inert during sintering.
The results of interaction between different mineral phases in iron ores and various fluxing materials reflected the processes of solid-state diffusion and initial liquid-phase formation before a large amount of liquid phase can be generated. This investigation fills a knowledge gap concerning the interaction between the mineral phases of representative goethitic ores and flux materials (CaO, MgO, Al2O3, and SiO2). The bulk sinter bed, from the local micro-areas, consists of fine ore particles or ore mineral phases and fluxing materials. Prior to a large amount of liquid phase generated, the chemical reaction on the contact interface between minerals and fluxing materials determines the lowest temperature at which the initial liquid phases generated. This study investigated the interactions between a mineral of iron ore and a single flux in micro-zone of sinter bed and revealed the fundamental mechanism of initial liquid-phase formation during sintering. In addition, this study revealed the localized solid-state diffusion of Mg2+ within limited micron level at high temperature during sintering, which questioned the function of Mg-bearing raw materials as conventional effective fluxing materials during sintering if its roles in the downstream ironmaking are not considered. Overall, the study helps to better understand the mechanism of the sintering process and the behavior of ore mineral phases during iron ore matching in sinter plant, and can support the industry in the development of better condition to energy optimization and better usage of limestone, Mg-bearing raw materials, and insights about quality.
Conclusions
The major minerals in a goethitic ore were determined and their interaction with different fluxing materials was examined by tablet experiments in the temperature range of 1200 to 1300 °C. The following conclusions are obtained.
-
(1)
The major mineral phases in the goethitic ore include goethite, hydrohematite, and quartz. Goethite phase containing finely distributed quartz forms the matrix of the goethitic ore. Other minerals are present in the matrix as core particles, including quartz, hydrohematite, quartz-dispersed goethite, and quartz-dispersed hydrohematite.
-
(2)
On heating during sintering, dehydration of goethite generates a porous structure and cracks in the ore body, facilitating the diffusion of flux and penetration of flux-containing liquid phase. The core particles in the ore body, including hydrohematite minerals, remain relatively dense and so their assimilation during sintering is slow. In addition, the dispersed fine quartz in the goethite matrix contributed Ca2+ diffusion due to the high affinity between SiO2 and CaO. Therefore, the goethite matrix with dispersed fine quartz and porous structure during sintering was the most reactive mineral phases in Ore C during sintering.
-
(3)
For the cores in Ore C, including quartz, hydrohematite, quartz-dispersed goethite, and so on, the dense structure restricted the fluxing materials or ions’ diffusion, and further limited the liquid-phase formation at high temperature.
-
(4)
The interaction between goethite and CaO plays a key role in initiating formation of liquid phase and assimilation of other minerals. Below 1225 °C, no liquid formation was observed, and CaO diffused into dehydrated goethite matrix by solid-state diffusion. At approx. 1225 °C, an initial liquid phase of calcium ferrite or its solution with silica and alumina was formed and the sintering process was enhanced. With increasing temperature, more liquid was quickly generated in the goethite phase. When the temperature reached 1300 °C, most of the goethite ore particle was melted, with only a few nuclei of dense structured cores remaining unmelted.
-
(5)
CaO is the most effective flux for liquid-phase generation during sintering. MgO diffuses into ore grains by solid-state diffusion and forms a solid solution, (Fe, Mg)O·Fe2O3, which does not initiate a liquid phase. Externally added Al2O3 and SiO2 are relatively inert fluxes during sintering.
References
E. R. Ramanaidou, R. C. Morris and R. C. Horwitz: Western Australia. Australian Journal of Earth Sciences, 2003, vol. 50, pp. 669-690.
R. Morris and M. Kneeshaw: Australian Journal of Earth Sciences, 2011, vol. 58, pp. 417-451.
R. C. Morris: Economic Geology, 1980, vol. 75, pp. 184-209.
R. C. Morris and E. R. Ramanaidou: Australian Journal of Earth Sciences, 2007, vol. 54, pp. 733-756.
J. Manuel: Iron Ore 2017 Workshop Presentation, 2017.
S. Wu, H. L. Han, H. X. Li, J. Xu, S. D. Yang, and X. Liu: International Journal of Minerals, Metallurgy, and Materials, 2010, vol. 17, pp. 11-16.
S. Wu, Y. Liu, J. Du, K. Mi, H Lin: Journal of University of Science and Technology Beijing(China), 2002, 24, 254-257.
D. Witchard: ISIJ international, 2000, vol. 40, pp. 647-653.
L. Yang: ISIJ international, 2005, vol. 45, pp. 469-476.
F. Zhang, D. Zhu and J. Pan: 8th International Symposium on High-Temperature Metallurgical Processing, 2017, pp. 615–625.
Z. Liu, J. Gao, R. Gao, H. Wen, J. Wang: Iron and Steel, 2005, vol. 2, pp. 19-23.
H. Hu: Metallurgy of Sichuan, 2004, vol. 5, 39-42.
Y. Pei, Z. Zhao and Z. Ma: Sintering and Pelletizing, 2011. Vol. 5, pp. 2-9.
T. Shen, W. Chen: Mining and Metallurgy, 2010, vol. 4, pp. 75-79.
C. E. Loo, L. T. Matthews, and J. Ostwald: Transactions of the Institution of Mining and Metallurgy, Section C, 1992, vol. 101, pp. 129-138..
J. Wang: Metallurgical Engineering, 2016, vol. 2, pp. 79-86.
H. Li, D. Pinson, P. Zulli, L. Lu, R. Longbottom, S. Chew, B. Monaghan, G. Zhang: Metallurgical and Materials Transactions B, 2020, 24: 1-15.
H. Li, D. Pinson, P. Zulli, L. Lu, R. Longbottom, S. Chew, B. Monaghan, G. Zhang: Metallurgical and Materials Transactions B, 2018, vol. 49, pp. 2285-2297.
H. Li, D. Pinson, P. Zulli, L. Lu, R. Longbottom, S. Chew, B. Monaghan, G. Zhang: Journal of Hazardous Materials, 2019, vol. 385(121592), pp. 1-13.
C. E. Loo: ISIJ International, 2005, vol. 45, pp. 436-448.
L. Lu: Iron Ore: Mineralogy, Processing and Environmental Sustainability, Elsevier, 2015, pp. 45-84.
E. W. Washburn: Physical Review, 1921, vol. 17, pp. 273-283.
R. M. German, Liquid Phase Sintering, Springer Science & Business Media, Berlin, Germany, 2013, pp. 13-41.
J. Carpenter, C. Bai, J. Escobedo-Diaz, J. Hwang, S. Ikhmayies, B. Li, J. Li, S. Monteiro, Z. Peng, M. Zhang, Characterization of Minerals, Metals, and Materials 2015, Springer, 2016, pp. 283-317.
V. D. Eisenhüttenleute and M. Allibert: Slag atlas, Verlag Stahleisen, Dusseldorf, Germany, 1995.
Acknowledgments
The authors gratefully acknowledge funding from the Australian Research Council Research Hub for Australian Steel Manufacturing (Project Number IH130100017) and the support of BlueScope Steel Ltd. The SEM/EDS observations were carried out at the Electron Microscopy Center of the University of Wollongong.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted September 11, 2020; accepted December 31, 2020.
Rights and permissions
About this article
Cite this article
Li, H., Pinson, D.J., Zulli, P. et al. Interaction Between Mineral Phases in a Goethitic Iron Ore and Fluxing Materials During Sintering. Metall Mater Trans B 52, 996–1011 (2021). https://doi.org/10.1007/s11663-021-02072-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02072-2