Abstract
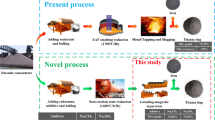
As the titanium industry rapidly develops, low-grade ilmenite resources are drawing global attention. The direct use of low-grade ilmenite can result in low production efficiency and heavy pollution. In addition, the production of high-titanium slag via electric furnace melting consumes significant energy and possesses low production efficiency. Therefore, a novel process with low energy consumption is necessary for producing ultra-grade slag (UGS) for chlorination. For low-grade ilmenite, semi-molten reduction and magnetic separation were suggested in this study. The effects of carbon content, reduction time, and Na2CO3 addition on the reduction and separation behavior were studied. The results showed that the addition of Na2CO3 favored the formation of a semi-molten state, which was more conducive for the diffusion, aggregation, and growth of the metal phase. In this regard, excess carbon was not helpful, and it weakened the growth of the metal phase. Wet grinding and magnetic separation were used for beneficiation of the reduced sample for efficiently separating the slag iron and preventing the formation of agglomerates between slag and metal. For the sample with a carbon dosage of 13 pct, Na2CO3 dosage of 8 pct, reduction temperature of 1673 K (1400 °C), and 90 minutes holding time, high-titanium slag with a TiO2 grade of 81.63 pct and iron content of 4.53 pct was produced, with the TiO2 recovery rate of 93.43 pct and the yields of 55.37 pct. High-titanium slag can be used as a high-quality raw material to produce UGS for chlorination by leaching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium resources are abundant in China; however, most Ti exists in middle- and low-grade ores with complex multi-metals such as V and Cr. The low-grade ore cannot be used directly due to environmental and economic concerns. In addition, high-grade titanium reserves are gradually being depleted with the rapid development of the titanium industry worldwide. The inefficiency in the utilization of low-grade titanium resources has attracted the attention of researchers.[1,2] Ilmenite contains considerable impurities that must be removed for producing titanium slag via electric furnace smelting. Titanium slag can be classified as ultra-grade slag (UGS) and high-grade slag (HGS). UGS is preferred for producing titanium dioxide via chlorination, while HGS, with high impurities, is mostly used in the sulfuric acid process. Both UGS and HGS are mainly produced using the electric furnace melting method, which accounts for more than 70 pct of the world’s titanium-rich raw material production.[3] The UGS is obtained when titanium slag produced using the electric furnace melting technique is leached. However, the production efficiency is low and more energy is required if low-grade ore is used during the electric furnace process. Therefore, it important to develop a novel and low energy consuming process for producing UGS from low-grade ilmenite for use in chlorination.
In the past decades, most studies on ilmenite focused on the reduction process. It was generally accepted that FeTiO3 is first reduced to Fe and TiO2 during the carbothermal reduction of ilmenite, and TiO2 is then reduced to a series of low-valence titanium oxides.[2,4,5,6,7,8,9,10,11,12] Studies on the enhanced reduction and separation of ilmenite have been conducted, including pre-oxidation treatment,[13,14,15,16,17] additive addition,[18,19,20,21,22,23] and mechanical activation,[24,25,26] to improve the reduction efficiency of ilmenite. A variety of additives were used in these studies, as shown in Table I. Additives (e.g., Na2B4O7, Na2CO3, Na2SO4, Fe-Si, FeS2) can improve the reduction efficiency of ilmenite and promote the growth of metal particles.
Therefore, based on previous studies, semi-molten reduction of ilmenite, followed by wet grinding and magnetic separation of slag from iron, was conducted to produce UGS with low-grade ores. This study aims to investigate the effect of Na2CO3 on the semi-molten reduction of ilmenite and the separation behavior of slag from iron. Finally, we developed a novel and low energy consuming process to produce UGS from low-grade ilmenite.
Experimental
Materials and Apparatus
The chemical composition and the X-ray diffraction pattern of ilmenite used in the experiment are shown in Table II and Figure 1, respectively, which indicate that the main phases in the ilmenite are FeTiO3 and Fe2O3. The particle size analysis of ilmenite is shown in Figure 2. Generally, the smaller the particle size of the reactants, the larger the specific surface area, which makes the reactants and reductants fully contact, thus promoting the reaction. However, when the particle size is too small, the permeability between reactants will decrease, which will affect the diffusion of the gas produced by the reaction and reduce the reduction effect. Therefore, the size of most of the particles falls within the range of 48–150 μm, which can not only guarantee the large enough specific surface area, but also not affect the reduction effect. The reducing agent used was graphite powder with ≥ 99.9 pct purity and < 13 μm particle size. The instrument used in the experiment is a high-temperature silicon molybdenum furnace with MoSi2 as the heater, as shown in Figure 3.
Experimental Procedure
Graphite powder was used as the reducer and sodium carbonate was used as the additive in the reduction experiment. The dosage of the reducer was set at 12, 13, and 14 wt pct and that of the Na2CO3 additives was set at 0, 2, 4, 6, 8, and 10 wt pct. According to previous studies, the temperature for the isothermal reduction was set at 1673 K (1400 °C), at which the semi-molten reduction of ilmenite can achieve better separation results.[20] All the materials were well mixed and then placed into a cylinder mold with a diameter of 16 mm to form tablets. The pressure of the cylinder mold was 8 MPa, and the weight of the individual sample was 15 g. The sample was dried at 378 K (105 °C) for 120 minutes, and then the alumina crucible containing the sample tablet was placed in an electric resistance furnace. The furnace was heated with flowing argon gas (0.6 L/min, purity: 99.99 pct). The reduced sample in the crucible was quickly removed from the furnace and it was rapidly cooled down after the sample was reduced according to the programmed temperature profile. The cooled sample cooled was beneficiated, the sample was then separated using the wet-magnetic separation technique, and the sample was analyzed using optical microscopy, backscattered electron scanning electron microscopy (BSE), energy-dispersive X-ray spectroscopy (EDS), and XRD.
Herein, the recovery rate of the titanium and the iron were defined as follows:
where \( m_{{{\text{TiO}}_{ 2} }} \) and \( m_{\text{Fe}} \) are the TiO2 and the iron content in high-titanium slag and iron concentrate after wet-magnetic separation, respectively; \( m_{{{\text{TiO}}_{ 2} }}^{0} \)and \( m_{\text{Fe}}^{0} \) are the TiO2 and the iron content in sample before wet-magnetic separation.
The high-titanium slag and the iron concentrate were obtained after wet-magnetic separation, so the yield of high-titanium slag and iron concentrate were defined as:
where \( \alpha_{{{\text{TiO}}_{2} }} \) and \( \alpha_{\text{Fe}} \) are the grade of titanium and iron in raw ore, respectively;\( \beta_{{{\text{TiO}}_{ 2} }} \) and \( \theta_{\text{Fe}} \) are the grade of titanium and iron in the high-titanium slag, respectively; \( \beta_{\text{Fe}} \) and \( \theta_{{{\text{TiO}}_{ 2} }} \) are the grade of iron and titanium in the iron concentrate, respectively.
The content of theoretical sodium oxide was defined as:
where \( m_{{{\text{Na}}_{ 2} {\text{CO}}_{ 3} }} \)is the quality of Na2CO3 in sample before reduction; \( m_{{\left( {\text{ilmenite}} \right)}} \) is the quality of sample after reduction; \( {\text{Mr}}_{{{\text{Na}}_{ 2} {\text{O}}}} \) and \( {\text{Mr}}_{{{\text{Na}}_{ 2} {\text{CO}}_{ 3} }} \) are the relative molecular weight of Na2O and Na2CO3, respectively.
Results
Thermodynamic Calculation
The changes in the content of the solid metal, solid slag, liquid metal, and liquid slag of the reduced product with their parameters were calculated using FactSage software, as shown in Figure 4. As shown in Figure 4(a), when the carbon content was 12 pct and 6 pct Na2CO3 was added, the content of the liquid slag phase and liquid metal phase increased with an increase in temperature. The sample almost completely melted when the temperature rose to 1773 K (1500 °C).
As shown in Figure 4(b), on the addition of 6 pct Na2CO3, the content of the liquid slag phase gradually decreased with an increase in the carbon content at a reaction temperature of 1673 K (1400 °C). This may be attributed to the iron in the liquid slag phase that was gradually reduced to solid metal iron as the carbon content increased, but the amount of the liquid slag phase was reduced to a stable value at 12 pct carbon content. This is because the reduction of iron in the liquid slag phase approached equilibrium. In addition, the titanium suboxide has a high melting point,[2,27] so more titanium oxide was reduced to titanium suboxide as the carbon content increased, resulting in a decrease in the amount of liquid slag.
Figure 4(c) shows that at a reaction temperature of 1673 K (1400 °C) and a 12 pct carbon content, the liquid slag content increased with the addition of Na2CO3, and the slag phase was almost completely converted to liquid with the addition of 8 pct Na2CO3,which indicates that the addition of Na2CO3 was beneficial to the formation of liquid slag.
Growth of Metal Phase
The optical images of a part of the reduced sample are shown in Figure 5. The upper right corner of the image shows the image of the reduced sample and the crucible. The image of the reduced specimen and the crucible when the Na2CO3 added was above 6 pct is shown in Figure 6. As shown in Figure 5, the metallic iron particle gradually increased with the increase in the amount of Na2CO3 and an increase in the holding time. Addition of Na2CO3 results in the formation of a semi-molten slag phase, which accelerates the rate of mass transfer, and therefore was beneficial for the aggregation and growth of the metal iron phase. As shown in Figure 6, when the amount of Na2CO3 added was more than 6 pct, the reduced sample showed a semi-molten state which became more obvious as the amount of Na2CO3 added increased, and the growth of the metal iron phase was more visible, as metal iron particles were easily observed.
Figure 5 shows that the proper addition of carbon could also promote the growth of the metal iron phase because the carburizing of metal iron reduced the melting temperature of the metal iron phase, thus promoting the accumulation and growth of the metal iron phase. However, when the carbon content exceeded 13 pct, the increase in carbon content did not favor the aggregation and growth of the metal iron phase. This is due to the high-valence titanium oxide was more easily reduced to the low-valence titanium oxide with a higher melting point by excessive carbon,[23,24] rather than formed sodium titanates with a low melting point, which led to the decrease of the slag phase with molten state. As shown in Figure 6, the semi-molten state of the reduced sample was not obvious when the carbon content 14 pct. It can be seen from Figures 5 and 6 that erosion of the crucible by the reduced sample occurred when Na2CO3 was added, and the erosion increased in severity as more Na2CO3 was added. This also shows that the addition of Na2CO3 would lead to the transformation of parts of the reduced sample molten state,[14] and this molten phase increases with the increase in the amount of Na2CO3 added.
X-ray Diffraction
The reduced samples were ground into titanium slag and the visible metal iron particles were sieved out. The metal iron was then separated from titanium slag using a wet-magnetic process. X-ray diffraction analysis of the titanium slag was conducted before and after wet-magnetic separation was carried out, and the results are shown in Figure 7. The main phases in the product were Fe, Ti3O5, SiO2, Na0.23TiO2, Na2Fe2Ti6O16, and M3O5 (M: Fe, Mg, Ti, etc.). It can be seen that the change in the carbon content and the addition of Na2CO3 had little effect on the phase transition of the slag before and after the wet-magnetic separation. In addition, the peaks attributed to metal iron gradually weakened with the increase in the amount of Na2CO3 before wet-magnetic separation. This is due to the sieving and removal of the visible metal iron particles in the sample that resulted in a decrease of the metal iron in the sample. Furthermore, it also indicated that the size of the metal iron particles increased with the increase of Na2CO3 to ease the sieving process.
Na0.23TiO2 and Na2Fe2Ti6O16 were observed on the diffract gram after the addition of Na2CO3. Sodium carbonate decomposes into sodium oxide and carbon dioxide when heated, and this promotes the gasification of carbon and then accelerates the reduction reaction.[13,18] Furthermore, the alkali metal can cause lattice distortion of iron oxide, which promotes lattice transformation and easily produces porous iron, and this accelerates the diffusion in the produced layer. The peak of the metal iron phase was weaker after wet-magnetic separation, and the peak was not observed in the case with 13 pct carbon content and 8 pct Na2CO3, which indicates that the wet-magnetic separation had a good impact. In addition, sodium-iron-titanates (Na2Fe2Ti6O16) and solid solution (M3O5 (M: Fe, Mg, Ti, etc.)) were observed in the titanium slag with the addition of Na2CO3, so the rest of the iron in the titanium slag existed in the form of sodium–iron–titanates[18] and solid solution.
Analysis of Elemental Content and Recovery Rate
The content of titanium and iron in the high-titanium slag and iron concentrate after wet-magnetic separation were chemically analyzed. The content and recovery rate of titanium and iron in the high-titanium slag and iron concentrate as well as the yields of high-titanium slag and iron concentrate are shown in Figure 8. At the same time, the theoretical and actual sodium contents in high-titanium slag were also analyzed, as shown in Figure 9.
It can be seen from Figure 8 that in the high-titanium slag, the TiO2 content and the recovery rate of TiO2 increased when the carbon contents were different and sodium carbonate content increased. The total iron content and the yield decreased with an increase in the sodium carbonate content. The total iron content, the recovery rate of iron, and the yield increased when the carbon contents were different with an increase in sodium carbonate, and the TiO2 content decreased with an increase in sodium carbonate. However, the recovery rate of TiO2 in the high-titanium slag and the total iron content in the iron concentrate decreased when the Na2CO3 content was higher than 8 pct and the carbon content was 12 and 13 pct, respectively. This is because large particles of iron were formed when the Na2CO3 content was over 8 pct, as shown in Figure 6, and the surface of the large particle of iron adhered to some titanium slag and it could not be separated; thus, the recovery rate of TiO2 in the high-titanium slag and the total iron content in the iron concentrate decreased. Figure 9 shows that the Na2O content increased in the high-titanium slag for different carbon contents with an increase of sodium carbonate. Compared with 12 and 14 pct carbon content, Na2O concentration is the lowest at 13 pct carbon content. Furthermore, some molten Na2O reacted with the alumina crucible to form NaAlO2, eroding the crucible. Some Na2O was hydrolyzed to form sodium salts,[28] which were removed during the process of water-cooling, so the actual sodium oxide content was lower than the theoretical value.
As shown in Figure 6, the excess carbon lowered the effect of sodium carbonate when the amount of carbon was 14 pct, and the aggregation and growth effect of metal iron was poor, which led to the poor separation effect of slag iron. Therefore, the recovery rate and concentration of TiO2 in high-titanium slag and the recovery rate and content of iron in the iron concentrate decreased. Finally, when the carbon content was 13 pct and the addition of 8 pct Na2CO3, high-titanium slag with a TiO2 grade of 81.63 pct, TiO2 recovery rate of 93.43 pct, and total iron content of 4.53 pct were obtained. Iron concentrate with a total iron grade of 95.89 pct, iron recovery rate of 94.73 pct, and TiO2 content of 1.22 pct were also obtained, and the yields of high-titanium slag and iron concentrate were 55.37 and 37.43 pct, respectively. High-titanium slag can be used as a high-quality raw material to produce UGS for the chlorination process.
The traditional electric furnace smelting process is always maintained at 1873 K to 2073 K (1600 °C to 1800 °C) for approximately 8 to 10 hours, and it contains 10 to 12 pct FeO in the slag to obtain high fluidity of the slag and ensure the separation of liquid iron and liquid slag. So the titanium slag with a TiO2 grade of 72 to 74 pct was obtained. Therefore, this semi-molten reduction process improves the smelting conditions (1673 K (1400 °C), 1.5 hours) to obtain high-grade titanium slag (81 to 82 pct TiO2) with low FeO content (5.8 to 6.1 pct FeO). However, the very fine particle size of titanium slag is a challenge when using this process. Further study will be required for the wet grinding process.
Discussion
Analysis of Reduction Mechanism
The reduction mechanism of the semi-molten reduction process is shown in Figure 10. During the reduction process, the reduction of iron oxide first occurred, and it resulted in a partial distribution of metal iron in the samples. Then, the Na2CO3 decomposed to Na2O and CO2, and the Na2O reacted with the titanium oxide to form sodium titanates with a low melting point. The molten phase was distributed in the sample during the reduction process, which led to the transformation of the reduced sample to a molten state. As shown in Figure 6. The molten phase was beneficial to the diffusion-migration of particles[29] and it increased the probability of contact between reactants, thus promoting the reaction. In addition, the metal iron produced by reduction diffused and migrated in the molten phase, and the metal iron was avoided to form a dense metal shell wrapped around the reactants, which affected the reduction process.
Generally, the diffusion-migration of the metal phase is more likely to occur in the molten phase than in the solid phase.[29,30] During the reduction process, the molten phase wrapped the core of the reactants as reduction proceeded. The molten phase formed the diffusion-migration channel in the product layer, which was beneficial to the diffusion of the reducing agent on the surface of the reactant’s core. In addition, the reduced metal iron diffused in the molten phase and combined with other metal iron to agglomerate in the subsequent process. This process continued repeatedly, and eventually, the metal iron grew to visible metal iron particles, which enabled the subsequent separation of slag iron. In addition, as shown in Figure 6, the effect of semi-molten slag on the sample was more obvious when the amount of Na2CO3 was increased, thus promoting the growth of metal iron. However, when the carbon content was 14 pct, the excess carbon also migrated into the molten phase, which resulted in the solidification of the molten phase, thus hindering the diffusion-migration of particles and further affecting the growth of metal iron.
Wet-magnetic Separation Analysis of Slag Iron
Presently, there are many studies on the mineral processing of low-grade ilmenite,[25,26] but there are few reports on the separation of slag and iron after the reduction of ilmenite. In the previous magnetic separation process, the reasons for the poor magnetic separation effect are shown in Figure 11. As shown in Figure 11(a), the particle size of metal iron was much smaller than that of the reduced sample obtained by magnetic separation. This is because some of the metal iron was not fully grown, which resulted in the partial separation of the slag iron; the partial titanium slag was magnetically separated by wrapping onto the metal iron, resulting in a poor magnetic separation effect. Additionally, as shown in Figure 11(b), the reduced sample was ground too finely before magnetic separation, although the slag iron was fully separated, the metal iron particles would be subjected to magnetic agglomeration during separation. This is because the particles were so fine that the slag and iron particles were similar in size, and the agglomeration process was wrapped in the titanium slag and formed an agglomeration state. In strong and weak magnetic environments, agglomerates that wrapped the titanium slag were formed. The agglomerates did not disintegrate even after stirring the slag.
During beneficiation, it was observed that the effect of the combined method was better than that of the single method, these combine methods include magnetic separation–flotation, heavy separation–flotation, magnetic separation–heavy separation, and heavy separation–magnetic separation–flotation–electric separation. Therefore, based on a previous study combined with experimental trials, a new type of wet-magnetic separation process was adopted, as shown in Figure 12. The reduced samples were ground in water, then the particle size of titanium slag reduced with the increase of grinding intensity. This is because of the relatively soft nature of titanium slag; therefore, the metal iron wrapped in titanium slag could be separated. The metal iron settled at the bottom of the container because of its relatively higher hardness and density. While the titanium slag with smaller particles was suspended in water to form a deep black suspension, then magnetic separation was performed. At this time, the metal iron and the titanium slag-containing iron were selected. The water was replaced, the sample was demagnetized, and re-ground for a cycle process like a → b → c → a. The high-titanium slag was obtained by filtration of the replaced suspension, and the filtrate could be reused. A cycle process like a → b → d → e → c → a was conducted until the slag iron was completely separated, and the iron concentrate was left in the container. This method enabled the full separation of the slag iron and also prevented the formation of agglomerates that may cover the surface of the titanium slag, and this enabled the complete separation of the slag iron.
Morphology Change Analysis of Titanium Slag
The morphology of the titanium slag before and after the wet-magnetic separation process is shown in Figure 13. The particle size of the titanium slag after wet-magnetic separation was smaller than that before wet-magnetic separation, which is due to further decrease in particle size caused by grinding in water during wet-magnetic separation. Metal iron particles were observed such as the areas marked by a in the micrograph of titanium slag before separation, but they were not observed after separation. This indicates a good separability of slag and iron, and metal iron could be removed from the titanium slag. There was still a small amount of iron in the titanium slag, this is because the slag would inevitably form a small amount of M3O5 (M: Fe, Mg, Ti, etc.) solid solution represented by b, c, d, and f, and sodium-iron-titanates (Na2Fe2Ti6O16) represented by c and d, which was also the main reason for the grade of titanium slag obtained. It can be seen from the regions marked as c, d, and e that Na, Ti, and O coexisted in some regions in the reduced sample, which indicates that there was a small amount of Na0.23TiO2 in the sample.
Conclusion
Theoretical calculations and experiments were conducted on the carbothermic reduction of ilmenite and separation of iron slag in a semi-molten state by adding sodium carbonate. The following conclusions were drawn from our study:
-
(1)
An increase in the Na2CO3 content and extension of holding time favored the growth of the metal phase. The addition of Na2CO3 favors the formation of a semi-molten state, which is more conducive to the diffusion, aggregation, and growth of the metal phase. The excess carbon-reduced titanium oxide was transformed into titanium suboxide and it migrated in the molten phase where it blocked the molten phase by filling more solid particles, which did not favor the formation of a semi-molten state, and it weakened the growth of the metal phase.
-
(2)
The addition of Na2CO3 favored the formation of a semi-molten state, which was more conducive for the diffusion, aggregation, and growth of the metal phase. Wet grinding and magnetic separation were used for beneficiation of the reduced sample for efficiently separating the slag iron and preventing the formation of agglomerates between slag and metal.
-
(3)
When carbon content of 13 pct and Na2CO3 dosage of 8 pct at reduction temperature of 1673 K (1400 °C) for holding time of 90 minutes, when the carbon content was 13 pct and the addition of 8 pct Na2CO3, high-titanium slag with a TiO2 grade of 81.63 pct, TiO2 recovery rate of 93.43 pct, and total iron content of 4.53 pct were obtained. Iron concentrate with a total iron grade of 95.89 pct, iron recovery rate of 94.73 pct, and TiO2 content of 1.22 pct were also obtained, and the yields of high-titanium slag and iron concentrate were 55.37 pct and 37.43 pct, respectively. High-titanium slag can be used as a high-quality raw material to produce UGS for the chlorination process.
References
C. S. Kucukkaragoz, and R. H. Eric: Miner. Eng., 2006, vol. 19, pp. 334-37.
R. Huang, X. W. Lv, C. G. Bai, Q. Y. Deng, and S. W. Ma: Can. Metall. Q., 2012, vol. 51, pp. 434-39.
M. Gueguin, and F. Cardarelli: Miner. Process. Extr. Metall. Rev., 2007, vol. 28, pp. 1-58.
B. Song, X. Lv, J. Xu, H. Miao, and K. Han: Int. J. Miner. Process., 2015, vol. 142, pp. 101-06.
A. A. Francis, and A. A. El-Midany: J. Mater. Process. Technol., 2008, vol. 199, pp. 279-86.
S. A. Rezan, G. Zhang, and O. Ostrovski: ISIJ Int., 2012, vol. 52, pp. 363-68.
G. Q. Zhang, and O. Ostrovski: Can. Metall. Q., 2001, vol. 40, pp. 489-97.
G. Q. Zhang, and O. Ostrovski: Can. Metall. Q., 2001, vol. 40, pp. 317-26.
H. P. Gou, G.-H. Zhang, X. Yuan, and K.-C. Chou: ISIJ Int., 2016, vol. 56, pp. 744-51.
M. A. R. Dewan, G. Zhang, and O. Ostrovski: ISIJ Int., 2010, vol. 50, pp. 647-53.
N. J. Welham, and J. S. Williams: Metall. Mater. Trans. B, 1999, vol. 30, pp. 1075-81.
M. I. Elguindy, and W. G. Davenport: Metall. Mater. Trans. B, 1970, vol. 1, pp. 1729-34.
D. S. Chen, B. Song, L. N. Wang, T. Qi, Y. P. Wang, and W. J. Wang: Miner. Eng., 2011, vol. 24, pp. 864-69.
W. Lv, X. Lv, J. Xiang, Y. Zhang, S. Li, C. Bai, B. Song, and K. Han: Int. J. Miner. Process., 2017, vol. 167, pp. 68-78.
P. L. Vijay, R. Venugopalan, and D. Sathiyamoorthy: Metall. Mater. Trans. B, 1996, vol. 27, pp. 731-38.
G. Q. Zhang, and O. Ostrovski: Int. J. Miner. Process., 2002, vol. 64, pp. 201-18.
H. P. Gou, G.-H. Zhang, and K.-C. Chou: ISIJ Int., 2015, vol. 55, pp. 928-33.
S. Z. ElTawil, I. M. Morsi, A. Yehia, and A. A. Francis: Can. Metall. Q., 1996, vol. 35, pp. 31-37.
C. Geng, T. Sun, H. Yang, Y. Ma, E. Gao, and C. Xu: ISIJ Int., 2015, vol. 55, pp. 2543-49.
W. Lv, C. Bai, X. Lv, K. Hu, X. Lv, J. Xiang, and B. Song: Powder Technol., 2018, vol. 340, pp. 354-61.
B. Song, X. Lv, H. H. Miao, K. Han, K. Zhang, and R. Huang: ISIJ Int., 2016, vol. 56, pp. 2140-46.
R. Huang, X. Lv, C. Bai, K. Zhang, and G. Qiu: Steel. Res. Int., 2013, vol. 84, pp. 892-99.
C. Li, and R. R. Merritt: Aust. J. Chem., 1990, vol. 43, pp. 1-9.
E. V. Bogatyreva, A. V. Chub, and A. G. Ermilov: J. Min. Sci., 2014, vol. 50, pp. 385-98.
C. Li, B. Liang, L.-h. Guo, and Z.-b. Wu: Miner. Eng., 2006, vol. 19, pp. 1430-38.
L. Zhang, H. Hu, L. Wei, Q. Chen, and J. Tan: Metall. Mater. Trans. B, 2010, vol. 41, pp. 1158-65.
M. Pourabdoli, S. Raygan, H. Abdizadeh, and K. Hanaei: Int. J. Miner. Process., 2006, vol. 78, pp. 175-81.
T. A. Lasheen: Hydrometallurgy, 2008, vol. 93, pp. 124-28.
J. Diao, X. Liu, T. Zhang, and B. Xie: Int. J. Min. Met. Mater., 2015, vol. 22, pp. 249-53.
H.G. Li: Metallurgical Principle, 1st ed., Science Press, Beijing, 2005, pp. 92-93.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Grant No. U1902217).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted August 3, 2020; accepted October 29, 2020.
Rights and permissions
About this article
Cite this article
Lv, X., Xin, Y., Lv, X. et al. High-Titanium Slag Preparation Process by Carbothermic Reduction of Ilmenite and Wet-Magnetic Separation. Metall Mater Trans B 52, 351–362 (2021). https://doi.org/10.1007/s11663-020-02027-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-02027-z