Abstract
Vacuum metallothermic reduction is the main method to produce magnesium (Mg) metal from Mg-containing ores. In the process, a mixture of ore and reductant material is charged in retorts, which provide a vacuum atmosphere of about 100 Pa (1 mbar) in the process. The accounts in the literature stated that the process is viable for the metallothermic reduction of strontium (Sr) as well. The main problem for the reduction of the Sr is the high affinity of Sr to oxygen. Moreover, the Sr is an important metal for Mg alloying. In the present study, production of Mg-Sr alloy from oxide raw materials was studied through the vacuum metallothermic process. Thus, it was aimed to develop a simple and commercially viable method to directly produce Mg-Sr alloys, and the Sr content would be in the form of Mg17Sr2 intermetallic alloy. In the experiments, calcined dolomite ore and SrO were used as raw materials. Investigated parameters were reductant type (FeSi and Al), process temperature, and process time on the recovery ratios of Mg and Sr in produced alloys. The highest recovery ratios, 97.1 pct for the Mg and 81.2 pct for the Sr, were obtained in the experiment conducted at 1250 °C for 480 minutes. The reductant material was the Al, and the Sr-Al addition ratio was 5 wt pct in the experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnesium (Mg) is a silvery white metal; it has two valance electrons, and its configuration is 1s2, 2s2, 2p6, 3s2. The residual 3s2 valance electron structure removes the Mg from the covalent bond structure, resulting in a structural material with the lowest average valence bond energy and with the lowest interatomic bond. The crystal structure of Mg is hexagonal-closed-packed (hcp).[1,2,3,4,5,6,7,8] Strontium (Sr) is one of the alkaline earth metals, and it belongs to group II of the periodic table with its atomic number 38. Strontium has allotropic transformation and it has three crystal structures: face-centered-cubic (up to 215 °C), hcp (from 215 °C to 605 °C), and body-centered-cubic (greater than 605 °C).[9,10] With respect to Hume–Rothery rules, it is possible to make Mg-Sr alloy. Because their ionic balance is (Mg−2, Sr+2), the difference between their atomic diameters is 3.2 pct, which is lower than the limitation of 15 pct.[11]
The world’s demand for Mg increases about 10 pct annually because it has the lowest density (1.738 g/cm3) among all structural metals. However, the mechanical properties and processing performances of Mg alloys (such as AZ series alloys) are not still enough for some important parts in transportation and in aviation industries in which high strength is critical.[12] In order to improve high-strength Mg alloys, a lot of processes have been investigated in the world. Grain refinement is an alternative method to enhance the strength of metals.[13] The Sr is a grain refiner for particularly Al-Si alloys, and it is widely used in commercial scale for that purpose. Also, it is stated in the literature that it has a remarkable grain refinement effect for Mg alloys as well.[14,15] Apart from its grain refinement effect, Sr as an alloying element can decrease the ratio of microporosity and remarkably enhance the heat resistance and the creep properties of Mg alloys.[16,17] The Sr is added to Al or Mg alloys in the form of Sr-containing master alloy (such as containing 5, 10, or 15 pct Sr) because it prevents the burning loss of pure Sr metal addition.[15,18] Direct reaction, metallothermic reduction, vacuum thermal reduction, and electrolysis methods are main methods to produce Mg-Sr master alloys.[13]
The main primary production method of Mg metal is the Pidgeon process. The process is carried out to produce Mg metal from calcined dolomite (MgCaO2, MgO·CaO) ores under vacuum atmosphere of approximately 100 Pa (1 mbar). FeSi (ferrosilicon) is used as reductant material in the conventional process. Moreover, it is reported that Al can be used as reductant material as well. To provide vacuum atmosphere, the reactant mixture is charged in steel airtight retorts. The main reactions of the process are given with Eq. [1] for FeSi reductant and with Eq. [2] for Al reductant. The process is conducted for nearly 6 hours and at temperatures between 1200 °C and 1250 °C. After the reaction, retorts are cooled to room temperature and the produced Mg metal (in the form of crown) and remaining slag phase are removed from them.[19,20,21,22,23] Moreover, it was reported that Sr metal can be produced through the metallothermic process from Sr oxide-containing raw materials (Eq. [3]).[24]
Nayeb-Hashemi and Clark investigated the Mg-Sr system and plotted the phase diagram. Mg17Sr2, Mg38Sr9, Mg23Sr6, and Mg2Sr phases occur up to 66 wt pct Sr content, respectively, and after that point, Sr[Mg] solid solution starts to form.[25] Zeng et al. investigated the effect of Sr addition on the mechanical properties of Mg alloys. The highest yield strength was obtained at the Sr addition of 0.01 pct. It shows that a slight amount of Sr addition is enough to enhance the mechanical properties of Mg alloys.[26] The Mg17Sr2 was the phase with the lowest Sr content observed in Nayeb et al.’s study. Therefore, in the present study, the production of a Mg-Sr alloy containing Mg17Sr2 intermetallic compound was sought through a simple and combined method (vacuum metallothermic process). The alloy would provide optimum mechanical properties with minimum Sr content.
Materials and methods
Before the experiments, some thermochemical modeling studies were carried out through the “Equilibrium” module of the FactSage 6.4 thermodynamic simulation software to predict possible reaction products and experimental parameters. The Equilibrium module uses the Gibbs energy minimization method for the calculations.[27]
In experimental studies, calcined dolomite samples (− 74 µm), which were obtained from domestic sources, were characterized by use of an atomic absorption spectrometer (AAS, Perkin Elmer Analyst 800) and chemical analysis methods (Table I). The SrO used had a purity of 99.50 pct (Alfa Aesar, − 149 µm). FeSi (ferrosilicon, − 150 µm) and Al (− 150 µm) were used as reductant materials in the experiments, and their chemical analysis results, which were measured by using AAS and chemical analysis techniques, are shown in Table II.
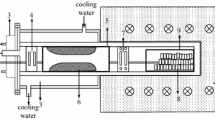
In the experiments, calcined dolomite, SrO, Al, and FeSi were weighed in stoichiometric proportions and the raw materials were mixed by using a turbula mixer for 30 minutes. Reduction experiments were mainly carried out by means of a lab scale furnace (in 1 L retort), but by using optimum reduction conditions, an experiment was duplicated in a semipilot scale (in 10 L retort). Both retorts were airtight and were made of 304 grade stainless steel (Figures 1(a) and (b)). For 1 L retort experiments, a homogenized mixture was put in the retort on alumina boats after briquetting, while mixtures were directly put in a 10 L retort without boats in the semipilot scale. All experiments were conducted under air atmosphere of 100 to 200 Pa. In order to condense the Mg and Sr vapors formed during reduction, a water-cooling system was used, which was located in the lids of both retorts. Both retorts were heated using an electrical resistance tube furnace. At the end of the experiments, the retorts were cooled to room temperature under vacuum to prevent burning of produced alloys at high temperatures with oxygen in the air. The reaction pressure in retorts was reduced by means of an ILMVAC-PK8D two-stage integrated rotary vane pump. The ILMVAC PIA 100 piezoelectric sensor was used as an integrated system to measure the vacuum values. In the experiments, the temperature measurement was performed using the 6RhPt-30RhPt (EL-18) thermocouple.
Schematic sketch of the (a) lab scale and (b) semipilot scale systems: (1) furnace, (2) stainless steel retort, (3) raw materials, (4) metal vapor condensation unit, (5) cooling water, (6) vacuum connector, (7) vacuum pump, (8) vacuum data logger, and (9) resistance (horizontal for lab scale and vertical for semipilot scale)
Reduction conditions of Mg-Sr alloys were investigated in the experiments to obtain the Mg17Sr2 intermetallic compound. Investigated parameters were reaction temperature (from 1100 °C to 1250 °C), reductant type (FeSi + Al and only Al), and duration (240 to 480 minutes).
In the first experimental series, 100 pct stoichiometric mixtures of calcined dolomite + FeSi and SrO + Al were prepared and the SrO + Al mixture was added to the calcined dolomite + FeSi mixture with increasing ratio from 2.5 to 10 pct by mass. Final mixtures were shaped in the form of pellets and were put in a 1 L retort with a total amount of 20 g. The effects of increasing temperature and SrO + Al addition ratios were investigated for 240 minutes.
In the second experimental series, only the Al was used as the reductant material for both calcined dolomite and SrO. Similar to the first experimental series, increasing the SrO + Al ratio and temperature were tried for a constant duration of 240 minutes.
Increasing the reaction duration from 240 to 480 minutes was the parameter that was investigated in the last experimental series of lab scale reduction experiments. In this series, the reductants used were only Al and FeSi + Al. Experiments were carried out at 1250 °C and for a SrO-Al addition ratio of 5 pct. These parameters were previously determined as optimum.
After lab scale experiments, to determine the efficiency of the developed process in laboratory scale, a semipilot scale experiment was conducted in 10 L retort. The constant experimental parameters were temperature of 1250 °C, duration of 240 minutes, and SrO-Al mixture addition ratio of 5 pct to the calcined dolomite-FeSi mixture. The total weight of reactants was 2 kg, and the experiment was conducted under a vacuum pressure of 100 to 200 Pa.
The recovery ratios of the Mg and Sr metals were calculated from reduction slag (residue) for 1 L retort experiments and from collected metal in the condensation zone (in the form of crown) for 10 L semipilot scale experiments. Related recovery ratios were calculated with Eqs. [4] and [5]:
where W0 refers to the weight of the reactant mixture, Me0 pct is the weight percent of Mg or Sr in the reactant mixture, W1 is the reduction slag weight, Me1 pct is the weight percentage of Mg or Sr in the reduction slag, W2 is the weight of the crown Mg-Sr alloy, and Me2 pct is the weight percent of Mg or Sr in crown.
AAS, chemical analysis, and X-ray diffraction (XRD spectrometry, PANalytical PW3040/60) were used for the characterization of obtained reduction slag and metal phases. Micrographs of produced alloy, in semipilot scale, were taken using optical microscopy (Olympus GX71) and scanning electron microscopy–energy dispersive spectroscopy (SEM-EDS, JEOLFootnote 1 JCM-6000Plus NeoScope operated at 15 kV) techniques.
Results and Discussion
Thermochemical Modeling
The first thermochemical simulation was plotted for the case in which FeSi was used as reductant for calcined dolomite and Al reductant was used for SrO (Figure 2). In Figure 2, the change of reaction products is given with the increase in reaction temperature under 100 Pa atmospheric pressure. Sr and Mg reduction starts at 750 °C. Mg reduction reaches equilibrium at a temperature slightly lower than 800 °C, while Sr reduction reaches equilibrium after 1150 °C. Reduced Mg and Sr phases are in the gas phase at the temperatures and atmospheric conditions at which they are reduced. The relationship between the vapor pressures of Mg and Sr and the process temperature is given in Supplementary Figure S-1 (refer to the Electronic Supplementary Material) (Fact Sage 6.4, reaction module).
The second thermochemical simulation was done for the case in which Al was used as reductant material for both calcined dolomite and SrO under 100 Pa atmospheric pressure with the increase in temperature (Figure 3). Both Mg and Sr start to be reduced at 750 °C. The Mg reduction reaches equilibrium at 920 °C, whereas the ratio of the Sr increases with increasing proportion up to 1250 °C. Also, a slight amount of reduced Ca is predicted to exist in reaction products after 850 °C. All metals are in the gas phase at temperatures they reduced.
Vacuum Metallothermic Reduction Experiments
In the first series of experiments, reduction behaviors of cocharged Mg and Sr in different mixtures were investigated for increasing temperature and for a SrO-Al addition ratio up to 10 pct. In these experiments, the required amount of FeSi in a stoichiometric ratio was used to reduce the Mg in calcined dolomite and the stoichiometric amount of aluminum was used to reduce Sr. The effects of temperature and the SrO-Al addition ratio on the Mg, Sr recovery are shown in Figures 4 and 5, respectively. The highest yields for both metals were obtained at 1250 °C. The recovery yield of 78.2 pct for Mg was obtained in the experiment at 1250 °C with the addition of 2.5 pct SrO-Al. The Mg recovery ratio was 78 pct by the addition of 10 pct SrO-Al to the mixture at the same temperature. The Sr recovery yield increased from 34.5 to 63.5 pct in the experiment conducted at 1250 °C. A clear effect on the recovery ratios of Mg and Sr was not determined for the increasing SrO-Al ratio. The amounts of MgO and SrO in reduction slag are given in Supplementary Figures S-1, S-2, and Table S-I. According to Supplementary Figure S-2, the lowest amount of MgO (12.8 pct) in reduction slag was obtained in the experiment at 1250 °C with 2.5 pct SrO-Al addition ratio. The amount of SrO was 1.3 pct for the same reduction slag (Supplementary Figure S-3).
XRD investigations of selected reduction slags are illustrated in Figure 6. Slags are from experiments conducted with 5 pct SrO-Al addition ratio. The main phases in the patterns are MgO, SrO·Al2O3, SiO2, FeSi, Fe3O4, and CaSiO4. In the pattern at 1250 °C, the intensity of the FeSi peak decreases, while the intensity of SiO2 increases. This result supports the reduction effect of FeSi being similar to chemical analysis results.
In the second series of experiments, Al was used as reductant for both calcined dolomite and SrO. The Mg recovery ratios are given in Figure 7, whereas the Sr recovery ratios are shown in Figure 8. In the experiments, the highest Mg yield (89.8 pct) was obtained at 1250 °C with 2.5 pct SrO-Al, while the highest Sr yield was 78.6 pct for 7.5 pct SrO-Al addition at 1250 °C. The results of the chemical analysis of reduction slags, formed as a result of experiments, are given in Supplementary Figure S-4, S-5, and Table S-II. The lowest MgO and SrO contents were obtained at 1250 °C with the addition of 2.5 pct SrO-Al by weight. In this experiment, the amount of MgO was measured as 5.3 pct and that of SrO was determined as 1.9 pct.
The XRD patterns of reduction slags (for 5 pct SrO-Al addition) are shared in Figure 9. It can be clearly seen that the amount of Mg in slags decreases with the increase in temperature up to 1250 °C, and the amount of oxide formed of used reducing agents increases. In the same figure, it is shown that the amount of SrO·Al2O3 phase represented by S at 1100 °C decreases as the temperature rises. The amount of Ca2(AlO3)2 phase represented by C was determined to increase with increasing temperature. This phase stops SrOAl2O3 formation and makes Sr recovery higher.
In the last experimental series, the reaction duration was investigated up to 480 minutes both for FeSi and Al and for only Al reductants. Experiments were done in the lab scale system and at 1250 °C for a SrO-Al addition ratio of 5 pct. 5 pct SrO-Al addition mixture was preferred because the highest Mg recovery ratio was previously obtained at this addition ratio as 79.3 pct. The results of the experiments that were conducted with FeSi (for calcined dolomite) and Al (for SrO) reductants are given in Figure 10, Supplementary Figure S-6, and Table S-III. The Mg recovery ratio increased from 79.3 pct after 240 minutes to 84.5 pct after 360 minutes and to 85.8 pct after 480 minutes. The efficiency of Sr in the same conditions increased from 61.25 to 65.8 pct after 360 minutes and 66.30 pct after 480 minutes. The amount of MgO in reduction slag (Supplementary Figure S-6 and Table S-III) decreased from 14.4 pct after 240 minutes to 7.57 pct after 480 minutes. Under the same conditions, the SrO amount was 2.7 pct in reduction slag after a reaction duration of 480 minutes. In the second stage of the experiments that were process duration investigated, Al was used as the reductant material to reduce both Mg from calcined dolomite and Sr from SrO. Other experimental parameters were the same as in the previous reduction experiments, in which duration was investigated under process temperature of 1250 °C, SrO-Al addition ratio of 5 pct, and process atmospheric pressure of 100 to 200 Pa. The results are given in Figure 11, Supplementary Figure S-7, and Table S-III. As can be seen, the recovery ratio of the Mg increased from 89.8 pct after 240 minutes to 97.1 pct after 480 minutes. In the experiment that was conducted for 480 minutes, the Sr recovery ratio obtained was 81.2 pct. The Sr recovery ratio was 75.1 pct after 240 minutes. It is obvious to see that increasing the temperature positively affected the Sr recovery. The recovery ratios after 360 minutes were close to the values after 480 minutes, which shows that reactions reach equilibrium between 360 and 480 minutes. Furthermore, the recovery ratios, which were obtained after 480 minutes for both metals, were the highest values calculated during the experiments. The amount of MgO in reduction slag decreased to 3.6 pct after 480 minutes, and the SrO amount was 1.7 pct for the same process duration (Supplementary Figure S-7 and Table S-III).
After laboratory scale experiments of Mg-Sr alloy reduction, a semipilot scale experiment was conducted to determine the viability of the developed technique at industrial scale. It was not possible to produce any metal phases in the lab scale system because of the volume of the retort and inefficient condensation. However, it was easy to see that the reduction of the Mg and the Sr took place as a result of reduction slag analyses. But reduced metals were in the gas phase and could not condense in the condensation zone of the retort due to the volume of the retort. The semipilot scale experiment was done under acceptable conditions to reduce the alloy such as at 1250 °C, for 240 minutes, and under 100 to 200 Pa atmospheric pressure in 10 L retort. The reductant materials were FeSi for calcined dolomite and Al for SrO, and an additional ratio of 5 pct for the SrO-Al mixture was used. The total weight of the reactant mixture was 2 kg. After the process was completed under the previously given conditions, the retort was left to cool to room temperature for 24 hours under vacuum. The retort was not opened before fully cooling to prevent the burning of reduced alloy at high temperatures with the oxygen in the air. Cooled retort was opened, and the reduced alloy (crown shape in condensation zone) and reduction slag were taken out. The photograph of the obtained alloy is given in Figure 12.
There were some metallic powders with crown alloy crystals, and those metallic powders burned while they were being taken out. But the remaining alloy (crown), which was the major part of the product, was still in a metallic state. The obtained alloy phase was characterized by means of optical microscopy and SEM-EDS techniques after metallographic sample preparation operations. The alloy was polished using 800-, 1200-, 1800-, and 2500-grit polishing discs, respectively, and a following etching was applied using an ethanol-nitric acid (2 vol pct) mixture. The optical microscope micrograph of the alloy is given in Figure 13. Dendritic Mg-Sr structures were observed at magnification 500 times.
SEM micrograph and EDS results of the alloy are shown in Figure 14 with the XRD pattern of the alloy. EDS analysis showed that the alloy consists of 89.62 pct Mg and 1.76 pct Sr with a slight amount of Ca (0.32 pct) and 8.30 pct oxygen. It was thought that the oxygen was the result of partial burning of the alloy. From the EDS results, the Mg recovery ratio was 71.32 pct, while the Sr recovery ratio was 41.24 pct. A similar result was observed and reported in the study conducted by Yang et al. In that study, the Mg content of the alloy was 98.1 pct, whereas the Sr content was 1.88 pct.[13] The XRD pattern indicates the alloy consisted of mainly Mg with a slight amount of Mg17Sr2 phase. However, a slight amount of MgO was observed in the alloy too as a result of its high affinity to oxygen. The XRD pattern was consistent with the SEM-EDS results.
Conclusions
Experimental studies were conducted to investigate the reduction conditions of Mg-Sr alloy from calcined dolomite and SrO raw materials for increasing reaction temperature, duration, and SrO + Al addition ratio. FeSi and Al powders were used as reductant materials. The experiments were done by using FeSi + Al and only Al reductants to see the effects of different reductant types. Thermochemical modeling studies were carried out using a FactSage 6.4 thermodynamic database to determine the most suitable experimental conditions before the reduction experiments. As a result of the studies, the minimum reduction temperatures of metals were determined and possible products that would occur as a result of reactions were determined. The evaporation temperatures of Mg and Sr were determined by calculating the cooling conditions of the test system. It is a thermodynamical requirement to conduct reduction experiments under vacuum atmosphere. The experiments, therefore, were conducted in airtight retorts under a vacuum atmosphere of 100 to 200 Pa. The reduction experiments were carried out in a lab scale system (in 1 L retort) first, and reduction efficiencies were calculated through amounts of MgO and SrO remaining in the reduction slag. Calcined dolomite and SrO mixtures were used as the raw materials, FeSi and Al were used together as reducing agents, and the effects of reduction temperature changes on metal recovery ratios were investigated. In this experimental group, the highest metal reduction efficiencies were obtained by adding 5 pct SrO of calcined dolomite at 1250 °C for 480 minutes. These values were 85.8 pct for Mg and 66.3 pct for Sr. In the final stage of the experiments, in which the reduction conditions of Mg and Sr were investigated, the mixture was reduced with Al for both oxide raw materials. In this experimental series, the highest recovery values were determined as 97.1 pct for Mg and as 81.2 pct for Sr at 1250 °C for 480 minutes with 5 pct SrO-Al addition ratio. After lab scale experiments, a semipilot scale experiment was conducted to see the viability of the developed method at industrial scale. In a semipilot scale experiment, 10 L retort was used at a pressure of 100 to 200 Pa for 240 minutes and at 1250 °C. Metallic structures, which were used to examine the resulting product quality, were collected in the water-cooled zone of the retort in the form of crown. As a result of the experiment, it was determined that the alloy contains 89.62 pct Mg and 1.76 pct Sr in the EDS analysis, which was obtained from the crown Mg-Sr alloy. In the experiment, the Mg recovery ratio was calculated as 71.32 pct and the Sr recovery ratio was determined as 41.24 pct.
Notes
JEOL is a trademark of Japan Electron Optics Ltd., Tokyo.
References
B.A. Chubukov, A.W. Palumbo, S.C. Rowe, M.A. Wallace, K.Y. Sun, and A.W. Weimer: Metall. Mater. Trans. B, 2018, vol. 49B, pp. 2209–18.
W. Li, J. Zhou, B. Ma, J. Wang, J. Wu, and Y. Yang: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 3152–60.
K.H. Matucha, ed.: Structure and Properties of Nonferrous Alloys, vol. 8, Materials Science and Technology—A Comprehensive Treatment, R.W. Cahn, P. Haasen, and E.J. Kramer, eds., VCH Weinheim, New York, 1996, p. 786.
Z.Z. Wang, X.C. He, Y.Q. Bai, Z.X. Ba, Y.M. Dai, and H.Z. Zhou: Int. J. Miner. Metall. Mater., 2012, vol. 19 (3), pp. 225–30.
M.M. Avedesian and H. Baker: Magnesium and Magnesium Alloys, ASM International, Materials Park, OH, 1999, pp. 12–25.
W. Li, J. Zhou, B. Ma, J. Wang, J. Wu, and Y. Yang: Metall. Mater. Trans. B, 2017, vol. 48B, pp. 2564–73.
K. Amundsen, T.K. Aune, P. Bakke, H.R. Eklund, J.Ö. Haagensen, C. Nicolas, C. Rosenkilde, S. Van den Bremt, and O. Wallevik: ULLMANN’S Encyclopedia of Industrial Chemistry: Magnesium, B. Elvers, ed., Wiley-VCH Verlag, Weinheim, 2003, pp. 1–26.
K. Andreassen, T.K. Aune, T. Haugerod, N.O. Hoy-Petersen, D. Oyma, O. Skane, and T. Vralstad: Handbook of Extractive Metallurgy, vol. II, Magnesium, F. Habashi, ed., Wiley-VCH, Weinheim, 1997, p. 981.
B. Elvers, volume ed.: ULLMANN’S Encyclopedia of Industrial Chemistry: Strontium and Strontium Compounds, J.P. MacMillan, J.W. Park, R. Gerstenberg, H. Wagner, K. Köhler, and P. Wallbrecht, eds., Wiley-VCH Verlag, Weinheim, 2000, pp. 473–80.
F. Habashi, ed.: Handbook of Extractive Metallurgy, vol. IV, Strontium, P. MacMillan, ed., Wiley-VCH, Weinheim, 1997, pp. 23–29.
W.D. Callister and D.G. Rethwisch: Material Science of Engineering: An Introduction, 8th ed., Wiley, Hoboken, NJ, 2009.
M. Yang, H. Li, W. Zhang, and T. Zhou: Adv. Mater. Res., 2012, vols. 403–408, pp. 20–23.
M. Yang, H. Li, W. Zhang and T. Zhou: Adv. Mater. Res., 2012, vols. 403–408, pp. 707–11
M. Yang, F. Pan, and L. Cheng: J. Mater. Eng. Perform., 2010, vol. 19 (7), pp. 1043–50.
M.B. Yang, F.S. Pan, R.J. Cheng, and A.T. Tang: Trans. Nonferr. Met. Soc. China, 2008, vol. 18 (1), pp. 52–58.
P. Mao, B. Yu, Z. Liu, F. Wang, and Y. Ju: J. Appl. Phys., 2015, vol. 117 (115903), pp. 1–11.
M. Aljarrah and M. Medraj: Calphad, 2008, vol. 32 (2), pp. 240–51.
X. GuoLiu, X. DongPeng, W. DongXie, and Q. YiWei: Mater. Sci. Forum, 2005, vols. 488–489, pp. 31–34.
O. Yücel, S. Yiğit, and B. Derin: Mater. Sci. Forum, 2005, vols. 488–489, pp. 39–42.
M. Bugdayci, A. Turan, M. Alkan, and O. Yucel: High Temp. Mater. Processes, 2018, vol. 37 (1), pp. 1–8.
R. Huang, L.V. Xiaodong, Q. Wu, and J. Zhang: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 816–24.
L.V. Xiao-Dong, R. Huang, Q. Wu, and J. Zhang: Can. Metall. Q., 2019, vol. 58 (4), pp. 419–26.
R. Huang, P. Liu, X. Qian, and J. Zhang: Vacuum, 2016, vol. 134, pp. 20–24.
M. Bugdayci, A. Turan, Y. Cucurachi, and O. Yücel: Strontium Production from Strontium Oxide Using Vacuum Aluminotermic Process. 18th Int. Metal. Mater. Congr. (IMMC2016) Proc., Istanbul, Turkey, 2016.
A.A. Nayeb-Hashemi and J. B. Clark (1986) Bull. Alloy Phase Diagr., 7 (2), 149–56.
X. Zeng, Y. Wang, W. Ding, A.A. Luo, and A.K. Sachdev: Metall. Mater. Trans. A, 2006, vol. 37A, pp. 1333–41.
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R. BenMahfoud, J. Melançon, A.D. Pelton, and S. Petersen: Calphad, 2002, 26(2), pp. 189–228.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted July 2, 2019.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bugdayci, M., Turan, A. & Yücel, O. Production of Magnesium-Strontium Alloys Through Vacuum Metallothermic Process. Metall Mater Trans B 51, 1254–1262 (2020). https://doi.org/10.1007/s11663-020-01825-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01825-9