Abstract
Super-gravity segregation was investigated to enrich and remove the low-content impurities from Al by solidifying Al under a super-gravity field. The macrosegregations of Fe (0.19 wt pct) and Si (0.09 wt pct) were remarkable within 1.5 cm along the direction of super gravity, and the concentration ratios between two sides under super gravity of 1000 g reached 4.05 and 2.80, respectively. The microstructures demonstrated that Fe- and Si-rich phases formed and gathered at the bottom along the direction of super-gravity field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Although recycled metals become more ubiquitous under the exhaustion of raw resources, their properties are not always excellent and stable like the new metals. The main reason is because the remained impurities from the environment, which are difficult or costly to be removed entirely, damage the performance of the recycled metals. Some researchers have found that a trace of some elements can decrease metal properties, which means that many recycled metals are used only as low-level products.[1]
Aluminum is the second-largest metal material and has one of the highest recovery rates among the metals. A small amount of impurities in its matrix can also worsen some properties of the metal.[1] Besides silicon, iron is the most pervasive impurity in aluminum. Because of its low solid solubility (0.05 wt pct), most formed Fe-rich phases together with other elements are detrimental to the mechanical properties.[2] Among the removal methods employed, the most popular strategy adopts the precipitation and sedimentation of the melting Al liquid and also requires the addition of other elements called chemical “neutralizers,” such as Mn, Co, etc. However, many additives are harmful to the Al alloy.[3] Zone refining, which is based on the theory of unidirectional solidification, is also an effective method to remove impurities from metal,[4] but it is adapted to the high-purity metals, not to the large-scale product. Although three-layer liquid electrolysis is an effective way to purify aluminum on a large scale, it is costly. So it makes sense to find a new way to remove the low-content impurities from metals rapidly and economically.
Macrosegregation caused by gravity, which is called gravity segregation, is ubiquitous in casting metal, and it is harmful to the properties of materials. But sometimes, the phenomenon can be enlarged by super gravity and used for other purposes. J. F. Löffler et al.[5] have used ultra-high gravity to discover new bulk metallic glasses. When the Mg-Al alloy was solidified under super gravity field of 60,000 g (g is the normal gravitational acceleration with the value of 9.81 m/s2), a significant stratification was found with respect to density, with primary phases crystallizing and sedimenting to the end and binary and ternary eutectic microstructures forming in the middle part of the sample. Hiromi Matsubara et al.[6] have studied the removal of Fe from Al alloy by solidification under a super-gravity field. The Fe concentration decreased from 2.07 to 0.27 wt pct. The results were attributed to the macrosegregation caused by super gravity during solidification. Unfortunately, the case of lower concentration was not studied, and the influence of a different super-gravity field also was not investigated. Even so, the research results still indicated that super-gravity segregation is a potential method for the large-scale purification of metals.
To better investigate super-gravity segregation as a purification means of metals, we studied the enrichment of Fe and Si in industrial pure aluminum through solidification under different super-gravity fields. The concentrations of Fe and Si in the raw material were 0.19 wt pct and 0.09 wt pct, respectively. The range of super-gravity field (calculated as the ratio of super-gravitational acceleration to normal-gravitational acceleration) in this work was 1 to 1000 g to investigate the influences of super gravity sufficiently.
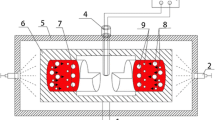
The super-gravity field was generated by the centrifugal apparatus in the experiment. Figure 1 shows the sketch of this apparatus, and the furnace was fixed into the centrifugal rotor. The sample was the industrial pure aluminum. The covering slag was the mixture of 45 wt pct sodium chloride and 55 wt pct potassium chloride, and both were analytical reagents.
Experimental procedures: First, 10 g Al was placed in an alumina crucible (ID = 17 mm) and covered with 3 g covering slag. The temperature of the furnace was raised to 1023.15 K (750 °C) and kept at this temperature for 10 minutes, and then the centrifugal apparatus was started up and adjusted to the specified angular velocity. The apparatus was kept rotating until the sample was cooled down to room temperature at 16 °C/min.
The sample was cut into halves along the direction of super-gravity, and one was burnished, polished, and then investigated by metallurgical microscope and electron probe microanalysis (EPMA-1600; Shimadzu Corporation, Kyoto, Japan). Four 2-mm-wide slices, whose positions were evenly distributed in the other half, were chipped out along vertical direction of super gravity, and then the concentrations of Fe and Si elements were tested by atomic absorption spectrometry. Figure 2(a) shows the positions of the slices for impurity concentration analysis inside the sample.
The longitudinal-sectional microstructures of different portions inside the sample showed that the outer portions were column crystal zone and the center was equiaxed crystal zone, which shows that samples was solidified by the mode like most of the ingot. Therefore, the solidification process could be considered as the casting process.
Although solidification under super gravity has been investigated by many researchers, most have focused on the microstructure of alloys, and almost all used the rigorous thermal-gradient furnace like the Bridgman furnace.[5,7,8] Here, the sample was placed at the flat temperature zone, and the heat was diffused slowly toward the surrounding. The heat transfer was close to that of the casting process; the solidification rate was much faster than that of unidirectional solidification or zone refining in metallurgical industry.
Figure 3 shows the bottom microstructures of samples solidified under different gravity field. Unlike being solidified under the normal gravity, plenty of clustering aciculate-crystals occurred at the bottom of the sample under the super gravity, and the amount increased with the increase of super-gravity strength. Moreover, the grain boundaries also became more obscure and thinner near the clusters.
An electron probe micro-analyzer (EPMA) analysis of the aciculate-crystal clusters showed the main components of the clusters were 82.80 pct aluminum, 15.97 pct iron, and 1.22 pct silicon in atom, which indicates that the clusters were the sediment phases of iron and silicon, and the main compound should be FeAl3 according to the element proportion. These results demonstrated that Fe and Si were enriched to the bottom.
The reason must be that super gravity prompted Fe and Si to gather and sedimentate to the bottom. In Hiromi Matsubara’s[6] research, the concentration of iron in the aluminum alloy was greater than that at the eutectic point (1.7 wt pct), and silicon concentration was also high (11 wt pct), so the initial newly born phase of solidification was Fe-rich solid phase, in which silicon formed the compound (Fe2SiAl8) with Fe. Because of its greater density than molten Al, the Fe-rich phase was propelled downside along the direction of super-gravity field.[6] However, the concentration of Fe was just 0.19 wt pct in our experiment, which is much lower than that of the eutectic point, and the concentration of Si was also only 0.09 wt pct; therefore, the initial newly born phase of solidification was not the Fe-rich solid phase.[2]
Because of microsegregation, iron and silicon always gather at the grain boundaries inside industrial pure aluminum. It was the solute distribution coefficients of impurities—less than 1 for Fe and Si in solid–liquid aluminum at such low content—that resulted in the impurity gathering. In the same way, Fe and Si partial concentrations in the liquid ahead of freezing interface would be greater than that in main liquid in solidification process. The concentration difference indicates a simultaneous difference in density. When propelled, the impurities migrated along the direction of the super-gravity field. With the concentration increasing at the bottom, the aciculate-crystal cluster began to form. In fact, macrosegregation caused by gravity is ubiquitous in large ingot, which is called gravity segregation, but here, the super-gravity field largely intensified that effect.[9] We described this as super-gravity segregation.
The principle of this macrosegregation is mostly like the one used in unidirectional solidification, which has been widely investigated. In contrast, it was super gravity that was the power of blend mode, instead of induction or mechanical agitation.[10] Because of its rigorous directivity and specificity to density difference, super gravity enriched Fe and Si to the bottom more efficiently than the other blend modes.
Figure 2(b) shows the distribution of Fe and Si inside samples solidified under a different gravity field. Each point in Figure 2(b) was the average value of three parallel samples. The distributions of the Fe and Si inside the sample along the direction of super-gravity field also indicated the enrichment of impurities. The concentrations increased from up to down along the direction of super-gravity, and the concentration gradient also became larger with the increase of super-gravity field. In contrast, there was almost no visible trend under normal gravity. When the super-gravity field was 1000 g, the concentration ratios of Fe and Si between the bottom and the top, over 1.5 cm along direction of super gravity, reached 4.05 and 2.80 cm, respectively. The gradient gave evidence of the enrichment of Fe and Si along the direction of the super-gravity field.
In practice, the bottom of aluminum ingot—where the iron and silicon impurities have enriched and sedimentated through super-gravitational solidification—can be cut away to remove the impurities. Compared with other methods such as zone refining, this method has the advantages of high speed and large capability. Thus, it is expected that the method can be used to enrich and separate rapidly the slight impurity element from metals.
Briefly, the super-gravity segregations of the main impurities Fe (0.19 wt pct) and Si (0.09 wt pct) were remarkable over 1.5 cm along the direction of super gravity, and the concentration ratios between two sides under super gravity of 1000 g reached 4.05 and 2.80, respectively. The microstructures and EPMA analysis demonstrated that Fe and Si gathered and formed the Fe-rich phase as the clusters of aciculate-crystals, at the bottom along the direction of super-gravity field. This made possible the easy removal of the low-content impurities. It was the super-gravity field that largely intensified the gravity segregation and resulted in the enrichment of impurity elements. This method is expected to be used to enrich and separate rapidly the slight impurity elements from metals.
References
P. Kim, C. Hayashi, K. Nakashima, Y. Yamada, K. Tachikawa, and T. Mitsugashira: Phys. Stat. Sol., 2002, vol. 189, pp. 149–58.
L.F. Mondolfo: Aluminum Alloys Structure and Properties, Butterworth, London, U.K., 1976, pp. 47–49.
J.W. Gao, D. Shu, J. Wang, and B.D. Sun: Scr. Mater., 2007, vol. 57, pp. 197–200.
E. Hashimoto and Y. Ueda: Mater. Trans., 1994, vol. 35, pp. 262–65.
J.F. Löffler and W.L. Johnson: Intermetallics, 2002, vol. 10, pp. 1167–75.
H. Matsubara, N. Izawa, and M. Nakanishi: J. Jpn. Inst. Light Met., 1998, vol. 48, pp. 93–97.
G. Müller and G. Neumann: J. Cryst. Growth, 1983, vol. 63, pp. 58–66.
G. Müller, G. Neumann, and W. Weber: J. Cryst. Growth, 1992, vol. 119, pp. 8–23.
G. Müller: J. Cryst. Growth, 1990, vol. 99, pp. 1242–57.
R.H. Zhou: Solidification Technique, China Machine Press, Beijing, China, 1998, pp. 436.
This work is supported by the Natural Science Foundation of China (50804043 and 50674011), the Knowledge Innovation Program of the Chinese Academy of Sciences (KGCX2-YW-321-2), and Science and Technology Cooperation Project of CAS and GuangDong (2009B091300047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted December 9, 2009.
Rights and permissions
About this article
Cite this article
Zhao, L., Guo, Z., Wang, Z. et al. Removal of Low-Content Impurities from Al By Super-Gravity. Metall Mater Trans B 41, 505–508 (2010). https://doi.org/10.1007/s11663-010-9376-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-010-9376-2