Abstract
Summary
Previous studies have shown that improving vitamin D status among the elderly may lead to an improvement in muscle mass and muscle strength. In our study, vitamin D supplementation showed significant improvements in vitamin D concentrations as well as appendicular muscle mass in pre-sarcopenic older Lebanese people. However, we found no significant effect on muscle strength.
Introduction
Improving vitamin D status might improve muscle function and muscle mass that lead to sarcopenia in older subjects. The aim of this randomized, controlled, double-blind study was to examine the effect of vitamin D supplementation on handgrip strength and appendicular skeletal muscle mass in pre-sarcopenic older Lebanese subjects. We also examined whether this effect differs in normal vs. obese subjects.
Methods
Participants (n = 128; 62 men and 66 women) deficient in vitamin D (25(OH)D = 12.92 ± 4.3 ng/ml) were recruited from Saint Charles Hospital, Beirut, Lebanon. The participants were given a supplement of 10,000 IU of cholecalciferol (vitamin D group; n = 64) to be taken three times a week or a placebo tablet (placebo group; n = 64) for 6 months. One hundred fifteen subjects completed the study: 59 had normal weight, while 56 were obese. Strength and functional assessment and biochemical analysis were performed at the start and after 6 months.
Results
Compared to placebo, the vitamin D supplemented group showed significant improvements in appendicular skeletal muscle mass (ASMM) (P < 0.001) but not in handgrip strength (P = 0.2901). ANCOVA for ASMM adjusting for obesity and including the interaction between obesity and vitamin D showed a significant interaction. The increase in ASMM with vitamin D in normal-weight subjects was higher than that of obese subjects (B = 35.09 vs. B = 2.19).
Conclusion
Treatment with vitamin D showed beneficial effects on appendicular muscle mass in pre-sarcopenic older Lebanese men and women. However, it had no effect on muscle strength relative to placebo. This trial was registered at isrctn.org as ISRCTN16665940.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The risk of falls and hip fractures increases in older people, primarily owing to a loss of skeletal muscle mass and strength, termed sarcopenia. Recent studies have revealed the effect of vitamin D on muscle health, in particular the consequence of its deficiency on muscle mass and muscle function in older people and patients with chronic diseases. It was suggested that low vitamin D status plays a central role in the risk of falls in older people, partly through its effect on muscle function [1,2,3,4]. Nevertheless, there remains an absence in the literature of a clear consensus on the link between vitamin D status and either muscle mass or strength. Previous trials, in particular randomized clinical trial, have generated contradictory conclusions on whether muscle strength is improved with vitamin D treatment [5,6,7,8,9]. Similarly, observational studies [10, 11] investigating this association have established conflicting findings. These different outcomes may reflect different baseline serum 25-hydroxyvitamin D (25(OH)D) levels in the populations studied, different period of vitamin D treatment, or different strength assessments used as outcome measures.
A related decrease in physical activity and metabolic disturbances results in sarcopenia being systematically associated with a significant increase in fat mass. For several reasons, obesity and sarcopenia go hand in hand [12]. Physical activity decreases with age, negatively affecting muscle mass and contractile function, and predisposing to weight gain, mainly as fat mass. Increased fat mass promotes insulin resistance and can produce a direct catabolic effect on skeletal muscle [13, 14]. Baumgartner et al. showed that increased fat mass was associated with Instrumental Activities of Daily Living (IADL) disability, which causes a decline in appendicular skeletal muscle in individuals [15]. Villareal et al. also reported that obese older adults had low relative muscle mass and low muscle strength per muscle area, which led to sarcopenia, in contrast to normal-weight older adults [16]. In addition, it has been reported that vitamin D supplementation optimizes body composition and muscle function outcomes, thereby reducing falls and fracture risk in sarcopenic obese people [17]. Despite these recent data, the effect of a long-term vitamin D supplementation on muscle mass and function in pre-sarcopenic versus pre-sarcopenic obese subjects had not yet been studied.
The main aim of the study is to analyze, in a 6-month randomized, controlled, double-blind study, the effect of vitamin D supplementation on handgrip strength and appendicular skeletal muscle mass in pre-sarcopenic older subjects. We also aim to test whether this effect differs in normal-weight pre-sarcopenic versus obese pre-sarcopenic older subjects.
Subjects and methods
Participants
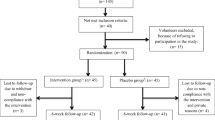
A total of 160 participants seen at the endocrinology and orthopedics outpatient clinics of Saint Charles Hospital (Fiyadiyeh-Lebanon) were asked to join the study between July and September 2015. The participants who were pre-sarcopenic (when skeletal muscle mass/height2 = 7.26 kg/m2 for males and 5.45 kg/m2 for females), deficient in vitamin D (25(OH)D < 20 ng/ml as per Institute of Medicine (IOM) recommendations), and having no medical history of type 2 diabetes were asked to join the study. Criteria for exclusion were non-pre-sarcopenic subjects, incidence of balance problems due to neurological disorders, renal failure, congestive heart failure and acute heart insufficiency as well as uncontrolled arterial hypertension or hypotension, use of sedative (that could affect balance), use of vitamin D supplementation, and primary hyperparathyroidism.
As shown in Fig. 1, out of 160 persons, 32 were not included, leaving 128 participants, who were randomized to receive vitamin D supplements or a placebo, 13 participants were lost to follow-up, and the remaining 115 (59 men and 56 women) completed the study. The first visit was in September 2015. They were given written informed consent to the study procedures, which were approved by the Ethics Committee of the institutional review board at Saint Charles Hospital, Lebanon. Study protocols were completed according to the Declaration of Helsinki ethical principles for medical research involving human subjects.
Study design and supplementation protocol
In this randomized, controlled, double-blind study, participants were randomized (using the simple randomization method) to receive either a supplement of 10,000 IU of cholecalciferol (Euro-Pharm International, Canada) or a placebo tablet (containing microcrystalline cellulose = 66.3%, starch = 33.2%, magnesium stearate = 0.5%, per serving) to be taken three times a week for a period of 6 months. After screening, the subjects were given a number (1–128) based on their order of inclusion in the study. A simple randomization method was conducted by the pharmacist by tossing a coin, and the participants were randomly assigned to two groups according to the European Working Group on Sarcopenia in Older People (EWGSOP) criteria: (i) pre-sarcopenic subjects receiving vitamin D (n = 64) and (ii) pre-sarcopenic subjects receiving the placebo (n = 64). Neither the investigator nor the subjects were aware of the group allocation; the pharmacist (in charge of the placebo tablets as well as the packing and coding of the supplements) was the only person to know to which group each participant belonged. The first subject was randomized to the placebo group. Afterward, the participants were allocated to either group until the expected sample number is achieved. At 3 months, a follow-up by phone calls was done with the subjects, and they were seen after 6 months of supplementation with vitamin D or placebo. They were also asked to return the bottles to measure the compliance by bottle counts.
Biochemical analyses and muscle assessments were performed at baseline and at 6 months. The study patients’ medical history and medical treatment were assessed from physicians’ records.
Primary outcomes
Muscle mass and strength assessment
Handgrip strength
Handgrip strength was used to measure muscle strength and is recognized to be positively associated equally with lower-extremity and upper-body strength in older persons [18, 19]. It was measured in the dominant hand with a Martin vigorimeter (Martin; Elmed, Addison, IL, USA), and the force was expressed in kilograms [20]. Before starting, the participant was allowed to perform one test trial. Measurement was then made in the seated position with elbow bent at 110° and upper arm parallel to but not pressed to the body. The width of the grip was adjusted to the size of the hand so that the middle phalanx relaxed on the inner grip. Each participant then performed three trials, and the best score was taken for analysis. Handgrip strength measurements were assessed by a registered nurse and were included in the analysis.
Appendicular skeletal muscle mass
Skeletal muscle mass was determined from bioimpedance analysis measurements (Tanita BC-418 Segmental Body Composition Analyzer, Illinois, USA) and expressed as appendicular skeletal muscle mass (ASMM, kg). Pre-sarcopenia is characterized when skeletal muscle mass is 2 standard deviations below the sex-specific young-normal mean for estimates of skeletal muscle mass [21, 22]. Cutoff thresholds for skeletal muscle mass indices are not given in the Lebanese literature: we took 7.26 and 5.45 kg/m2 as cutoff points for sarcopenia in males and females, respectively [21].
25(OH)D
25(OH)D concentration was measured by radioimmunoassay (DiaSorin, Stillwater, MN); inter-assay CV was < 8%. The reference range for normal values of 25(OH)D concentrations should be 30–90 ng/mL, and values < 20 ng/ml were considered as hypovitaminosis.
Secondary outcomes
Anthropometric measurements
Body composition (weight, fat mass, and muscle mass) was assessed using the same body composition analyzer (Tanita BC-418 Segmental Body Composition Analyzer, Illinois, USA). Anthropometric measurements, including weight (kg), height (m), and fat mass (kg), were made on a screening visit to determine eligibility. Body mass index (BMI) was calculated using the standard formula (body weight in kilograms divided by square of body height in meters). Subjects were classified as obese when BMI was ≥ 30 kg/m2. Pre-sarcopenic subjects were classified using the definition described previously in the text. Pre-sarcopenic obesity was diagnosed when subjects met the criteria for both pre-sarcopenia and obesity using these definitions. Waist circumference was measured at the iliac crest while the subject is standing.
Biochemical analysis
Blood specimens were collected after a 12-h overnight fast at baseline and after 6 months of supplementation. The aliquots of the serum samples were centrifuged at room temperature for 20 min (1500×g), then frozen and stored at − 80 °C until analyzed. PTH was measured using a two-site immunoradiometric assay with an NH2-terminal monoclonal antibody as capture (Fitzgerald Industries International Inc., USA); inter-assay CV was < 10%. Serum creatinine was measured using the Jaffe kinetic alkaline picrate reaction (Interpretation and Techniques, Lea and Febiger, Philadelphia); inter-assay CV was < 10%. Creatinine reflects skeletal muscle mass, since it is a breakdown product of creatine phosphate in muscle [23].
A short questionnaire to assess dietary vitamin D intake was given to each participant at the start of the study [24]. The subjects’ dietary vitamin D intake ranged between 4.5 and 6.5 μg per day.
Physical activity assessment
A Physical Activity Scale for the Elderly (PASE) was used to assess the physical activity of each participant. PASE involves ten items used to recognize leisure (walking, sports, muscular strength/endurance), household (housework, home repair, lawn work, outdoor gardening, caring for others) and occupational related activity, and duration of activity over a 1-week period. The total PASE score was calculated by multiplying the duration of each activity (hours/week) or participation (yes/no) by the established item weights to end up with an overall physical activity score for the week. The higher is the score, the greater is the physical activity. This tool was used at the beginning of the study and after 6 months of supplementation.
Sample size calculation
Sample size calculation was carried out to address the main objective of the study. The minimum sample size required to detect an effect size of 0.6 between the vitamin and control group, a power of 80%, and a significance level of 0.025 (taking into account two primary outcomes: grip strength and muscle strength) is 55 subjects per arm. Therefore, the total sample size needed is 110 subjects. To account for a 10% loss to follow-up, 128 subjects were targeted. Sample size calculation was done using GPower version 3.0.10.
Statistical analysis
Statistical evaluations were completed using SPSS version 21 (SPSS Inc., Chicago, IL). Thirteen subjects did not have follow-up data at 6 months (four in the vitamin D group and nine in the placebo group). Subjects’ baseline characteristics such as age, gender, height, weight, BMI, and smoking were compared between those who dropped and those who completed the study. The only significant difference was in gender where all the dropouts were males. Complete cases (N = 115) were used in the analyses. Two main outcomes were considered: handgrip strength and appendicular skeletal muscle mass. Relative changes in the outcomes from baseline to 6 months were computed where relative change = [(follow-up − baseline) / baseline] × 100. The paired samples t test was used to determine the changes in the outcomes within the same group (time point 0 and time point 6 months) and the independent samples t test was used for between group comparisons. To test whether the effect of the intervention differs between the two obesity groups (normal weight/obese), a two-way analysis of variance was used on the relative changes in the outcomes; the two factors considered in the two-way ANOVA were obesity (normal/obese) and the intervention (vitamin D/placebo). We also considered the interaction between the two. Finally, analysis of covariance (ANCOVA) was run, also on the relatives changes as outcomes, to further adjust for the effect of other subjects’ characteristics (such as vitamin D, obesity, smoking, physical activity); gender was included in all models. Interactions between obesity and vitamin D supplementation were included in the ANCOVA models. Parameter estimates, P values, and 95% confidence intervals (CI) were reported. A P value of < 0.05 was considered statistically significant.
Results
The baseline characteristics for participants are shown in Table 1. The participants were all vitamin D-deficient (The IOM considers a serum level of 25(OH)D below 20 ng/ml as a deficiency) [25], and their age ranged from 70 to 79 years. Out of 128 subjects randomized at baseline of the treatment period, 64 subjects received vitamin D pills, and 64 subjects received placebo pills. Thirteen subjects were lost to follow-up after the first visit, and the remaining 115 completed the study. Their average weight was 83.59 ± 14.79 and average BMI 29.42 ± 5.49. Based on their BMI, 59 subjects were classified as having a normal weight (51.3%) and 56 (48.7%) as obese. Fifty-three percent were smoking persons. All subjects had no history of diabetes or coronary artery disease. The subjects’ physical activity level was conveyed as low (according to the PASE tool), because they reported non-participation in sports (strength and endurance exercises, lawn and yard work, and heavy housework), home repairs, gardening, and caring for another person. The only physical engagement was walking (once per week) and light housework.
Primary outcomes
Among the 115 subjects with complete follow-up data, the mean serum 25(OH)D concentration increased significantly from baseline (10.13 ± 2.87 ng/mL) to 6 months (27.98 ± 3.83 ng/mL) in the vitamin D supplemented group (P = < 0.001). As for the placebo group, the mean serum 25(OH)D concentration showed a slight but significant increase from baseline (10.56 ± 3.14 ng/mL) to 6 months (15.71 ± 5.70 ng/mL) (P = < 0.001). The mean percent change from baseline to 6 months in the vitamin D supplemented group (194.59 ± 76.55) was significantly different from that of the placebo group (48.83 ± 31.37) (P = < 0.001). Regarding the handgrip strength, the mean values increased significantly from baseline (21.42 ± 5.48 kg) to 6 months (22.27 ± 6.60 kg) in the vitamin D supplemented group (P = 0.007). Whereas, the change observed in the placebo group from baseline (20.64 ± 5.51 kg) to 6 months (20.7 2 ± 5.24 kg) was not significant (P = 0.656). No further differences between groups were observed (P = 0.290). As for the ASMM in kilogram, the mean values increased significantly from baseline (21.58 ± 6.53 kg) to 6 months (22.23 ± 5.85 kg) in the vitamin D supplemented group (P = 0.001). The change in the placebo group showed no significance from baseline (16.83 ± 3.11 kg) to 6 months (16.92 ± 3.25 kg) (P = 0.203). The mean percent change between the vitamin D supplemented group (3.01 ± 2.38) and the placebo group (0.46 ± 3.30) was significantly different, with a P = < 0.001 (Table 2).
Two-way ANOVA
Two-way ANOVA was carried out to assess whether the effect of the intervention on ASMM, handgrip strength, and 25(OH)D differs between normal and obese subjects. Results of the two-way ANOVA showed a significant interaction between vitamin D and obesity on ASMM (F (1, 111) = 30.27, P < 0.001). Simple main effects analysis showed that although a statistically significant increase was observed with vitamin D compared to placebo in both normal-weight and obese subjects, the effect size was much higher in the normal-weight group (ES = 1.57 vs. 1.32). The interaction between obesity and vitamin D was not significant on the change in grip strength. The main effect analysis showed that both main effects were also not significant. A significant interaction was also observed for 25(OH)D (F (1, 111) = 73.46, P < 0.001). The effect size in the obese group was larger than the effect size of the normal-weight group (ES = 0.96 vs 3.66) (Table 3).
Analysis of covariance
To adjust for other subjects’ characteristics such as age, gender, and smoking status, ANCOVA was conducted. Gender and variables that were significant at the univariate level were entered in the ANCOVA model. The ANCOVA model for ASMM included vitamin D supplementation, obesity, their interaction, and gender. Since the interaction between obesity and vitamin D was significant (P < 0.001), two ANCOVA models were run for normal-weight and obese subjects separately. In the model including subjects with normal weight, the significant predictors of ASMM were vitamin D supplementation (B = 35.09, P < 0.001, 95% CI = 26.48–43.71) and gender with a higher percent change observed in males compared to females (B = 32.98, P < 0.001, 95% CI = 24.38–41.50). For obese subjects, only vitamin supplementation was significant (B = 2.19, P < 0.001, 95% CI = 1.27–3.10). The effect of vitamin D supplementation was much higher in subjects with normal weight. Estimated means of ASMM percent change for every group from the ANCOVA models are shown in Table 4.
As for the handgrip strength, no variables (including gender) showed significant associations at the univariate level and therefore no ANCOVA model was run.
Regarding 25(OH)D, the ANCOVA model included vitamin D, obesity and their interaction, and gender. In the ANCOVA model, gender was not significant while the interaction between vitamin D and weight was significant (B = 90.34, P > 0.001); thus, two models were run separately for subjects with normal weight and obese subjects. Vitamin D was significant in both groups with a greater effect in normal weight as compared to obese subjects (B = 188.18, P < 0.001, 95%CI = 168.15–208.2 vs. B = 104.83, P < 0.001, 95% CI = 73.11–136.56). Estimated means of 25(OH)D percent change for every group from the ANCOVA models are shown in Table 4.
Secondary outcomes
The mean weight of the subjects decreased from baseline (85.45 ± 16.67) to 6 months (85.09 ± 16.6) in the vitamin D supplemented group, but this change was not significant (P = 0.06). As for the placebo group, the change observed from baseline to 6 months was significant (P < 0.001). The mean percent change from baseline to 6 months in the vitamin D supplemented group (− 0.42 ± 0.39) was significantly different from that of the placebo group (− 1.89 ± 1.27) (P = 0.001). The same pattern was observed for BMI. As for waist circumference, the change was significant in both groups (P = 0.001). The fat mass decreased significantly from baseline (31.26 ± 7.83) to 6 months (30.18 ± 7.82) in the vitamin D supplemented group (P = 0.001) and from 29.65 ± 6.30 to 27.72 ± 5.96 in placebo group (P = 0.001). Also, the mean percent change from baseline to 6 months in the vitamin D supplemented group (− 3.55 ± 5.67) was significantly different from that of the placebo group (− 6.25 ± 6.62) (P = 0.021). Also, the lean body mass increased significantly from baseline (54.19 ± 9.05) to 6 months (54.91 ± 9.35) in the vitamin D supplemented group (P = 0.005). The change in placebo group was also significant (P = 0.002). As for the mean percent change from baseline to 6 months, the vitamin D supplemented group (1.33 ± 3.31) was not significantly different from that of the placebo group (0.72 ± 6.49) (P = 0.196). The mean serum PTH concentration decreased significantly from baseline (60.71 ± 12.22) to 6 months (49.68 ± 9.99) in the vitamin D supplemented group (P < 0.001) and from 63.56 ± 10.56 to 61.38 ± 10.11 in the placebo group (P = 0.001). The mean percent change from baseline to 6 months in the vitamin D supplemented group was significantly different from that of the placebo group (P < 0.001). Mean change from baseline to 6 months in serum creatinine concentration was not significant in both the vitamin D and the placebo group and between the two (Table 3).
Discussion
This study assessed the effects of a 6-month vitamin D supplementation on handgrip strength and appendicular skeletal muscle mass in pre-sarcopenic older subjects. After the 6 months supplementation, only appendicular skeletal muscle mass increased significantly in the vitamin D supplemented group, whereas no significant effect on muscle strength was shown in the vitamin D group relative to placebo.
There have been many intervention studies in the area of vitamin D and muscle physiology as well as physical performance. However, less than one third of these studies meet the criteria for a randomized clinical trial. Some of these previous studies suggested a relationship between low vitamin D concentration and poor muscle function [26, 27]. The present study is the first, based on a Lebanese population, to investigate the effect of vitamin D supplementation on pre-sarcopenia in older normal-weight and obese people using a randomized controlled trial design.
We clearly show that vitamin D supplementation improved muscle mass in older men and women but did not have a significant effect on muscle strength. The gain in appendicular skeletal muscle mass that we observed after 6 months of supplementation is in line with El-Hajj et al. (2006). They found an increase in lean and muscle mass in younger age (adolescent girls) after vitamin D supplementation, but no significant change was seen in grip strength [28]. Similarly, a recent study on sarcopenic older adults revealed a significant increase in muscle mass after vitamin D supplementation (combined with leucine-enriched whey protein) [29]. Conversely, some cross-sectional studies found no consistent association between vitamin D status and skeletal muscle mass [10, 11, 30]. One explanation could be that higher proportion of these studies were performed in elderly institutions, and vitamin D supplementation was perceived to increase muscle mass among studies reporting lower baseline serum 25(OH)D levels when compared with higher ones.
Our study showed no beneficial effect on muscle strength after the intervention of vitamin D, although a significant increase in handgrip strength was perceived in the treatment group, but this change was not significantly different compared to the placebo group. Although our findings come in accordance with many previous studies [6, 10, 31, 32, 33], they are not consistent with several cross-sectional studies on vitamin D status and muscle function [5, 34, 35] that showed beneficial effect of vitamin D treatment on muscle strength or physical performance. However, there are many distinguishing aspects that may account for these differences. First, comparing results from different studies is slightly obstructed by differences in subject demographics, as well as the study design, and the chemical form of vitamin D used during supplementation. Moreover, vitamin D deficiency is only one of the conditions that can alter muscle function in older adults [36, 37], which is explained by previous research that even in healthy elderly people, the decline in muscle strength with age was not prevented by vitamin D treatment [6, 9, 38]. Besides, severe comorbidity combined with a low physical activity level may cause muscle weakness and thus functional impairment, which cannot be enhanced by treating a coexisting vitamin D deficiency [8].
Our data showed a reduction in fat mass as well as body weight and waist circumference after 6 months of vitamin D supplementation. These findings are consistent with those of Shahar et al. (2014), who demonstrated in their recent study that women experienced more loss in body weight and body fat and had a greater reduction in waist circumference after vitamin D supplementation [39]. Khozravi et al. (2018) revealed lately that 6 weeks vitamin D supplementation with doses 50,000 IU/week reduced significantly the mean of BMI, weight, and waist circumference among overweight and obese women [40]. However, conflicting results have been seen [41, 42]. Some studies have not shown significant association between vitamin D, BMI, body fat, and waist circumference. Probably, its main reason could be that the participants included in these studies were not all vitamin D-deficient.
Aging is associated with major modifications in body composition that reveal marked muscle loss and elevated fat mass [43]. Obesity is also known to be linked with low blood vitamin D concentration, and previous studies in community-dwelling older adults have established a strong relationship between vitamin D status and physical performance [43, 44]. Evidence for effects of vitamin D supplementation on pre-sarcopenic obesity is still lacking. Earlier studies suggested that obese individuals had lower 25(OH)D concentrations and elevated serum parathyroid hormone concentrations than normal-weight persons [43, 44]. The reason could be that vitamin D is a fat-soluble vitamin and is immediately stored in adipose tissue and so could be hidden in the fatty tissues of obese persons [43,41,45]. In our study, serum vitamin D concentrations were significantly different between obese and normal-weight individuals at baseline (results not shown) and the effect of vitamin D supplementation was smaller in obese subjects.
Earlier studies demonstrated a positive relationship between high BMI and increased risk of impaired functional status among community-dwelling elderly people [46, 47]. Several mechanisms might explain the link between vitamin D status and muscle strength. First, 1, 25(OH)D connects to vitamin D receptor (VDR) in the nucleus and creates a complex with the retinoid receptor. This whole complex triggers gene transcription, which leads to a sequence of genomic outcomes associated with muscle function. Furthermore, 1,25(OH)D is able to bind a membrane receptor that stimulates the activation of a protein kinase (mitogen-activated protein kinase) and phospholipase C, which triggers non-genomic effects [48]. 1,25(OH)D regulates calcium homeostasis in muscle fibers by inducing calcium flux. This regulates calcium signaling, which may control contractile force in differentiated muscle fibers [49]. In addition, a low vitamin D status is associated with a lipid-related degeneration of muscle cells, i.e., lipotoxicity, which affects muscular strength in older persons with vitamin D deficiency [50,48,52]. In our study, vitamin D concentration showed a significant interaction between vitamin D and obesity on ASMM (F(1, 111) = 30.27, P < 0.001), in addition to a significant decrease in fat mass after vitamin D supplementation (P = 0.001). Finally, vitamin D regulates insulin secretion and action in insulin-dependent tissues. Vitamin D deficiency may therefore lead to insulin resistance and muscle metabolic abnormalities [51,49,53].
Many studies have suggested that an elevated blood PTH concentration is a risk factor for sarcopenia in older men and women [3, 5]. Visser et al. [3] concluded in their study that subjects with higher PTH concentrations were more likely to lose grip strength and tended to lose more ASMM. In a prospective study, Verrault et al. [31] observed a relationship between higher PTH levels and loss of hip flexor and knee extensor strength. In addition, vitamin D insufficiency associated with secondary hyperparathyroidism increased risk of sarcopenia [54]. However, Kim et al. [2] concluded that there was no longer any association between PTH and sarcopenia after further adjustment for the subjects’ BMI. This indicates that the association between PTH and sarcopenia may be partially mediated by obesity [55]. In our study, lower 25(OH)D values were observed in obese subjects (results not shown) which is the most probable reason for their elevated PTH levels, as has been demonstrated in earlier studies that considered 25(OH)D and PTH levels in obese individuals [56,54,58]. Hamoui et al. [59] have shown that morbidly obese patients have low 25(OH)D concentrations, together with an incidence of hyperparathyroidism. We note that a chronic vitamin D deficiency leads to an enlargement of the parathyroid gland and a rise in PTH secretion. The gland may stay engorged even after improvement of vitamin D status [60].
The main strength of this work is that it is the first randomized, double-blind, placebo-controlled trial to study the effect of vitamin D supplementation on muscle strength and muscle mass in obese and normal-weight pre-sarcopenic Lebanese subjects. Moreover, we had minimal loss-to-follow-up of participants; around 90% of the randomized subjects completed the study. However, our study has some limitations. First, the period of supplementation was relatively short, though sufficient to identify positive ameliorations in muscle mass. Second, sun exposure and dietary vitamin D intake were a potential confounder in our study. Vitamin D status is related to sunlight exposure. Records for sun exposure were not available, but blood collection was during fall/winter, when older people are not usually exposed to much sunlight, compared with spring/summer.
In conclusion, our study showed no association between serum 25(OH)D concentration and muscle strength but rather between serum 25(OH)D concentration, appendicular muscle mass, and fat mass. Fat mass and muscle are interrelated in the development of disease in older adults, obesity and sarcopenia worsening each other’s outcomes on disability and morbidity. Nutritional supplementation may thus help subjects with pre-sarcopenia, regardless of their BMI. However, other longitudinal studies with longer durations of supplementation are needed to confirm these findings.
Abbreviations
- ASMM:
-
Appendicular skeletal muscle
- BMI:
-
Body mass index
- PTH:
-
Parathyroid hormone
- 25(OH)D:
-
25-Hydroxyvitamin D
References
Domingues-Faria C, Boirie Y, Walrand S (2017) Vitamin D and muscle trophicity. Curr Opin Clin Nutr Metab Care 2
Kim MK, Baek KH, Song KH, Kang M, Park CY, Lee WY, Oh KW (2011) Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab 96:10
Visser M, Deeg D, Lips P (2003) Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab 88:12
Bischoff-Ferrari HA, Dietrich T, Oray EJ, Hu FB, Zhang Y, Karlson AW, Dawson-Hughes B (2004) Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥ 60 y. Am J Clin Nutr 80(3):752–758
Verhaar HJ, Samson MM, Jansen PA, de Vreede PL, Manten JW, Duursma SA (2000) Muscle strength, functional mobility and vitamin D in older women. Aging 12:455–460
Grady D, Halloran B, Cummings S, Leveille S, Wells L, Black D, Byl N (1991) 1,25-Dihydroxyvitamin D3 and muscle strength in the elderly: a randomised controlled trial. J Clin Endocrinol Metab 73:1111–1117
Zhu K, Austin N, Devine A, Bruce D, Prince R (2010) A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc 58(11):2063–2068
Janssen HCJP, Samson MM, Verhaar HJ (2002) Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr 75:611–615
Johnson KR, Jobber J, Stonawski BJ (1980) Prophylactic vitamin D in the elderly. Age Ageing 9:121–127
Ceglia L, Chiu GR, Harris SS, Araujo AB (2011) Serum 25-hydroxyvitamin D concentration and physical function in adult men. Clin Endocrinol 74:370–376
Annweiler C, Beauchet O, Berrut G, Fantino B, Bonnefoy M, Herrmann FR, Schott AM (2009) Is there an association between serum 25-hydroxyvitamin D concentration and muscle strength among older women? Results from baseline assessment of the EPIDOS study. J Nutr Health Aging 13(2):90–95
Roubenoff R (2000) Sarcopenic obesity: does muscle loss cause fat gain? Ann N Y Acad Sci 904:553–555
Reaven GM (1988) Role of insulin resistance in human disease. Diabetes 37:1595–1597
Roubenoff R (2003) Catabolism of aging: is it an inflammatory process? Curr Opin Clin Nutr Metab Care 6:295–299
Baumgartner RN, Wayne SJ, Water DL, Jansse I, Gallagher D, Morley JE (2004) Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 12:1995–2004
Villareal D, Banks M, Sienerc C, Sinacore D, Klein S (2004) Physical frailty and body composition in obese elderly men and women. Obes Res 12:912–919
Scott D, Daly RM, Sanders KM, Ebeling PR (2015) Fall and fracture risk in sarcopenia and dynapenia with and without obesity: the role of lifestyle interventions. Curr Osteoporos Rep 13:4
Viitasalo JT, Era P, Leskinen AL, Heikkinen E (1985) Muscular strength profiles and anthropometry in random samples of men aged 31–35, 51–55 and 71–75 years. Ergonomics 28:1563–1574
Avlund K, Schroll M, Davidsen M, Løvborg B, Rantanen T (1994) Maximal isometric muscle strength and functional ability in daily activities among 75-year-old men and women. Scand J Med Sci Sports 4:32–40
Desrosiers J, Hébert R, Bravo G, Dutil É (1995) Comparison of the Jamar dynamometer and the Martin vigorimeter for grip strength measurements in a healthy elderly population. Scand J Rehabil Med Suppl 27:137–143
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763
Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ (2014) Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 68(9)
SB Heymsfield, Arteaga C, McManus J, Smith SM (1983) Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr 37:478–494
Hedlund L, Brekke HK, Brembeck P, Augustin H (2014) A short questionnaire for assessment of dietary vitamin D intake. European Journal of Nutrition & Food Safety 4(2):150–156
Institute of Medicine (2011) Dietary reference intakes for calcium and vitamin D. The National Academics Press, Washington, DC
Pfeifer M, Begerow B, Minne HW, Schlotthauer T, Pospeschill M, Scholz M, Lazarescu AD, Pollahne W (2001) Vitamin D status, trunk muscle strength, body sway, falls, and fractures among 237 postmenopausal women with osteoporosis. Exp Clin Endocrinol Diabetes 109:87–92
Dhesi JK, Bearne LM, Moniz C, Hurley MV, Jackson SH, Swift CG, Allain TJ (2002) Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res 17:891–897
El-Hajj G, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, Choucair M, Arabi A, Vieth R (2006) Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab 91:405–412
Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo MET, Mets T, Seal C, Wijers SL, Ceda GP, de Vito G, Donders G, Drey M, Greig C, Holmbäck U, Narici M, McPhee J, Poggiogalle E, Power D, Scafoglieri A, Schultz R, Sieber CC, Cederholm T (2015) Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 16:740–747
Marantes I, Achenbach SJ, Atkinson EJ, Khosla S, Amin S (2011) Is vitamin D a determinant of muscle mass and strength? J Bone Miner Res 26(12):2860–2871
Verrault R, Semba RD, Volpato S, Ferrucci L, Fried LP, Guralnik JM (2002) Low serum vitamin D does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc 50:912–917
Bunout D, Barrera G, Leiva L, Gattas V, Pía de la Maza M, Avendaño M, Hirsch S (2006) Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol 41:746–752
Goodpaster BH, Park SW, Harris TB, Kritchchesky SB, Nevitt M, Schwartz AV et al (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study, for the Health ABC Study. J Gerontol A Biol Sci Med Sci 61:1059–1064
Gloth FM, Smith CE, Hollis BW, Tobin JD (1995) Functional improvement with vitamin D replenishment in a cohort of frail, vitamin D-deficient older people. J Am Geriatr Soc 43:1269–1271
Sørensen OH, Lund BI, Saltin B, Lund BJ, Andersen RB, Hjorth L, Melsen F, Mosekilde L (1979) Myopathy in bone loss of ageing: improvement by treatment with 1-hydroxycholecalciferol and calcium. Clin Sci 56:157–161
Grimby G (1995) Muscle performance and structure in the elderly as studied cross-sectionally and longitudinally. J Gerontol 17–22(74):50A
Brooks SV, Faulkner JA (1994) Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc 26:432–439
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293(18):2257–2264
Shahar DR, Schwarzfuchs D, Fraser D, Vardi H, Thiery J, Fiedler GM, Blüher M, Stumvoll M, Stampfer MJ, Shai I, DIRECT Group (2010) Dairy calcium intake, serum vitamin D and successful weight loss. Am J Clin Nutr 92(5):1017–1022
Khosravi ZS, Kafeshani M, Tavasoli P, Zadeh AH, Entezari MH (2018) Effect of vitamin D supplementation on weight loss, glycemic indices, and lipid profile in obese and overweight women: a clinical trial study. Int J Prev Med 9:63
Kim M, Na W, Sohn C (2013) Correlation between vitamin D and cardiovascular disease predictors in overweight and obese Koreans. J Clin Biochem Nutr 52:167–171
Sneve M, Figenschau Y, Jorde R (2008) Supplementation with cholecalciferol does not result in weight reduction in overweight and obese subjects. Eur J Endocrinol 159:675–684
Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S (1985) Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest 76:370–373
Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH (1988) Low circulating vitamin D in obesity. Calcif Tissue Int 43:199–201
Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TRE (1981) Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr 34:2359–2363
Friedmann JM, Elasy T, Jensen GT (2001) The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc 49:398–403
Jensen GL, Friedmann JM (2002) Obesity is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc 50:918–923
Halfon M, Phan O, Teta D (2015) Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int:953241 11 pages
Dirks-Naylor AJ, Lennon-Edwards S (2011) The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol 125:159–168
Tagliafico AS, Ameri P, Bovio M, Puntoni M, Capaccio E, Murialdo G, Martinoli C (2010) Relationship between fatty degeneration of thigh muscles and vitamin D status in the elderly: a preliminary MRI study. AJR Am J Roentgenol 194(3):728–734
Park SH, Lee KS, Park HY (2010) Dietary carbohydrate intake is associated with cardiovascular disease risk in Korean: analysis of the third Korea National Health and Nutrition Examination Survey (KNHANES III). Int J Cardiol 139:234–240
Oh JH, Kim SH, Kim JH, Shin YH, Yoon JP, Oh CH (2009) The level of vitamin D in the serum correlates with fatty degeneration of the muscles of the rotator cuff. J Bone Joint Surg (Br) 1587–1593(1602):91
Pittas AG, Lau J, Hu FB, Dawson-Hughes B (2007) The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab 92:2017–2029
De SouzaGenaro P, de Medeiros Pinheiro M, Szejnfeld VL, Martini LA (2015) Secondary hyperparathyroidism and its relationship with sarcopenia in elderly women. Arch Gerontol Geriatr 60(2):349–353
Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P (2005) Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 90:4119–4123
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72:690–693
Cipriani C, Pepe J, Piemonte S, Colangelo L, Cilli M, Minisola S (2014) Vitamin D and its relationship with obesity and muscle. Int J Endocrinol 2014:841248
Mithal A, Bonjour JP, Boonen S, Burckhardt P, Degens H, El Hajj Fuleihan G, Josse R, Lips P, Morales Torres J, Rizzoli R et al (2013) Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int 24(5):1555–1566
Hamoui N, Anthone G, Crookes PF (2004) Calcium metabolism in the morbidly obese. Obes Surg 14:9–12
Olgaard K, Lewin E (2006) Can hyperparathyroid bone disease be arrested or reversed? Clin J Am Soc Nephrol 1:367–373
Acknowledgments
The authors thank Saint Charles Hospital and the participants for their contribution to the study.
Funding
This work did not receive any grant support.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: E.H.C. and W.S. equally contributed to the conception and design of the research; E.H.C. contributed in the conduct of the study and data collection; F.S. contributed in the statistical analysis of the data; E.H.C., F.S., C.J.M., B.Y., and W.S. contributed to the interpretation of the data; E.H.C., C.J.M., B.Y., and W.S. had primary responsibility for final content; and E.H.C. and W.S. drafted the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Hajj, C., Fares, S., Chardigny, J.M. et al. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: a randomized controlled trial. Arch Osteoporos 14, 4 (2019). https://doi.org/10.1007/s11657-018-0553-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-018-0553-2