Abstract
The origin of boron in boron-rich salt lakes in the Tibetan Plateau is highly controversial. In this study, we carried out a detailed study on boron geochemistry and isotope composition of lake sediments collected in Zigetang Co, central Tibet. Evaporites had high boron concentrations of 172.3–418.6 μg/g and δ11B values of −8.2‰ to −3.3‰, suggesting a non-marine origin for the saline lake. The boron isotopic fractionation factor, α, between evaporite and brackish water (αevaporite–brackish) decreased systematically with depth, from 0.9942 at the top of the drill core to 0.9893 at the bottom; the linear variation between αevaporite–brackish and depth reflects boron isotopic fractionation associated with progressive crystallization. The positive correlation between δ11B versus [B] and δ11B versus depth in the evaporite phase reflects pH and boron speciation in the solution control on the adsorption of boron, and B(OH)3 species incorporated preferentially into Mg(OH)2 precipitation at high pH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Elements in lake sediments can be used to investigate the contributions of different forcing mechanisms to changes in sedimentary environments. Boron’s highly soluble character and the large relative mass difference between 10B and 11B lead to significant isotopic fractionation (Wei et al. 2014). Hence, boron concentration and isotopic composition have been used as proxies for tracing mass transfer processes and reconstructing depositional environments (Chetelat et al. 2009). Experimental research of inorganic carbonate precipitation has been carried out to determine the dependence of boron isotopic composition of carbonate under different conditions (Xiao and Wang 2006). In this study, we explored evolution processes of Zigetang Co by studying boron isotopic composition of the evaporite phase in sediments collected from a drill core. This research will deepen understanding of the environmental evolution of salt lakes of the Tibetan Plateau.
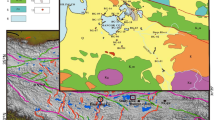
2 Geology of the study area
Zigetang Co (altitude 4561 m a.s.l.; in Tibetan, “Co” means “lake”) is in the central Tibetan Plateau, southern Tanggula Mountains, and belongs to Amdo County, Xizang Autonomous Region, China (Fig. 1). Zigetang Co is a meromictic saline endorheic lake with an area of 191.4 km2, formed along the Bangong Lake–Dongqiao–Nujiang River fault zone in the late Pliocene (Jin et al. 2016). No longer receiving glacial meltwater input, the water supply depends on seasonal inflows, precipitation, and springs at the southwestern edge of the lake. The average water depth is 19.2 m with a maximum of 38.9 m. Oxygen content at 15–22 m depleted rapidly to 0 at 22 m with pH values between 9.9 and 10.2 (measured with Yellow Spring Instrument 6600 V2 multisensor sonde in 2012).
3 Methods and results
Around 0.2 g of solid sediment was weighed and dissolved by the following procedure: sediment samples were placed in a 50-ml centrifuge tube and repeatedly soaked for 24 h with boron-free water (20 mL) until all the adsorbed boron had been removed (concentration of boron was less than 10 ng/g). The dissolved part from each step was collected as the evaporite phase. The minerals in the sediment samples were identified by X-ray diffraction and found to consist of mixed layers of aragonite and calcite with lesser amounts of quartz, illite, dolomite, and kaolinite. Boron isotopic composition of lake water samples varied from +2.3‰ to +2.9‰, with a mean value of +2.5‰. Samples from Zigetang Co sediments had relatively high boron concentrations from 172.3 to 418.6 μg/g; δ11B values varied from −3.3‰ to −8.2‰, indicating a non-marine origin for the saline lake.
4 Discussion
4.1 Boron isotopic fractionation occurred during crystallization of salts in evaporite phase
Boron contents and isotopic compositions varied systematically along the depth of the drill core in the evaporite phase: δ11B values decreased from −3.3‰ at the top to −8.2‰ at the bottom of the drill core at 8.9 m in depth. Similar trends have been observed in the sediments from DSDP Hole 477 in the Guaymas Basin (Spivack et al. 1987). Calculated isotopic fractionation factors between evaporite and brackish water (αevaporite–brackish) ranged from 0.9893 to 0.9942. Since boron isotopic fractionation was found to be controlled by speciation of boron in both the solution and mineralogy, and to vary with pH in the brine, the linear correlations of αevaporite–brackish values and depth should reflect the boron isotopic fractionation associated with progressive crystallization.
4.2 Isotopic composition of boron in lake sediments
Hobbs and Reardon (1999) suggested that the change in boron speciation could cause increased incorporation of boron at higher pH values. Palmer (1987) showed that the amount of adsorbed boron by sediment is determined by the activities of B(OH)3, B(OH) −4 , and OH−, and their affinity coefficients. Due to the low affinity coefficient of B(OH)3 at low pH, the amount of adsorbed boron is also lower at low pH, and increases with the increase of B(OH) −4 that comes with increasing pH (Keren et al. 1981). The amount of adsorbed boron increased rapidly due to the relatively strong affinity of clays for B(OH) −4 . However, boron adsorption decreased rapidly due to competition by OH− for the adsorption sites, since OH− concentration increases with increasing pH (Umeda et al. 1981). Zigetang Co is a typical saline lake dominated by Na+ and HCO3 −, with pH between 9.9 and 10.2. Mg precipitates may have low solubility as Mg(OH)2 at higher parent solution pH. Xiao (2006) proposed that during synthetic carbonate precipitation, the incorporation of B(OH)3 is attributed to the formation of Mg(OH)2 at the higher pH of a calcifying microenvironment. In this study, the boron concentration increased from 172.3 μg/g at the bottom layer to 418.6 μg/g at the top layer, and δ11B also increased, from −8.2‰ to −3.3‰. Linear correlations of δ11B versus [B] and δ11B versus depth were observed (Fig. 2). The increase of boron uptake into the Zigetang Co sediments is likely due to the coprecipitation of borate-boron with calcium carbonate or the adsorption of boron by Mg(OH)2 precipitation. Adsorption of B(OH)3 is accompanied by greater fractionation than is B(OH) −4 (e.g. αevaporite–brackish of 0.9893 in the bottom of the drill core and 0.9942 at the top), which is consistent with the formation of a tetrahedrally coordinated adsorbed species (Palmer et al. 1987). The activity of borate ions is important to the coprecipitation of borate-boron with calcium carbonate; the activity coefficient of B(OH)3 increases and that of B(OH) −4 decreases with increasing concentration of dissolved chemical species, as the coprecipitation of B(OH) −4 is dominant for aragonite and that of B(OH)3 for calcite. So, the amount of borate-boron coprecipitated with calcite increases with increasing salinity and pH, while it decreases with aragonite. Consequently, the δ11B of adsorbed boron is dependent on pH, and controlled by the speciation of boron—the different complexes formed at the solid surface—and by the chemical compositions of the brackish water.
5 Conclusions
Boron isotope geochemistry in evaporite sediments collected from Zigetang Co was investigated. The δ11B values and boron concentrations in the evaporite phase varied from −8.2‰ to −3.3‰, and 172.3–418.6 μg/g, respectively, indicating a non-marine origin of the saline lake. The geochemical information recorded in the evaporite phase of the sediments was interpreted as following: (1) Values of αevaporite–brackish decreased from 0.9942 to 0.9893 along the depth of the drill core. The linear correlations of the αevaporite–brackish values and depth suggest that boron isotopic fractionation occurred during progressive crystallization; and (2) The positive correlation between δ11B and [B] in the evaporite phase suggests pH and boron speciation in the solution control the adsorption of boron by clay, and the incorporation of B(OH)3 into Mg(OH)2 precipitation mainly depends on the pH of the solution.
References
Chetelat B, Liu CQ, Gaillardet J, Wang QL, Zhao ZQ, Liang CS, Xiao YK (2009) Boron isotopes geochemistry of the Changjiang basin rivers. Geochim Cosmochim Acta 73:6084–6097
Hobbs MY, Reardon EJ (1999) Effect of pH on boron coprecipitation by calcite: further evidence for nonequilibrium partitioning of trace elements. Geochim Cosmochim Acta 63:1013–1021
Jin CF, Nther FG, Li SJ, Jia GD, Peng PA, Gleixner G (2016) Reduced early Holocene moisture availability inferred from D values of sedimentary n-alkanes in Zigetang Co, Central Tibetan Plateau. Holocene 26:556
Keren R, Gast RG, Baryosef B (1981) pH-Dependent boron adsorption by Na-Montmorillonite. Soil Sci Soc Am J 45:45–48
Palmer MR, Spivack AJ, Edmond JM (1987) Temperature and pH controls over isotopic fractionation during adsorption of boron on marine clay. Geochim Cosmochim Acta 51:2319–2323
Spivack AJ, Palmer MR, Edmond JM (1987) The sedimentary cycle of the boron isotopes. Geochim Cosmochim Acta 51:1939–1949
Umeda M, Iwata K, Obut OT, Sennikov NV, Izokh NG, Ermikov VD (1981) Boron adsorption by clay minerals using a phenomenological equation. Clays Clay Miner 29:198–204
Wei HZ, Jiang SY, Tan HB, Zhang WJ, Li BK, Yang TL (2014) Boron isotope geochemistry of salt sediments from the Dongtai salt lake in Qaidam Basin: boron budget and sources. Chem Geol 380:74–83
Xiao YK, Wang L (2006) An unusual isotopic fractionation of boron in synthetic calcium carbonate precipitated from seawater and saline water. Sci China Ser B Chem 49:454–465
Acknowledgements
This work was jointly supported by the National Basic Research Program (973 project) of China (2013CB956401), and the National Natural Science Foundation of China (Grant Nos. 41210004, 41661144042).
Author information
Authors and Affiliations
Corresponding authors
Additional information
11th International Symposium on Geochemistry of the Earth’s Surface.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, C., Zhao, Z. et al. Boron isotope geochemistry of Zigetang Co saline lake sediments, Tibetan Plateau. Acta Geochim 36, 437–439 (2017). https://doi.org/10.1007/s11631-017-0185-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0185-z