Abstract
In this study, an efficient method for in vitro regeneration of Plumbago europaea was developed using direct and indirect organogenesis. Accordingly, micropropagation and regeneration were obtained on Murashige and Skoog (MS) medium supplemented with different concentrations and combinations of plant growth regulators. The effects of explant type and plant growth regulators on shoot organogenesis of P. europaea were evaluated. For the nodal explants, MS medium containing 0.5 mg/l TDZ (11.62 shoots per node) was the best medium for high frequency of micropropagation. In comparison, the highest percentage of direct organogenesis (70%) and number of shoots per explants (14.6) were acquired for the internode explants using 0.5 mg/l TDZ and 0.1 mg/l IAA. The obtained data revealed that TDZ is the most effective cytokinin for the direct shoot organogenesis. The highest indirect organogenesis rate was observed using 2 mg/l BA and 0.1 mg/l NAA for the internode explant. The maximum number of roots was distinguished on ½ MS medium containing 0.5 mg/l IBA (6.42). The rooted plantlets were gradually hardened and acclimatized under ex vitro conditions. As an important outcome, the active compound plumbagin was found mainly in the root tissues of the micro-propagated and regenerated plantlets. Taken all together, this study achieved a successful protocol for in vitro regeneration of P. europrea and could be considered for large-scale multiplication of this important medicinal plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plumbago europaea L. (Plumbaginaceae) is a valuable medicinal plant which is native to the Mediterranean region, Europe, north Africa, and south west Asia. It is the only species of the genus Plumbago growing wild in Iran (Rechinger and Schiman-Czeika 1974). Roots of P. europaea are known to have the capacity to produce large amounts of plumbagin as a bioactive naphthoquinone (Al-Nuri et al. 1994; Muhammad et al. 2009; Iwashina 2013). Plumbagin has been reported to possess antimicrobial, anticancer, antifertility, pesticidal, and antimalarial activities (Kubo et al. 1983; Likhitwitayawuid et al. 1998; Ding et al. 2005; Kuo et al. 2006; Nair et al. 2016).
Propagation of P. europaea by seeds is difficult due to poor seed quality, lower germination rate, and low seedling survival under natural field conditions (Chaplot et al. 2005). Plant cell, tissue, and organ cultures can be regarded as suitable alternative approaches to conserve many valuable plants and propagate sufficient amount of plants. In vitro propagation approaches, as asexual propagation methods, have some superiority over traditional techniques of propagation, including short production time, no dependence on season constraints, and production of disease-free plantlets. Furthermore, in vitro plant propagation requires low amounts of plant material with minor effect on wild populations (Nowakowska et al. 2020). Plants propagated via tissue culture are genetically identical to the original plant and are uniform in size, shape, and yield. Production of specific compounds/proteins using cell and tissue culture systems can be triggered by means of elicitors. Moreover, endangered or threatened species of plants can be cultivated for preservation and other purposes (Bhatia et al. 2015).
Plumbago species begin to produce restricted amounts of plumbagin between 2 and 6 yr of age (Kitanov and Pashankov 1994). Therefore, it is necessary to develop an efficient and improved protocol for production of sufficient yield of this bioactive secondary metabolite. Several studies have been conducted for callus induction, organogenesis, and regeneration of Plumbago species (Harikrishnan and Hariharan 1996; Selvakumar et al. 2001; Das and Rout 2002; Jose et al. 2007; Bhadra et al. 2009; Gopalakrishnan et al. 2009; Haque et al. 2012; Chatterjee and Ghosh 2015). In vitro propagation of P. rosea was previously attained through organogenesis from different types of explants such as leaves, shoot tips, axillary buds, and somatic embryos. However, no report is available on the in vitro propagation of P. europaea. Therefore, we aimed to identify the most responding explants as well as suitable plant growth regulators and their optimum concentrations for effective in vitro regeneration of P. europaea.
Cytokinins like BA, kinetin, zeatin, and TDZ may help the cultured tissues to produce adventitious shoots. BA and TDZ have been also used as growth regulator to induce shoots from the explants and for rapid in vitro regeneration (Moshtaghi 2020). Although shoot regeneration responses vary depending on the type of explant and cultivar, in most cases exposure of explants to cytokinins may promote shoot production (Panizza and Tognoni 1992; Ghorbani et al. 2021). TDZ, a synthetic phenylurea, possesses powerful cytokinin-like activity. It has been frequently used in the in vitro plant regeneration systems (Murthy et al. 1998; Pourebad et al. 2015). The purpose of this study was to determine an efficient and rapid shoot organogenesis protocol for P. europaea by investigating the effect of explant types and plant growth regulators.
Materials and methods
Plant material and culture condition
The seeds of P. europaea were obtained from Research Institute of Forests and Rangelands, Tabriz, Iran. The seeds were surface sterilized and cultured on the MS medium for germination. All the media used in this study were supplemented with 3% (w/v) sucrose and solidified with 0.7% (w/v) agar. The pH of media was adjusted to 5.8. All the cultures were incubated at 25°C and under a 16/8-h light/dark photoperiod in the growth chamber with the light intensity of 40 mmol m−2s−1 using cool white fluorescent lamps. Three-wk-old in vitro grown seedlings were used as a source of leaf, internode, and nodal explants for shoot organogenesis studies.
In this study, two experiments were designed for propagation purposes. In the first experiment, the effects of BA and TDZ on direct shoot organogenesis were evaluated using nodal explants of P. europaea. Nodal (contain auxiliary bud) explants were vertically cultured onto the full-strength MS medium containing the different concentrations of BA or TDZ (0.5, 1, and 2 mg/l). After 3 wk of culture, clusters of multiplied shoots in BA or TDZ containing media were transferred to basal solid MS medium without hormones to eliminate the effects of cytokinins on growth and development of the buds. After 2 wk of shoot elongation, shoots were transferred to root-inducing culture media.
In the second experiment, MS culture medium containing different concentrations and combinations of cytokinins (TDZ and BA) and auxins (NAA and IAA) was used for efficient plant regeneration from leaf and stem (internode) explants. In addition, a hormone-free medium was considered as control. Media were poured in sterilized culture vessels. The leaves and internodes from 3-wk-old plants were cut and cultured on solidified MS medium supplemented with different combinations of IAA, NAA, BA, and TDZ consisting of BA (0.5, 1, 2 mg/l), TDZ (0.5, 1, 2 mg/l), NAA (0, 0.05, 0.1 mg/l), and IAA (0.1, 0.5 mg/l). The explants were incubated under the same condition as for the first experiment. After callus or shoot induction, explants were transferred to MS media without hormones or supplemented with 0.5 or 1 mg/l BA for adventitious shoot induction.
Rooting and acclimatization
In order to develop root system, regenerated shoots were excised from the parent plants and transferred to the MS and 1/2 MS media containing different concentrations of NAA or IBA. In most of the treatments, root induction was observed after 7 to 10 d. After root induction, the explants were transferred to the hormone-free media. The frequency and the average number of roots in each treatment were recorded after 4 wk of culture.
The in vitro rooted and healthy plants were separated from rooting medium and their roots were washed to remove adhering gel. They were then transplanted to plastic pots containing garden soil and sand at a ratio of 3:1 and covered with the bottle. Plants were covered with transparent plastic bags to ensure high humidity. They were kept in a culture room for 2 wk, and then transferred to the shaded greenhouse until achievement of the appropriate growth. Their survival rate was measured for a period of 4 wk in greenhouse conditions. Each experiment was conducted in three repetitions. The obtained data were analyzed statistically using Duncan’s multiple range test.
Quantitative determination of plumbagin
In order to examine the ability of roots and aerial parts of micro-propagated and regenerated plants to produce plumbagin, dried samples were ground to fine powder using a mortar and pestle. Each sample (1 g) was extracted three times with 10 ml of chloroform for 3 d. After passage through a filter paper, the solvent evaporated by a rotary evaporator. The residue was dissolved in 5 ml of methanol and 50 μl of methanolic extract of sample was injected into C8 analytical column (250 mm * 4.6 mm, particle size 5 μm; PerfectSil, MZ-Analysentechnik, Mainz, Germany). A Breeze HPLC system from Waters Corporation (Milford, MA) was used at a wavelength of 270 nm. The mobile phase was a mixture of methanol:water (80:20) and run at the isocratic condition with a flow rate of 0.9 ml min−1. Quantitative analysis of plumbagin was carried out based on the peak area of the sample and the known concentrations of standard. The area under the peaks of plumbagin was integrated and converted to concentration using its calibration curve.
Results and discussion
Tissue culture and micropropagation using nodal explants
In the first experiment, nodal explants were cultured on the MS medium supplemented with different concentrations of BA or TDZ. One of the most widely used strategies for micropropagation is axillary bud proliferation, upon which the nodal segments harboring axillary buds are cultured to regenerate the shoots (Nowakowska et al. 2020). Organized meristems such as shoot tips and axillary buds are less prone to spontaneous genetic changes because meristems are more resistant to genetic changes than disorganized tissues (Krishna et al. 2016).
A rapid in vitro propagation using nodal explants was observed in the MS medium containing 0.5 mg/l TDZ with the highest percentage of micropropagation rate (87%) and maximum number of shoots (11.62 shoots per node) (Fig. 1). The nodal explants directly produced multiple shoot buds and developed new plantlets. The medium containing TDZ was more efficient for micropropagation than the medium supplemented with BA (Figs. 1 and 2). Based on previous reports, TDZ was proved to be superior for micropropagation of Cardiospermum halicacabum and P. zeylanica (Jahan and Anis 2009; Ceasar et al. 2013). Micropropagation was not observed on the control medium (without growth regulator), which confirmed the significance of cytokinins in the stimulation of the cell division and organogenesis.
Applying the exogenous cytokinins and removing the apical meristem promote apical dominance reduction, lateral bud break, and adventitious shoot induction in plant tissue culture (Banthorpe et al. 1986; Panizza and Tognoni 1992; Babaei et al. 2014).
Choosing the optimal concentration and type of plant growth regulator is a critical step for efficient regeneration. In fact, TDZ has been established as a substituted phenyl urea-type cytokinin and an important regulator for morphogenic responses, inducing in vitro adventitious bud and shoot organogenesis (Jiang et al. 2005; Yucesan et al. 2007; Sajid and Aftab 2009). It has been confirmed that the impact of TDZ concentrations can be effectively dependent on the plant species, explant status, and culture condition (Guo et al. 2011). Shoot regeneration using TDZ was studied in some species of Plumbago (Patidar et al. 2013; Sharma and Agrawal 2018). In some cases, the application of TDZ showed negative effects on shoot development and resulted in glassy structure production, stem elongation, and morphology abnormalities (Huetteman and Preece 1993). In the current study, to prevent the adverse effects of long-term exposure to TDZ, explants were transferred onto the TDZ-free MS medium for proliferation, development, and the elongation of the induced shoots. The efficient role of TDZ on micropropagation has been shown for plant species such as rose and Pterocarpus marsupium (Barna and Wakhlu 1995; Hussain et al. 2007).
Micropropagation through axillary bud proliferation has proven to be a handy tool for the conservation of valuable medicinal plant resources (Pierik 1997; Malathy and Pai 1998;Selvakumar et al. 2001; Najaf-Abadi and Hamidoghli 2009; Corral et al. 2011; Nowakowska et al. 2020). Although the technique of nodal micropropagation has been reported previously for some species of Plumbago such as P. indica (Gradner 1991) and P. zeylanica (Selvakumar et al. 2001; Chandravanshi et al. 2014), such a procedure has so far not been used for P. europaea. In this paper, we present, for the first time, a rapid protocol for the in vitro propagation of this plant through axillary bud culture.
Organogenesis and regeneration using leaf and internode explant
The results of the second experiment showed that among the 34 hormonal treatments, organogenesis occurred by using 11 and 20 treatments for leaf and internode explants, respectively. No callus induction and regeneration were observed in the control explants. The obtained results revealed that optimal concentration and combination of both cytokinin and auxin had an essential role in the regeneration of shoots from leaf and internode explants of P. europaea.
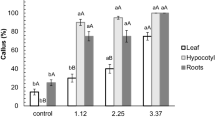
Using NAA + BA and IAA + BA combinations, callus production was established in both types of explants (Fig. 3). The shoots were induced indirectly via callus from internode and leaf segments of plants (Fig. 4). The highest indirect organogenesis rate was observed using 2 mg/l BA and 0.1 mg/l NAA for the internode explant (Figs. 5 and 6).
By application of TDZ + NAA and TDZ + IAA, direct shoot organogenesis occurred (Fig. 7). The highest percentage of direct shoot organogenesis (14.6 seedlings from each explant) was attained by 0.5 mg/l TDZ and 0.1 mg/l IAA in the internode explants (Figs. 5 and 6). TDZ in combination with NAA or IAA displayed the best impact on direct shoot organogenesis from the leaf and internode of P. europaea.
Direct shoot organogenesis process of P. europaea. (a–c) Induction of buds and shoots from internode explants on MS medium containing 0.5 mg/l TDZ and 0.1 mg/l IAA. (d, e) Induction of buds and shoots from leaf explants on MS medium containing 0.5 mg/l TDZ and 0.1 mg/l IAA. (f–h) Transfer to the hormone-free MS medium.
Based on the obtained results, significant effects of explant type and plant growth regulator on callus induction and organogenesis of P. europaea were confirmed (Figs. 3 and 5). These data were consistent with the former reports showing that culture media containing TDZ in combination with IAA exhibited the highest efficiency for the direct organogenesis in different plant species (Kasula et al. 2008; Nazari et al. 2016). Cytokinins are the most important growth regulators for plant regeneration (Song et al. 2011). The type of cytokinin could be regarded as one of the most important factors affecting the organogenesis of shoots. The mechanism of TDZ action has not yet been fully elucidated. Based on a suggestion, TDZ may induce organogenesis by direct stimulation of tissue. In contrast, the second conception expresses that TDZ stimulates intrinsic cytokinins and thereby facilitates the organogenesis of shoot (Huetteman and Preece 1993).
Other important factors including plant genotypes, type of explant, growth regulator combination, and medium composition play important roles in the efficiency of in vitro organogenesis (Tomsone and Gertnere 2003). Our results confirmed the preceding reports indicating the significant role of plant growth regulators and type of explants on regeneration of shoots (Asghari et al. 2012). Maximum organogenesis rate was observed in stem explants, and therefore stem explants were more efficient than leaf explants for callus formation and, shoot organogenesis (Figs. 5 and 6). The direct shoot organogenesis of P. zeylanica was studied and the outcomes confirmed that leaves were the best explants for the organogenesis (Rout et al. 1999). These results were in fair agreement with the findings of previous reports (Türker et al. 2010; Biswas et al. 2012). It was also observed that leaf explants showed the lower callus induction in Solanum tuberosum compared to the stem explants (Kumlay and Ercisli 2015). The diverse responses of the explant types may be due to the differences in the levels of the endogenous hormones, in the meristematic activity of the cells, in the physiological condition of explants, and in the expression levels of genes encoding hormone receptors (Close and Gallagher-Ludeman 1989; Kaul et al. 1990; Sujatha and Mukta 1996; Kaewpoo and Te-chato 2009). Even in a precise genotype, plant growth regulators may exhibit different effects based on the genotype of cells (Annadana et al. 2000).
Rooting and acclimatization
Regenerated shoots (4–5 cm) were transferred onto the half- and full-strength MS medium containing NAA and IBA. Consequently, maximum number of root per shoot (6.3±0.4) occurred in 1/2 MS medium containing 0.5 mg/l IBA (Figs. 8 and 9). IBA is a common plant growth regulator suggested for root induction specially in low concentrations in many plants such as bamboo (Alagumanian et al. 2004), Jatropha curcas (Singh et al. 2010), Cannabis sativa (Lata et al. 2010), and Stevia rebaudiana (Alhady 2011). These data were also in line with the former finding, which showed a better rooting response on 1/2 MS than MS medium for P. zeylanica (Ceasar et al. 2013). The increase in root number and length is very important for the acclimatization to ex vitro conditions, as well as for the water and nutrient uptake of plantlets (Sanavy and Moeini 2003). The hardening of plantlets was important for the transplantation of plantlets to the field. In our study, plantlets were transferred into pots covered by plastic for 2 wk to maintain 100% relative humidity. After 2 wk, the humidity was gradually decreased by opening and finally removing the bags. After 1 mo, the hardened plantlets were field-transferred successfully with 100% survival rate (Fig. 9). For maintenance of root function in free conditions, the environment of root has been changed gradually.
Capacity of the regenerated plantlets for plumbagin biosynthesis
The results of the HPLC analysis showed that the regenerated and micro-propagated plantlets were able to produce plumbagin in their roots (1.54 and 1.92 mg g−1 dry weight, respectively), which was considerably higher than the amount in their aerial parts (0.22 and 0.15 mg g−1 dry weight) (Table 1). Of note, the differences between the plumbagin quantities for same organs of regenerated and micro-propagated plantlets were not statistically significant. Similarly, the roots of wild plants contained higher plumbagin content (1.78 mg g−1 dry weight) than the aerial parts (0.10 mg g−1 dry weight). Although the plumbagin content of roots in both wild and propagated plants showed no significant difference (Table 1), the time period required for the in vitro plant propagation was much shorter than for wild plants. Besides, using this technique, the dependence of plants to nature and the mass destruction of natural resources will be reduced. However, further research is needed to improve massive in vitro platelet production. In our previous works, we found high plumbagin content in hairy roots (3.3 mg g−1 dry weight) and in suspension cell culture (0.9 mg g−1 dry weight) (Beigmohamadi et al. 2019; 2020). Nevertheless, the advantage of the in vitro propagation to hairy root culture is the faster and higher biomass production (fresh weight and dry weight). Thus, the current work presenting the remarkable production of plumbagin beside the micropropagation, shoot organogenesis, and regeneration of P. europaea by tissue culture techniques may serve as a basis for further in vitro researches.
Conclusion
Our study explains the role of TDZ for shoot organogenesis of P. europaea which could be useful for large-scale plant multiplication and conservation. We found three key factors for enhancing successful regeneration: (1) source of explants, (2) suitable combination and concentration of growth regulators, and (3) culture conditions. The most effective methods for rapid proliferation of plants in tissue culture are direct regeneration and micropropagation, which saves time and stability of genetics and reduces the somaclonal diversity. It could be used also for genetic transformation and breeding through a biotechnological approach. Our results revealed that roots of in vitro propagated plants of P. europaea are able to produce remarkable quantities of plumbagin. Conclusively, this outcome established the potential of the regenerated plants of P. europaea for the large-scale commercial production of plumbagin and its derivatives using the tissue and organ cultures.
Change history
23 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11627-021-10238-5
References
Al-Nuri MA, Hannoun MA, Zatar NA, Abu-Eid MA, Al-Jondi WJ, Hussein AI, Ali-Shtayeh MS (1994) Plumbagin, a naturally occurring naphthoquinone: its isolation, spectrophotometric determination in roots, stems, and leaves in Plumbago europaea L. Spectrosc lett 27:409–416
Alagumanian S, Perumal VS, Balachandar R, Rameshkannan K, Rao M (2004) Plant regeneration from leaf and stem explants of Solanum trilobatum L. Curr Sci 86:1478–1480
Alhady M (2011) Micropropagation of Stevia rebaudiana Bertoni. A new sweetening crop in Egypt. Glob J Biotechnol Biochem 6:178–182
Annadana S, Rademaker W, Ramanna M, Udayakumar M, De Jong J (2000) Response of stem explants to screening and explant source as a basis for methodical advancing of regeneration protocols for chrysanthemum. Plant Cell Tissue Organ Cult 62:47
Asghari F, Hossieni B, Hassani A, Shirzad H (2012) Effect of explants source and different hormonal combinations on direct regeneration of basil plants (Ocimum basilicum L.). Aust J Agric Eng 3:12–17
Babaei N, Abdullah P, Ashikin N, Saleh G, Lee Abdullah T (2014) An efficient in vitro plantlet regeneration from shoot tip cultures of Curculigo latifolia, a medicinal plant. Sci World J 2014:1–9
Banthorpe DV, Branch SA, Njar VC, Osborne MG, Watson DG (1986) Ability of plant callus cultures to synthesize and accumulate lower terpenoids. Phytochemistry 25:629–636
Barna K, Wakhlu A (1995) Effects of thidiazuron on micropropagation of rose. In Vitro Cell Dev Biol Plant 31:44–46
Beigmohamadi M, Movafeghi A, Jafari S, Sharafi A (2020) Potential of the genetically transformed root cultures of Plumbago europaea for biomass and plumbagin production. Biotechnol Prog 36:1–6
Beigmohamadi M, Movafeghi A, Sharafi A, Jafari S, Danafar H (2019) Cell suspension culture of Plumbago europaea L. towards production of plumbagin. Iran J biotechnol 17:46–54
Bhadra S, Akhter T, Hossain M (2009) In vitro micropropagation of Plumbago indica L. through induction of direct and indirect organogenesis. Plant Tissue Cult Biotechnol 19:169–175
Bhatia S, Sharma K, Dahiya R, Bera T (2015) Modern applications of plant biotechnology in pharmaceutical sciences, 1st edn. Academic Press, Cambridge, Massachusetts
Biswas KK, Mohri T, Kogawara S, Hase Y, Oono Y (2012) An improved system for shoot regeneration from stem explants of Lombardy poplar (Populus nigra L. var. italica Koehne). Am J Plant Sci 3:1181–1186
Ceasar SA, Ayyanar M, Ignacimuthu S (2013) An improved micropropagation protocol for Plumbago zeylanica L. An important medicinal plant. Asian J Biol Sci 6:214–220
Chandravanshi M, Sahu Y, Agrawal A, Raja W (2014) In vitro micropropagation of important commercial medicinal plant: Plumbago zeylanica. Adv Biol Res 8:139–142
Chaplot B, Vadawale A, Jhala J, Barve D (2005) Clonal propagation of value added medicinal plant-Safed musli Chlorophytum borivilianum). Recent Progress in Medicinal Plants, Govil JN and Singh VK (Eds.), Studium Press, LLC: Texas, USA, pp. 383-388
Chatterjee T, Ghosh B (2015) Simple protocol for micropropagation and in vitro conservation of Plumbago zeylanica L.: an important indigenous medicinal plant. Int J Bio-resour Stress Manag 6:68–75
Close K, Gallagher-Ludeman L (1989) Structure-activity relationships of auxin-like plant growth regulators and genetic influences on the culture induction response in maize (Zea mays L.). Plant Sci 61:245–252
Corral P, Mallón R, Rodríguez-Oubiña J, González ML (2011) Multiple shoot induction and plant regeneration of the endangered species Crepis novoana. Plant Cell Tissue Organ Cult 105:211–217
Das G, Rout G (2002) Plant regeneration through somatic embryogenesis in leaf derived callus of Plumbago rosea. Biol Plant 45:299–302
Ding Y, Chen ZJ, Liu S, Che D, Vetter M, Chang CH (2005) Inhibition of Nox-4 activity by plumbagin, a plant-derived bioactive naphthoquinone. J Pharm Pharmacol 57:111–116
Gopalakrishnan M, Janarthananm B, Sai GL, Sekar T (2009) Plant regeneration from leaf explants of Plumbago. Plant Tissue Cult Biotechnol 19:79–87
Gradner U (1991) Plumbago indica, choice of growth media, fertilization and in vitro propagation. Deutscher Gartenbau, Germany FR
Guo B, Abbasi BH, Zeb A, Xu L, Wei Y (2011) Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotechnol 10:8984–9000
Ghorbani S, Kosari-Nasab M, Mahjouri S, Talebpour AH, Movafeghi A, Maggi F (2021) Enhancement of in vitro production of volatile organic compounds by shoot differentiation in Artemisia spicigera. Plants 10:208
Haque F, Hassan AS, Jahan MAA, Roy SK (2012) In vitro shoot proliferation and plant regeneration of Plumbago indica L. (Ractochita), a rare medicinal shrub of Bangladesh. Bangladesh J Sci Ind Res 47:197–202
Harikrishnan K, Hariharan M (1996) Direct shoot regeneration from nodal explants of Plumbago rosea Linn. - a medicinal plant. Phytomorphology 46:53–58
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Hussain H, Krohn K, Ahmad VU, Miana GA, Green IR (2007) Lapachol: an overview. Arkivoc 2007:145
Iwashina T (2013) Flavonoid properties of five families newly incorporated into the order Caryophyllales. Bull Natl Mus Nat Sci 39:25–51
Jahan AA, Anis M (2009) In vitro rapid multiplication and propagation of Cardiospermum halicacabum L. through axillary bud culture. Acta Physiol Plant 31:133–138
Jiang B, Yang Y-G, Guo Y-M, Guo Z-C, Chen Y-Z (2005) Thidiazuron-induced in vitro shoot organogenesis of the medicinal plant Arnebia euchroma (Royle) Johnst. In Vitro Cell Dev Biol Plant 41:677–681
Jose B, Satheeshkumar K, Seeni S (2007) A protocol for high frequency regeneration through nodal explant cultures and ex vitro rooting of Plumbago rosea L. Pak J Biol Sci 10:349–355
Kaewpoo M, Te-chato S (2009) Influence of explant types and plant growth regulators on multiple shoot formation from Jatropha curcas. Sci Asia 35:353–357
Kasula K, Prasad S, Umate P, Gadidasu K, Abbagani S (2008) Efficient TDZ and IAA-assisted plant regeneration from cotyledon and leaf explants of Capsicum annuum L. - one-step protocol for shoot bud differentiation and elongation. Int J Plant Dev Biol 2:114–117
Kaul V, Miller RM, Hutchinson JF, Richards D (1990) Shoot regeneration from stem and leaf explants of Dendranthema grandiflora Tzvelev (syn. Chrysanthemum morifolium Ramat.). Plant Cell Tissue Organ Cult 21:21–30
Kitanov G, Pashankov P (1994) Quantitative investigation on the dynamics of plumbagin in Plumbago europaea L. roots and herb by HPLC. Pharmazie 49:1–6
Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK (2016) Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 6:54
Kubo I, Uchida M, Klocke JA (1983) An insect ecdysis inhibitor from the African medicinal plant, Plumbago capensis (Plumbaginaceae); a naturally occurring chitin synthetase inhibitor. Agric Biol Chem 47:911–913
Kumlay AM, Ercisli S (2015) Callus induction, shoot proliferation and root regeneration of potato (Solanum tuberosum L.) stem node and leaf explants under long-day conditions. Biotechnol Biotechnol Equip 29:1075–1084
Kuo P-L, Hsu Y-L, Cho C-Y (2006) Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther 5:3209–3221
Lata H, Chandra S, Khan IA, ElSohly MA (2010) High frequency plant regeneration from leaf derived callus of high Δ9-tetrahydrocannabinol yielding Cannabis sativa L. Planta Med 76:1629–1633
Likhitwitayawuid K, Kaewamatawong R, Ruangrungsi N, Krungkrai J (1998) Antimalarial naphthoquinones from Nepenthes thorelii. Planta Med 64:237–241
Malathy S, Pai J (1998) Micropropagation of Ixora singaporensis (Linn.): An ornamental shrub. Curr Sci 75:545–547
Moshtaghi N (2020) Tissue and cell culture of saffron. Elsevier, Saffron, pp 229–246
Muhammad HM, Saour KY, Naqishbandi AM (2009) Quantitative and qualitative analysis of plumbagin in the leaf and root of Plumbago europaea growing naturally in Kurdistan by HPLC. Iraqi J Pharm Sci 18:54–59
Murthy B, Murch S, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant 34:267–275
Nair SV, Baranwal G, Chatterjee M, Sachu A, Vasudevan AK, Bose C (2016) Antimicrobial activity of plumbagin, a naturally occurring naphthoquinone from Plumbago rosea, against Staphylococcus aureus and Candida albicans. Int J Med Microbiol 306:237–248
Najaf-Abadi AJ, Hamidoghli Y (2009) Micropropagation of thornless trailing blackberry (‘Rubus sp’.) by axillary bud explants. Aust J Crop Sci 3:191–194
Nazari F, Khosh-Khui M, Azadi P (2016) A simple and efficient direct shoot organogenesis method using leafy petiole explants in Gerbera jamesonii ‘royal soft pink.’ Int J Hortic Sci Technol 3:51–58
Nowakowska M, Pavlović Ž, Nowicki M, Boggess SL, Trigiano RN (2020) In vitro propagation of an endangered Helianthus verticillatus by axillary bud proliferation. Plants 9:712
Panizza M, Tognoni F (1992) Micropropagation of lavandin (Lavandula officinalis Chaix× Lavandula latifolia Villars cv. Grosso). High-Tech and Micropropagation III. Springer, pp 295-305.
Patidar S, Tripathi M, Tiwari G, Chundawat R, Pandey A, Patidar H, Pandey G (2013) In vitro micropropagation of Plumbago zeylanica Linn. through nodal segment and leaf explants. Plant Cell Biotech Mol Biol 14:72–83
Pierik RLM (1997) In vitro culture of higher plants. Springer science & business media
Pourebad N, Motafakkerazad R, Kosari-Nasab M, Akhtar NF, Movafeghi A (2015) The influence of TDZ concentrations on in vitro growth and production of secondary metabolites by the shoot and callus culture of Lallemantia iberica. Plant Cell Tissue and Organ Cult 122:331–339
Rechinger KH, Schiman-Czeika H (1974) Plumbaginaceae. In: Rechinger KH (ed), Flora Iranica, No. 108, Akademische Druck und Verlagsanstalt, Graz, pp 2-3
Rout G, Saxena C, Samantaray S, Das P (1999) Rapid plant regeneration from callus cultures of Plumbago zeylanica. Plant Cell Tissue Organ Cult 56:47–51
Sajid ZA, Aftab F (2009) Effect of thidiazuron (TDZ) on in vitro micropropagation of Solanum tuberosum L. cvs. Desiree and Cardinal. Pak J Bot 41:1811–1815
Sanavy S, Moeini MJ (2003) Effects of different hormone combinations and planting beds on growth of single nodes and plantlets resulted from potato meristem culture. Plant Tissue Cult 13:145–150
Selvakumar V, Anbudurai P, Balakumar T (2001) In vitro propagation of the medicinal plant Plumbago zeylanica L. through nodal explants. In Vitro Cell Dev Biol Plant 37:280–284
Sharma U, Agrawal V (2018) In vitro shoot regeneration and enhanced synthesis of plumbagin in root callus of Plumbago zeylanica L.—an important medicinal herb. In Vitro Cell Dev Biol Plant 54:423–435
Singh A, Reddy MP, Chikara J, Singh S (2010) A simple regeneration protocol from stem explants of Jatropha curcas—a biodiesel plant. Ind Crop Prods 31:209–213
Song JY, Mattson NS, Jeong BR (2011) Efficiency of shoot regeneration from leaf, stem, petiole and petal explants of six cultivars of Chrysanthemum morifolium. Plant Cell Tissue Organ Cult 107:295
Sujatha M, Mukta N (1996) Morphogenesis and plant regeneration from tissue cultures of Jatropha curcas. Plant Cell Tissue Organ Cult 44:135–141
Tomsone S, Gertnere D (2003) In vitro shoot regeneration from flower and leaf explants in Rhododendron. Biol Plant 46:463–465
Türker AU, Yücesan B, Gürel E (2010) Adventitious shoot regeneration from stem internode explants of Verbena officinalis L., a medicinal plant. Turk J Biol 34:297–304
Yucesan B, Turker AU, Gurel E (2007) TDZ-induced high frequency plant regeneration through multiple shoot formation in witloof chicory (Cichorium intybus L.). Plant Cell Tissue Organ Cult 91:243–250
Funding
This work was supported by the Zanjan University of Medical Sciences, Zanjan, Iran (grant number: A-12-848-5) and the University of Tabriz, Tabriz, Iran. All authors have agreed to the order of authorship for this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
The original online version of this article was revised: The name of coauthor Ali Movafeghi was presented incorrectly (as “Movafeghi Ali”) in this article as originally published.
Rights and permissions
About this article
Cite this article
Beigmohamadi, M., Movafeghi, A., Jafari, S. et al. Efficient in vitro organogenesis, micropropagation, and plumbagin production in Plumbago europaea L.. In Vitro Cell.Dev.Biol.-Plant 57, 820–830 (2021). https://doi.org/10.1007/s11627-021-10224-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-021-10224-x