Abstract
The somatic embryogenesis receptor kinase (SERK) gene has been extensively studied in many plant species due to its role in conferring embryogenic competence to somatic cells. The oil palm (Elaeis guineensis Jacq.) full-length SERK I (EgSERK I) cDNA was first isolated from cell suspension culture using RACE-PCR. Total length of EgSERK I cDNA was 2378 bp in length with a 5’UTR region (358 bp) longer than 3’UTR region (130 bp) and the ORF was 1890 bp (629aa). The deduced amino acid sequence of EgSERK I contained protein domains commonly present in reported SERK proteins, including the hallmark proline-rich region and C-terminal domains. EgSERK I was most highly expressed in leaf explants and also detected in all tested tissues, including vegetative tissues, reproductive tissues, embryogenic tissues, and non-embryogenic tissues, suggesting that it may have a broad role in plant growth and development. Expression of EgSERK I in leaf explant was upregulated by minimal auxin concentration at the initial 6 h of incubation in callus induction media. EgSERK I mRNA was detected in the adjacent cells of the vascular tissues in the midvein region of leaf explants which serves as the callus initiation point of callogenesis in oil palm. Collectively, our findings suggest that the EgSERK I gene is involved in the callus initiation stage of oil palm somatic embryogenesis by transducing the signal to switch on the dedifferentiation process, triggering cellular reprogramming to form callus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil palm (Elaeis guineensis Jacq.) is a monocotyledonous plant of the palm family Arecaceae, originating from West Africa (Corley and Tinker 2003). It is a perennial crop with a 25-yr life cycle that produces fresh fruit bunches throughout the year. Oil palm is regarded as the most effective oil yielding crop, which is 11 times more effective than soybean as it occupies the least world farm land (0.23%) compared to soybean (2.14%). Thus, oil palm is a species of particular economic importance and is a major cash crop. Palm oil is accounted for 33% of total edible oil production worldwide (Basiron 2011). The demand for palm oil is expected to rise further due to its potential to produce biodiesel and the increasing consumption from the growing world population. Hence, cultivating high-yielding palms with desired traits is a pre-requisite to improve the productivity, utilization, and quality of palm oil. Micropropagation of oil palm via somatic embryogenesis offers an attractive approach to produce uniform planting materials with desired characteristics through the cloning of elite and true-to-type palms (Paranjothy and Othman 1982; Muniran et al. 2008). The demand for clonal plantlets is expected to increase due to its high-yielding performance of at least 25% more oil than commercial seedlings (Tan et al. 2003). However, large-scale in vitro clonal propagation of oil palm is still problematic as somatic embryogenesis in oil palm is still inconsistent, unpredictable, and genotype-dependent (De Touchet et al. 1991; Duval et al. 1995). These obstacles have become a major bottleneck in the current oil palm tissue culture technique.
Somatic embryogenesis is the formation of an embryo from somatic cell(s) that have undergone re-differentiation upon acquisition of embryogenic competency, which will eventually give rise to whole plants under favorable experimental conditions (Namasivayam 2007). The acquisition of embryogenic competence by somatic cells and the development of somatic embryos require the reprogramming of gene expression patterns due to chromatin remodeling and methylation changes (Fehér 2005). Efforts in unraveling the mechanisms of gene regulation during this developmental process have resulted in the discovery of an array of genes that are activated or differentially expressed during somatic embryogenesis (Chugh and Khurana 2002). Among the genes that have been isolated, the Somatic Embryogenesis Receptor Kinase gene, also known as SERK, was deemed to confer embryogenic competency to somatic cells (Schmidt et al. 1997; Hecht et al. 2001).
SERK encodes a leucine-rich repeat containing receptor-like kinase (LRR-RLK) protein. This protein plays an important role in the transduction of extracellular signals by phosphorylating intracellular target proteins during somatic embryogenesis (Schmidt et al. 1997). SERK genes are highly conserved among different species. They share the same intron/exon structure that contains 10–11 exons coding for the domains found in the SERK protein (Schmidt et al. 1997; Hecht et al. 2001). Extensive studies have been carried out in characterizing SERK genes’ expression from different plants since the discovery of the first SERK gene from Daucus carota (DcSERK) as a potential marker to determine embryogenic competence in somatic cells (Schmidt et al. 1997). While similar observations were reported in Dactylis glomerata (Somleva et al. 2000), Arabidopsis thaliana (Hecht et al. 2001), and Musa acuminata (Huang et al. 2010), SERK genes’ expression of other species demonstrated a broader expression pattern extending to vegetative tissues, reproductive tissues, and non-embryogenic tissues. For instance, transcripts of both ZmSERK I and 2 of Zea mays were expressed in embryogenic and non-embryogenic calluses but absent in somatic embryos (Baudino et al. 2001). TnSERK of Trifolium nigrescens was expressed significantly higher in embryogenic culture compared to non-regenerative cultures (Pilarska et al. 2016) while TcSERK of Theobroma cacao expressed in both mature somatic and zygotic embryos (Santos et al. 2005). Other SERK genes were only expressed up to globular stage embryos in D. carota (Schmidt et al. 1997) and D. glomerata (Somleva et al. 2000); up to the heart stage in A. thaliana (Hecht et al. 2001); up to the scutellar stage in Brachypodium distachyon (Oliveira et al. 2017). In Citrus unshiu (Shimada et al. 2005) and Rosa hybrida (Zakizadeh et al. 2010), SERK genes were detected throughout somatic embryogenesis until the plantlet stage, showing a broader expression profile with relatively uniform expression in all plant tissues including flower, stem, leaf, and fruit.
Addition of exogenous auxin especially 2, 4-dichlorophenoxyacetic acid (2-4D) and naphthaleneacetic acid (NAA) to the media is reported to elevate the expression of SERK genes substantially. This phenomenon was observed in D. carota (Schmidt et al. 1997), A. thaliana (Hecht et al. 2001), and Citrus sinensis (Ge et al. 2010), suggesting that auxin might be a factor regulating the expression of SERK genes. This finding correlates with SERK’s role as a potential biomarker for the acquisition of embryogenic competence since auxin is usually used as a potent inducer of the embryogenic response that inhibits cell elongation but enhances cell division during somatic embryogenesis (Campanoni and Nick 2005). In addition to that, SERK genes also have been reported to play a role in brassinosteroid signaling (Santiago et al. 2013; Imkampe et al. 2017), defense signaling transduction (Huang et al. 2010), and most recently reported to control anther cell fate determination (Li et al. 2017b). These data collectively provide some evidence that the expression of SERK is related to the induction of embryogenesis while may play a broader and unknown role in plant growth and development.
The above findings have propelled this study to isolate the first SERK gene orthologue of oil palm (EgSERK I), and to further unravel SERK’s function during oil palm somatic embryogenesis. As such, we report here the cloning and characterization of EgSERK I cDNA as well as the effects of exogenous auxin on EgSERK I expression in an in vitro system.
Materials and methods
Plant materials
Six-month-old oil palm cell suspension culture (CSC) derived from line 3196 (dura x pisifera) was purchased from Felda Agricultural Services Sdn. Bhd, Kuala Lumpur, Malaysia, and used for the isolation of EgSERK I cDNA. Samples used for expression analysis using real-time PCR were of the same genotype (dura x pisifera) obtained from different sources as detailed below. For tissue-specific expression analysis, leaf explant (LE), white embryoid (WE), green embryoid (GE), globular, torpedo, haustorium, germinating embryo, female flower (FF), and male flower (MF) were provided by the Malaysian Palm Oil Board, Selangor, Malaysia (MPOB). Root (R), meristems (M), and mature leaves (ML) were harvested from 2-mo-old oil palm (dura x pisifera) seedlings obtained from Sime Darby Plantation Sdn. Bhd., Petaling Jaya, Malaysia. Embryogenic callus (EC) and non-embryogenic callus (NEC) derived from leaf explant (dura x pisifera) were kindly provided by Applied Agricultural Resources Sdn. Bhd., Petaling Jaya, Malaysia. Embryogenic callus are usually spherical nodular or friable-like which are identified morphologically for further subculture onto embryo development media. The non-embryogenic callus is selected based on its translucent and slimy morphology at the time of sampling. Samples used in the study of exogenous auxin effects and expression of EgSERK I are detailed as below.
Auxin treatment on leaf explant
Young leaves or spear leaves contained within a cylinder of older leaf petioles or leaf cabbage in the center of the palm canopy of two different oil palm ortets (dura x pisifera) that were obtained from Terengganu, Malaysia, were used as explants for tissue culture in the auxin treatment. The cabbage was cut more than 10 cm above the meristem with an approximate bulge length of 40–70 cm depending on the ortet age (Rohani et al. 2003). The leaf spears are then cut into three or four segments of 9 ± 1 cm long, which are referred to as zones. Zone 1 is the nearest to the spear base and zone 2 is at 9 ± 1 cm apart (Rohani et al. 2003). In this study, zone 1 and zone 2 leaf explants were cultured on MS medium supplemented with different concentrations of auxin, denoted as A, 10 μgL−1 2, 4-dichlorophenoxyacetic acid (2-4D) and 1 mgL−1 naphthaleneacetic acid (NAA); B, 10 μgL−1 2-4D and 10 mgL−1 NAA; and C, 10 μgL−1 2-4D and 50 mgL−1 NAA for 1 wk. The expression level of EgSERK I was assayed at different times, at 0 h (To), 6 h (6h), 2 d (2d), and at 7 d (7d) after auxin treatment.
Cloning of the full-length EgSERK I cDNA
An expressed sequence tag (EST), EgZE011B02, derived from an oil palm zygotic embryo cDNA library (provided by MPOB) was analyzed using the BLAST tool to UniProt (http://www.uniprot.org) and conserved domain search (CDS) tool from the National Center of Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov). Two specific primers (TSP- forward and TSP- reverse; Table 1) that corresponded to the conserved protein kinase domain were used for the cloning of EgSERK I cDNA. The full-length EgSERK I cDNA was obtained by rapid amplification of the cDNA End-PCR (RACE-PCR) technique using the CapFishing Full-length cDNA kit (Seegene, Seoul, Korea) according to the manufacturer’s instructions. Total RNA from cell suspension culture was extracted according to Rochester et al. (1986) and used to synthesize the first strand cDNA by using the M-MLV reverse transcriptase Superscript II (Invitrogen, Waltham, MA), following the protocol suggested by the manufacturer.
The deduced EgSERK I amino acid sequence was subjected to protein domain analysis via the PSORT (Prediction of Protein Sorting Signals and Localization Sites in Amino Acid Sequences; http://psort.nibb.ac.jp; Nakai and Horton 1999) and PROSITE (www.expasy.org/prosite; De Castro et al. 2006). Multiple alignment of EgSERK I amino acid sequence and SERK proteins from other plants was performed using the CLUSTAL W tool (Thompson et al. 1994) in the BioEdit Sequence Alignment Editor Version 7.0.5.3 software (Hall 1999). The phylogenetic tree was constructed using the Neighbor-Joining (NJ) distance method in MEGA 4 (Molecular Evolutionary Genetic Analysis version 4; Tamura et al. 2007) based on the conserved region spanned between residues 86 and 585, which included the LRR region and the kinase domain of the deduced protein from the EgSERK I sequence. Bootstrapping analysis of 1000 replicates was performed to estimate the confidence level of the monophyletic groups.
Southern hybridization analysis
The CTAB method was used to isolate genomic DNA from oil palm leaves (dura x pisifera). Genomic DNA (30 μg for each reaction) was digested overnight with BamHI and HindIII at 37°C, and TaqI at 65°C for 1 h following the instructions of the manufacturer (Promega, Fitchburg, WI). The digested genomic DNA samples were fractionated on a 0.8% (w/v) agarose gel and blotted onto a pre-wet Hybond-N+ membrane (Amersham Biosciences, Buckinghamshire, UK) using an alkaline blotting method. The DNA gel blot was hybridized with two different non-radioactive probes corresponding to the region in between the subdomain VII-XI to 3’UTR region (UTR probe) and the complete ORF region (ORF probe), using the NEBlot Phototope Kit according to the instructions of the manufacturer (New England Biolabs, Hitchin, UK). The blots were hybridized overnight (around 16 h) at 60°C with the 3’UTR probe and at 65°C with the ORF probe, respectively. The membrane was rinsed twice in low stringency washing solution (2X SSC, 0.1% (w/v) SDS) for 5 min at room temperature to wash off the excess probe. The non-specifically bound probe was then washed off by higher stringency wash (0.5X SSC, 0.1% (w/v) SDS) at 60°C (UTR probe) or 65°C (ORF probe) twice for 15 min each.
Real-time PCR
The relative expression of EgSERK I was measured using real-time quantitative RT-PCR method (Applied Biosystems, Foster City, CA) with the ABI PRISM 7000 Sequence Detection System (Applied Biosystems) according to the manufacturer’s instructions. Three endogenous reference gene candidates were selected using the geNORM method (Vandesompele et al. 2002). The three endogenous reference gene with the highest M-values were EA1332 (EY406625.1), PD380 (unknown protein; Acc: EL684405.1) and PD569 (superoxide manganese dismutase; Acc: EL682210.1). Specific primers and probes were designed and synthesized by Sigma-Proligo (Sigma-Aldrich, St. Louis, MO). The primer set was designed to amplify the region between 604 bp and 689 bp, which overlapped with the SPP (Ser-Pro-Pro) region, a hallmark of the EgSERK I gene, to avoid amplification of other plant receptor-like kinases (RLKs). The probe was designed within the region flanked by the forward and reverse primers and was labeled with a reporter dye, FAM (6-carboxyfluorescein) and a quencher dye, TAMRA (6-carboxytetramethylrhodamine) at the 5′ and 3′ end, respectively. First-strand cDNA was synthesized using the Quantitect Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Independent qRT-PCR runs were conducted with four technical replicates and results were analyzed using the comparative delta Ct method (Vandesompele et al. 2002). Student’s t test was conducted to evaluate the statistical significance in the differences observed in the EgSERK I gene expression between young leaf and control (T0).
RNA in situ hybridization
In situ hybridization (ISH) was carried out according to the protocol described by Ooi et al. (2012). Riboprobes were synthesized using the Ampliscribe™ T3 and T7 Flash™ Transcription Kit (Epicentre® Biotechnologies, Madison, WI) following the manufacturer’s instructions. Sense and antisense probes for both 3’UTR and ORF regions of EgSERK I were generated by designing gene-specific primers incorporating the minimum T3 promoter sequence (5’-AATTAACCCTCACTAAAGG-3′) and T7 promoter sequence (5’-TAATACGACTCACTATAGG-3′), respectively, that are needed for efficient transcription (Table 1). Sense riboprobes for both 3’UTR and ORF were used as negative control. Elongation factor 1-α (EL687602) of oil palm was used as a positive control. The NBT-BCIP stained sections were observed with the digitized automated Leica DM6000 B research microscope.
Results and discussion
Cloning and sequence analysis of EgSERK I
The full-length cDNA sequence of EgSERK I (GenBank accession no.: KJ607989) is 2378 bp with the 5’UTR region (358 bp) longer than the 3’UTR region (130 bp); the putative ORF is 1890 bp encoding a 629 amino acid (aa) sequence. The putative protein prediction analysis revealed that the deduced amino acid sequence of EgSERK I encodes a number of protein domains that are typically found in the SERK protein of other plants. These include the signal peptide, leucine zipper, leucine-rich repeats, proline-rich region, transmembrane region, kinase domain, and C-terminal domain (Fig. 1a). The EgSERK I protein was predicted as a type I single pass cell membrane protein due to the presence of a transmembrane domain (between residues 242 and 265) located between a non-cytoplasmic region (1–241 aa) and a cytoplasmic region (266–629 aa).
Sequence analysis of EgSERK I gene. (a) Schematic drawing of predicted protein domains present in EgSERK I protein. Exons are represented as blocks and drawn to scale. SP signal peptide; Zip leucine zipper; LRR leucine-rich repeat; SPP serine-proline-proline; TM transmembrane region; C C-terminal kinase. (b) Prediction of signal peptide sequence and its confirmatory analysis. (c) Multiple sequence alignment of predicted amino acid sequence showing sequence similarity of EgSERK I with reported SERK proteins sequences from other plants. The shaded area indicates the ZIP domain conserved among EgSERK and CnSERK proteins. Region highlighted in black is the protein kinase ATP-binding region signature. The region italicized with gray font is the serine/threonine protein kinase active site signature. The conserved threonine present in the activation loop (A-loop) of plant serine/threonine type RLKs is shown in bold. EgSERK (AIC09100.1) from E. guineesis, CnSERK (AAV58833) from C. nucifera, OsSERK1 (AAU88198) from O. sativa; japonica cultivar-group, ZmSERK1 (CAC37638) from Z. mays, AtSERK1 (NP_177328) from A. thaliana, DcSERK (AAB61708) from D. carota.
At the N-terminal region of the putative protein sequence of EgSERK I, the first 28 aa code for a hydrophobic signal peptide domain with a cleavage point located between 28th aa and 29th aa as predicted by SignalP 3.0 Server (http://www.cbs.dtu.dk/services/SignalP, Fig. 1b). This is followed by a leucine zipper sequence (ZIP) and leucine-rich repeats (LRR). This LRR region is the primary N-terminal domain of the extracellular domain and is known as a platform for the binding of ligands and protein-protein interactions (Kobe and Deisenhofer 1994; Fig. 1c). The next region is the serine-proline-proline (SPP) region which is a unique feature for all SERK proteins that differentiates SERK from other LRR-RLK proteins (Schmidt et al. 1997; Fig. 1c). The intracellular domain consists of a protein kinase domain and a C-terminal domain. This protein kinase domain comprises 11 conserved subdomains of Ser/Thr protein kinases with a protein kinase ATP-binding region signature and the serine/threonine protein kinase active site signature (Fig. 1c). The EgSERK I protein shares the same conserved 29 aa residues in the activation loop (A-loop) with AtSERK 2, CnSERK, OsSERK 1, and ZmSERK 1. This similarity in the A-loop suggests that EgSERK I may share common functions with other reported SERK proteins. Finally, the C-terminal domain; which is rich in leucine residues, is the second special feature of SERK proteins after the SPP region. It involved in mediating the protein-protein interaction necessary for transmission of an intracellular phosphorylation cascade (Schmidt et al. 1997).
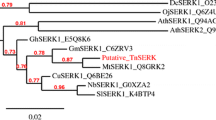
Sequence comparison between the deduced EgSERK I polypeptide and other SERK proteins revealed that SERK proteins are indeed well conserved across monocot and dicot plants, with the highest percentage of identity found in the kinase domain at the C-terminal while the lowest percentage of identity was found in the signal peptide and leucine zipper regions (Table 2). The deduced amino acid sequence of EgSERK I shows the highest identity to the SERK of Cocos nucifera (Table 2) as indicated in the phylogenetic analysis whereby EgSERK I is tightly clustered with the SERK of C. nucifera with a bootstrap value of 100 (Fig. 2).
Southern analysis
Southern analysis was carried out to determine the copy number of the EgSERK I gene in the oil palm genome. By comparing the hybridization results obtained with the ORF and UTR probes, many of the ORF-hybridized fragments were of the same size with the UTR-hybridized fragments (Fig. 3A). There were also a few additional ORF-hybridized fragments in each lane (Fig. 3B, black arrow). The restriction maps of the UTR and ORF probes showed that only one BamHI restriction site is present in the UTR probe, three BamHI restriction sites in the ORF probe but no HindIII restriction site was present in both probes (Fig. 3C). However, more than two hybridized fragments were observed on genomic DNA digested with BamHI and HindIII when hybridized with UTR and ORF probes. This multiple banding pattern suggests that the oil palm genome likely contains more than one SERK gene as reported in various plant species. This observation supported by previous studies indicating that the SERK gene belongs to a multigene family in plants like A. thaliana (AtSERK1-5; Hecht et al. 2001), R. hybrida (RhSERK 1-4; Zakizadeh et al. 2010), Triticum aestivum (TaSERK1-3; Singla et al. 2008), Vitis vinifera (VvSERK1-3; Schellenbaum et al. 2008), Z. mays (ZmSERK1–3; Baudino et al. 2001), and Oryza sativa (OsSERK1–2; Ito et al. 2005).
Autoradiograph of Southern blots analysis hybridized with UTR probe (a) and ORF probe (b) of EgSERK I. Signals present in both a and b (white arrows). Signals present in b only (black arrows). Schematic restriction map of BamHI present in EgSERK I cDNA (c). Genomic DNA of oil palm leaves was digested with BamHI and HindIII.
Expression analyses of EgSERK I – Expression of EgSERK I is developmentally regulated
Overall, the expression pattern of EgSERK I was different from A. thaliana (Hecht et al. 2001) and D. carota (Schmidt et al. 1997) whereby their expression was generally found only in embryogenic tissues. In oil palm, EgSERK I mRNA was highly expressed in leaf explants (or young leaves) and lowest in roots; low expression of EgSERK I mRNA was detected in cell suspension culture, white and green embryoids. EgSERK I mRNA was expressed in both embryogenic and non-embryogenic callus at relatively similar levels (Fig. 4). This expression pattern was also observed in C. sinensis whereby the CitSERK1-like gene was highly expressed in young leaves and moderately expressed in both embryogenic and non-embryogenic callus and various kinds of tissues at different levels (Ge et al. 2010). HvSERK1 and HvSERK3 of Hordeum vulgare were recently reported to express highly in leaves (Li et al. 2017a). In M. truncatula, Nolan et al. (2009) reported that the expression of MtSERK I was associated with the procambial cells of vascular tissue. Prior to that, AtSERK I transcripts of A. thaliana were also found in the procambium and immature vascular cells (Kwaaitaal and de Vries 2007). The above findings collectively explain the observation of high expression levels of EgSERK I mRNA in young leaf as this tissue contains midveins and a network of veins which are mainly made up from vascular bundles. This leads to the suggestion that the expression of EgSERK I is not only restricted to embryogenic tissues but its expression also extends to vegetative and reproductive tissues. It further implies that the EgSERK I gene has a broad role in plant growth and development.
Relative expression patterns of EgSERK I in different oil palm tissues (A to O) by real-time PCR. (A) leaf explant (LE); (B) embryogenic callus (EC); (C) non-embryogenic callus (NEC); (D) cell suspension culture (CSC); (E) white embryoid (WE); (F) green embryoid (GE); (G) globular; (H) torpedo; (I) haustorium; (J) germinating embryo; (K) female flower (FF); (L) male flower (MF); (M) meristem (M); (N) mature leaf (ML); (O) root (R). Relative expression levels were normalized to housekeeping genes (EA1332, PD380, and PD569). Data are means ± SE from three independent experiments (n = 3 each). *P < 0.05 (t test) compared to LE.
Expression of SERK genes was detected during somatic and zygotic embryogenesis at variable levels. In D. carota, the SERK gene expressed in the embryogenic mass until the formation of small globular somatic embryos of up to 100 cells but ceased thereafter (Schmidt et al. 1997). Meanwhile, AtSERK I of A. thaliana was expressed for a longer period, in all cells of the developing zygotic embryo until the heart stage (Hecht et al. 2001). Recently, transcripts of BdSERK 1 (B. distachyon) were found in all developmental stages from globular until scutellar stages (Oliveira et al. 2017). This transient expression pattern of SERK observed during embryogenesis of A. thaliana and D. carota suggested that SERK genes may be generally and primarily involved in the induction of somatic and zygotic embryogenesis but not in the later stages of embryogenesis (Schmidt et al. 1997; Hecht et al. 2001). Nevertheless, AcvSERK of Adiantum capillus-veneris was expressed throughout the embryo development process but declined during shoot formation (Li et al. 2015). In oil palm, it is difficult to identify the four morphological stages of embryogenesis (globular, heart, torpedo, and cotyledonary). Thus, the four different morphotypes of somatic embryos (globular, torpedo, haustorium, and germinating embryo) isolated from embryogenic callus cultures were deemed to resemble the four morphological stages typically found in most dicot plants (Rohani and Ong-Abdullah 2003). EgSERK I transcripts were detected in all four morphotypes at different levels, with germinating embryoids showing the highest expression at threefold above that observed in torpedo-shaped embryoids (Fig. 4). This set of samples was used to study the location of EgSERK I transcripts using in situ hybridization (ISH). However, no positive signals were detected in all the four morphotypes (data not shown). This may be due to the low abundance of EgSERK I transcripts. Similar observations have been reported in M. truncatula (Nolan et al. 2009) and C. sinensis (Ge et al. 2010) whereby the transcripts of MtSERK I and CitSERK1-like were detected throughout the somatic embryogenesis process from globular embryo until germinating embryo. Nolan et al. (2009) then suggested the association of MtSERK I expression with the developmental changes involving cellular reprogramming of somatic cells during somatic embryogenesis. It provides evidence to the observation of continuous minimal expression of EgSERK I is linked to the developmental changes of somatic cell during somatic embryogenesis through cellular reprogramming.
EgSERK I is upregulated by auxin
In oil palm tissue culture, exogenous application of auxins has proven to efficiently induce callus formation (Roowi et al. 2010). The combinations of 2-4D and NAA are used because these two auxins are strong promoters for callus induction and growth. Generally, 2-4D is used at very low concentrations to prevent somaclonal variation (Saieed et al. 1994). Young leaf was commonly used as explant in oil palm tissue culture due to its high successful rates in callusing (Rohani et al. 2003; Roowi et al. 2010). To elucidate the effect of auxin on EgSERK I expression, we examined the expression level of EgSERK I in auxin-treated leaf tissue. An upregulation of EgSERK I expression (2.5-fold) was detected after 6 h of auxin exposure, regardless of the exogenous auxin concentration and explant zones used (Fig. 5). However, the transcript levels of EgSERK I was subsequently decreased in all three auxin concentrations for both zones (Fig. 5). Similar expression levels of EgSERK I across three different auxin concentrations implies that a minimal auxin concentration (10 μgL−1 2-4D and 1 mgL−1 NAA) was able to trigger expression of EgSERK I. This observation coincides with the upregulation expression pattern of three TaSERK genes (Singla et al. 2008) and SERK I ortholog of Gossypium hirsutum (Cao et al. 2017) after 2 and 3 h of incubation in MS media supplemented with 2-4D, respectively. This initial 2-4D ‘shock’ is crucial in triggering reprogramming and dedifferentiation of explant cells, which respond swiftly to the addition of auxin, as soon as after 2 h of incubation, to form callus (Sharma et al. 2008). It provides insight to the observation of increased expression of EgSERK I in leaf tissue after 6 h of auxin incubation as initial excitation period for somatic cells of oil palm leaf explant, to reprogram and dedifferentiate to form callus.
Relative expression patterns of EgSERK I in zone 1 (nearest to the spear base) and zone 2 (9 ± 1 cm apart zone 1) of leaf explants on MS media supplemented with three different concentrations of NAA at different time points. (A) 1 mgL−1 NAA; (B) 10 mgL−1 NAA; (C) 50 mgL−1 NAA. T0 before treatment, 6H 6 h after treatment, 2D 2 d after treatment, 7D 7 d after treatment. Relative expression levels were normalized to housekeeping genes (EA1332, PD380, and PD569). Data are means ± SE from three independent experiments (n = 4 each). *P < 0.05 (t test) compared to T0.
EgSERK I is associated with callus initiation in oil palm
Somatic embryos are commonly originated from cells adjacent to vascular bundles of leaf as reported in D. carota and D. glomerata (Schmidt et al. 1997; Somleva et al. 2000). In oil palm, callusing was initiated when perivascular cells started dividing in vascular tissues near the midvein and cutting edge of the young leaf after about 3–6 mo on callus induction auxin added media (Nur Fatihah et al. 2012). This callus is termed as primary callus that serves as a platform for the formation of various forms of secondary calluses, namely nodular callus and rooty callus after about 4 mo in culture. Nodular callus is the favored type of callus owing to its capability to produce friable embryogenic callus which eventually will form somatic embryos (Nur Fatihah et al. 2012). In addition to that, a more recent study on morphological and anatomical changes during acquisition and development of oil palm somatic embryogenesis (Gomes et al. 2017) has provided more evidence to the findings reported by Nur Fatihah et al. (2012). Callus formation and development were associated with vascular bundle adjacent to the edge of the excised explants, with greater contact between vascular tissues and auxin present in media. Embryogenic callus was subsequently formed at the periphery of the parenchymal tissue of primary callus (Gomes et al. 2017).
Here, we examined the EgSERK I mRNA localization in young leaf and suspension culture through in situ hybridization. Positive signals were detected in the midvein of young leaf which is mainly made up of vascular bundles, sclerenchyma tissue, and phloem, using both ORF and UTR riboprobes (Fig. 6B–C). In suspension culture, EgSERK I mRNA was detected in relatively large and vacuolated parenchymal cells located in the middle region of primary callus (Fig. 6E–F). Similar expression pattern was reported in M. truncatula and C. sinensis where MtSERK I was expressed in cells surrounding vascular tissues at the cutting edges of the leaf explant (Nolan et al. 2009), and CitSERK1-like transcript was mainly found in the vascular cells of different embryos and tissues (Ge et al. 2010).
Localization of EgSERK I transcript visualized by in situ hybridization. Young leaf hybridized with sense riboprobe (negative control) of UTR (A), antisense riboprobe UTR (B), and antisense riboprobe ORF (C). Suspension culture hybridized with sense riboprobe (negative control) of UTR (D), antisense riboprobe UTR (E), and antisense riboprobe ORF (F). The purple stain or deposit shows the positive hybridization signal. VB vascular bundle, ST sclerenchyma tissue, PH phloem, MZ meristematic zone, PC parenchyma cells. A–CBar 380 μm; D–EBar 500 μm; Fbar 200 μm
To collate the expression profile of the EgSERK I gene, we showed that the EgSERK I gene was highly expressed in young leaf and upregulated in leaf tissue after 6 h of exposure to auxin. EgSERK I transcripts were found to be located in cells surrounding vascular bundles including vascular tissues at the cutting edge of leaf explants, and it was detected in parenchymal cells located in the middle region of primary callus, which coincides with the callus initiation point of oil palm. This collectively suggests that the EgSERK I protein might be involved in transducing signal to switch on the dedifferentiation process to trigger cellular reprogramming in perivascular cells and sclerenchyma cells of vascular bundles in leaf explant to form primary callus. A different set of genes will then turn on to confer embryogenic competence in selected cells of the primary callus clump to form embryogenic cells that eventually giving rise to somatic embryos. These data support the hypothesis that EgSERK I may play an important role during the callus initiation stage in oil palm tissue culture.
Conclusion
The first full-length cDNA of SERK I in oil palm was successfully isolated and is 2378 bp in length. The transcript comprised a 5’UTR region (358 bp), 3’UTR region (130 bp), and an ORF region (1890 bp) encoding a 629 aa protein. The overall expression results suggest that EgSERK I may be associated with callogenesis by triggering cell reprogramming and dedifferentiation of perivascular cells and sclerenchyma cells surrounding vascular bundles in leaf explant to form primary callus. In addition to that, EgSERK I plays a broader role to maintain plant growth and development. More work on the functional analysis of the EgSERK I gene is needed to elucidate the underlying molecular network of oil palm callogenesis which will eventually lead to better understanding of somatic embryogenesis in oil palm.
References
Basiron Y (2011) Pakistan-Malaysia palm oil trade: why buy Malaysian palm oil. Paper presented at Global Oils and Fats 7, Gaylord National Resort, National Harbour, MD, 2011 on 19–20 September 2011
Baudino S, Hansen S, Brettschneider R, Hecht V, Dresselhaus T, Lörz H, Dumas C, Rogowsky P (2001) Molecular characterization of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta 213:1–10
Campanoni P, Nick P (2005) Auxin-dependent cell division and cell elongation. 1-naphthaleneacetic acid and 2, 4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol 137:939–948
Cao A, Zheng Y, Yu Y, Wang X, Shao D, Sun J, Cui B (2017) Comparative transcriptome analysis of SE initial dedifferentiation in cotton of different SE capability. Sci Rep 7:8583
Chugh A, Khurana P (2002) Gene expression during somatic embryogenesis-recent advances. Curr Sci 83:715–730
Corley RHV, Tinker PB (2003) The oil palm, 4th edn. Blackwell Science, Oxford
De Castro E, Sigrist CJA, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N (2006) ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res 34(suppl_2):W362–W365
De Touchet B, Duval Y, Pannetier C (1991) Plant regeneration from embryogenic suspension cultures of oil palm (Elaeis guineensis Jacq.). Plant Cell Rep 10:529–532
Duval Y, Engelmann F, Durand-Gasselin T (1995) Somatic embryogenesis in oil palm (Elaeis guineensis Jacq.). In: Somatic embryogenesis and synthetic seed I. Springer, Berlin, Heidelberg, pp 335–352
Fehér A (2005) Why somatic plant cells start to form embryos? In: Mujib A, Samaj J (eds) Somatic embryogenesis. Springer, Berlin, Heidelberg, pp 85–101
Ge XX, Fan GE, Chai LJ, Guo WW (2010) Cloning, molecular characterization and expression analysis of a SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE gene (CitSERK 1-like) in Valencia sweet orange. Acta Physiol Plant 32:1197–1207
Gomes HT, Bartos PM, Scherwinski-Pereira JE (2017) Dynamics of morphological and anatomical changes in leaf tissues of an interspecific hybrid of oil palm during acquisition and development of somatic embryogenesis. Plant Cell Tissue Organ Cult 131:269–282
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hecht V, Vielle-Calzada J, Hartog MV, Schmidt ED, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis somatic embryogenesis receptor kinase I gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816
Huang X, Lu XY, Zhao JT, Chen JK, Dai XM, Xiao W, Chen YP, Chen YF, Huang XL (2010) MaSERK 1 gene expression associated with somatic embryogenesis competence and disease resistance response in banana (Musa spp.). Plant Mol Biol Rep 28:309–316
Imkampe J, Halter T, Huang S, Schulze S, Mazzotta S, Schmidt N, Manstretta R, Postel S, Wierzba M, Yang Y, Walter MAM (2017) The Arabidopsis leucine-rich repeat receptor kinase BIR3 negatively regulates BAK1 receptor complex formation and stabilizes BAK1. Plant Cell 29:2285–2303
Ito Y, Takaya K, Kurata N (2005) Expression of SERK family receptor-like protein kinase genes in rice. Biochim Biophys Acta (BBA) Gene Struct Expr 1730:253–258
Kobe B, Deisenhofer J (1994) The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci 19:415–421
Kwaaitaal MACJ, de Vries SC (2007) The SERK 1 gene is expressed in procambium and immature vascular cells. J Exp Bot 58:2887–2896
Li X, Fang YH, Han JD, Bai SN, Rao GY (2015) Isolation and characterization of a novel SOMATIC EMBRYOGENESIS RECEPTOR KINASE gene expressed in the fern Adiantum capillus-veneris during shoot regeneration in vitro. Plant Mol Biol Report 33:638–647
Li Y, Liu C, Guo G, He T, Chen Z, Gao R, Xu H, Faheem M, Lu R, Huang J (2017a) Expression analysis of three SERK-like genes in barley under abiotic and biotic stresses. J Plant Interact 12:279–285
Li Z, Wang Y, Huang J, Ahsan N, Biener G, Paprocki J, Thelen JJ, Raicu V, Zhao D (2017b) Two SERK receptor-like kinases interact with EMS1 to control anther cell fate determination. Plant Physiol 173:326–337
Muniran F, Bhore SJ, Shah FH (2008) Micropropagation of Elaies guineensis Jacq. ‘Dura’: comparison of three basal media for efficient regeneration. Indian J Exp Biol 46:79–82
Nakai K, Horton P (1999) PSORT: a program for detecting the sorting signals of proteins and predicting their subcellular localization. Trends Biochem Sci 24:34–35
Namasivayam P (2007) Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell Tissue Organ Cult 90:1–8
Nolan KE, Kurdyukov S, Rose RJ (2009) Expression of the SOMATIC EMBRYOGENESIS RECEPTORLIKE KINASE 1 (SERK1) gene is associated with developmental change in the life cycle of the model legume Medicago truncatula. J Exp Bot 60:1759–1771
Nur Fatihah MY, Sharifah Shahrul RS, Ong-Abdullah M, Ho CL, Parameswari N (2012) A time course anatomical analysis of callogenesis from young leaf explants of oil palm (Elaeis guineensis Jacq.). J Oil Palm Res 24:1330–1341
Oliveira EJ, Koehler AD, Rocha DI, Vieira LM, Pinheiro MVM, de Matos EM, da Cruz ACF, da Silva TCR, Tanaka FAO, Nogueira FTS, Otoni WC (2017) Morpho-histological, histochemical, and molecular evidences related to cellular reprogramming during somatic embryogenesis of the model grass Brachypodium distachyon. Protoplasma 254:2017–2034
Ooi SE, Lee FC, Ong-Abdullah M (2012) A rapid and sensitive in situ RNA hybridization method for oil palm tissues. J Oil Palm Res 24:1235–1239
Paranjothy K, Othman R (1982) In vitro propagation of oil palm. In: Fujiwara A (ed) Proceeding 5th international congress of plant tissue and cell culture. Malaysian Palm Oil Board, Kuala Lumpur, pp 747–748
Pilarska M, Malec P, Salaj J, Bartnicki F, Konieczny R (2016) High expression of SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE coincides with initiation of various developmental pathways in in vitro culture of Trifolium nigrescens. Protoplasma 253:345–355
Rochester DE, Winer JA, Shah DM (1986) The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J 5:451–458
Rohani O, Ong-Abdullah M (2003) Study of different morphotypes of somatic organized structures of oil palm. In: Proceedings of the 2003 PIPOC international palm oil congress (agriculture). Malaysian Palm Oil Board, Kuala Lumpur, pp 751–758
Rohani O, Zamzuri I, Tarmizi AH (2003) Oil palm cloning. In: MPOB protocol, MPOB technology, vol 26. Malaysian Palm Oil Board, Kuala Lumpur, Malaysia, pp 1–29
Roowi SH, Ho CL, Alwee SS, Ong-Abdullah M, Napis S (2010) Isolation and characterization of differentially expressed transcripts from the suspension cells of oil palm (Elaeis guineensis Jacq.) in response to different concentration of auxins. Mol Biotechnol 46:1–19
Saieed NT, Douglas GC, Fry DJ (1994) Induction and stability of somaclonal variation in growth, leaf phenotype and gas exchange characteristics of poplar regenerated from callus culture. Tree Physiol 14:1–16
Santiago J, Henzler C, Hothorn M (2013) Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science 341:889–892
Santos MO, Romano E, Yotoko KSC, Tinoco MLP, Dias BBA, FJL A˜o (2005) Characterisation of the cacao somatic embryogenesis receptor-like kinase (SERK) gene expressed during somatic embryogenesis. Plant Sci 168:723–729
Schellenbaum P, Jacques A, Maillot P, Bertsch C, Mazet F, Farine S, Walter B (2008) Characterization of VvSERK1, VvSERK2, VvSERK3 and VvL1L genes and their expression during somatic embryogenesis of grapevine (Vitis vinifera L.). Plant Cell Rep 27:1799–1809
Schmidt EDL, Guzzo F, Toonen MAJ, de Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Sharma SK, Millam S, Hein I, Bryan GJ (2008) Cloning and molecular characterisation of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta 228:319–330
Shimada T, Hirabayashi T, Endo T, Fujii H, Kita M, Omura M (2005) Isolation and characterization of the somatic embryogenesis receptor-like kinase gene homologue (CiSERK1) from Citrus unshiu Marc. Sci Hortic 103:233–238
Singla B, Khurana JP, Khurana P (2008) Characterization of three somatic embryogenesis receptor kinase genes from wheat, Triticum aestivum. Plant Cell Rep 27:833–843
Somleva MN, Schmidth EDL, de Vries SC (2000) Embryonic cells in Dactylis glomerata L. (Poaceae) explants identified by cell tracking and by SERK expression. Plant Cell Rep 19:718–726
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: a molecular evolutionary genetic analysis MEGA software version 4.0. Mol Biol Evol 24:1596–1599
Tan CC, Wong G, Soh AC, Hor TY, Chong SP, Gopal K (2003) Experiences and lessons from oil palm clonal evaluation trials and commercial test plantings. In: Proceedings of the 2003 PIPOC International Palm Oil Congress. Malaysian Palm Oil Board, Kuala Lumpur, pp 1093–1119
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vandesompele J, Preter KD, Pattyn F, Poppe B, Roy NV, Paepe AD, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–11
Zakizadeh H, Stummann BM, Lütken H, Müller R (2010) Isolation and characterization of four somatic embryogenesis receptor-like kinase (RhSERK) genes miniature potted rose (Rosa hybrida cv. Linda). Plant Cell Tissue Organ Cult 101:331–338
Acknowledgements
This work was funded by the Malaysian Palm Oil Board (MPOB). The authors thank the MPOB for providing tissue culture materials and to Dr. Yeap Wan Chin for her constructive suggestions and support.
Author information
Authors and Affiliations
Contributions
FCL, MOA, CLH, and PN designed and planned the experiments in this study. FCL carried out the experiments and wrote the manuscript. FCL and SEO developed the modified RNA in situ hybridization method. All authors discussed and were involved in results interpretation. All authors contributed to drafting and improving of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Lee, FC., Ong-Abdullah, M., Ooi, SE. et al. Cloning and characterization of Somatic Embryogenesis Receptor Kinase I (EgSERK I) and its association with callus initiation in oil palm. In Vitro Cell.Dev.Biol.-Plant 55, 153–164 (2019). https://doi.org/10.1007/s11627-018-9942-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-018-9942-x