Abstract
Cryopreservation is a valuable technique for the long-term conservation of plant germplasm and complementary to traditional seed storage methods. However, critical factors such as seed moisture content should be optimized before using this technique as a safe strategy for storing seeds such as those of Nicotiana spp. This study aimed to determine the effect of desiccation on physiological and biochemical indicators associated with germination and vigor in cryopreserved seeds of Nicotiana tabacum cv. Sancti Spíritus 96 (SS-96). The germination and vigor of seeds with a range of moisture content were assessed using electrolyte leakage and accelerated aging tests. In addition, these physiological indicators were related to the oxidative state of the seeds, in terms of the rate of O2 ·− generation and the H2O2 content, and the activity of enzymatic antioxidants superoxide dismutase and catalase. The cryopreserved seeds of N. tabacum SS-96 with a moisture content of 2.1% exhibited higher vigor probably due to the retention of membrane integrity, reflected by lower levels of lipid peroxidation and electrolyte leakage associated with the absence of oxidative stress. The results suggest 2.1% as the optimal moisture content for the storage of seeds of this cultivar, both at cryogenic temperatures and at 5°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 1980s, several studies on cryopreservation have been conducted worldwide to complement traditional seed storage methods (Stanwood and Bass 1981; González-Benito et al. 1998; Walters et al. 2004; Veiga-Barbosa et al. 2013). Plant cryopreservation techniques normally use liquid nitrogen (LN, − 196°C) as the cryogen due to its relatively low cost. The objective is to reach temperatures below − 130°C to achieve low molecular kinetic energy conditions and extremely slow diffusion, so that chemical reactions are practically halted (Pritchard 2007). Cryopreservation of whole seeds may be a very valuable strategy for the long-term conservation of tropical and subtropical forest species, as it avoids problems related to embryo isolation and in vitro handling. However, a prerequisite is that the seeds are desiccation tolerant (Gonzalez-Arnao et al. 2014).
Although protocols have been established for the storage of desiccation-tolerant seeds in LN, in some species, loss of viability is observed as a consequence of damage incurred during cryopreservation (Volk et al. 2006; Pritchard 2007; Veiga-Barbosa et al. 2013). The moisture content (MC) at which seeds are cryopreserved is considered to be a critical factor in the development of cryopreservation protocols (Verdier et al. 2013; Michalak et al. 2015). In fact, many reports suggest that seed MC and hence desiccation tolerance are primary factors that seed banks need to consider to maintain seed quality over time and ensure rapid and homogeneous germination and seedling emergence upon retrieval from storage (Li and Pritchard 2009; Mira et al. 2010).
Numerous biochemical and cellular events are associated with desiccation tolerance, including the ability to quench reactive oxygen species (ROS) (Bailly et al. 2008). ROS react with cell macromolecules, such as proteins, lipids, and nucleic acids, and cause damage and disruption of cellular function. Oxidative stress is most often associated with peroxidation of membrane lipids, followed by membrane disintegration and cell death (Veselovsky and Veselova 2012; Liu et al. 2016). The ability of seeds to withstand drying and low temperatures, and to remain viable for long periods, may be related to their ability to eliminate ROS to avoid harmful events such as lipid peroxidation (Guéraud et al. 2010; Veselovsky and Veselova 2012).
Another important issue is related to ultra-dry seeds (below 5% MC fresh weight basis), which are unable to regenerate antioxidants during storage (Groot et al. 2012). However, additional studies are needed to quantify the rate of decline in antioxidant capacity during seed storage in relation to moisture level, temperature, and oxygen pressure. Moreover, Groot et al. (2015) recommended that genebanks store dry seeds under anoxic conditions to prolong their longevity during ex situ conservation. Visscher et al. (2016) suggested that understanding any interaction between ultra-drying, and the gaseous environment is crucial for a critical assessment of the storage potential of seeds under artificial or natural ultra-dry conditions.
There are few reports on the cryopreservation of Nicotiana seeds worldwide. For example, Touchell and Dixon (1994) only managed to regenerate plants of Nicotiana occidentalis W. from the germination of isolated embryos in vitro after cryopreservation of whole seeds and obtained low levels of survival through conventional propagation methods. Additionally, Walters et al. (2004) observed a significant reduction in germination of four accessions of Nicotiana tabacum after 14 yr of exposure to LN, although they did not report the conditions in which the material was cryopreserved. Moreover, no evidence was found in the literature consulted for studies that evaluated the effects of preconditioning treatments on the viability and vigor of tobacco seeds recovered from LN.
Therefore, in the present study, the effect of desiccation on physiological and biochemical indicators associated with the germination and vigor of cryopreserved seeds of N. tabacum L. cv. Sancti Spíritus 96 were evaluated to determine the optimum MC for its conservation at cryogenic temperatures.
Materials and Methods
Plant material
Experiments were conducted with seeds of N. tabacum L. cultivar Sancti Spíritus 96 (SS-96) collected 35 d after anthesis in 2014 at the Tobacco Experimental Station, Cabaiguán, Cuba (latitude 22° 04′ 43″ N, longitude 79° 29′ 50″ W, and altitude 134 m). The seeds were immediately used in the experiments described below.
Seed MC determination
Seed MC was determined by the constant temperature (ISTA 2005) drying method, at 103°C for 4 h with three replicates of 0.5 g of seeds each, and was expressed as a percentage of the fresh mass.
Hydration and desiccation of seeds
Seeds, with an initial MC of 12.5%, were humidified or dried to 18.0, 13.0, 8.0, 4.2, 2.1, and 1.0%. The humidification was carried out in the vapor phase in a desiccator (62200, Biotech SL®, Madrid, Spain) above distilled water at 25°C. The desiccation was carried out in hermetically sealed desiccators at 25°C, with self-indicator silica gel orange (13767, Sigma-Aldrich® Quimica SL, Madrid, Spain), in 1:20 ratio (seed mass/silica gel mass).
Seed storage
Samples of 0.2 g (n = 3) of seeds at each MC were sealed in 2.0-mL cryovials (Nalgene®, Thermo Fisher Scientific®, Paisley, UK) and introduced directly into a storage chamber at 15% relative humidity and 5°C (− LN) (Fitotron® SGC 120, Weisstechnik®, Germany) or into liquid nitrogen Dewars (+ LN). After 30 d, the cryovials were recovered and rewarmed in a water bath held at 40°C for 5 min.
Germination assays
Germination tests were performed according to the paper germination method described by Rao et al. (2007), with four replicates of 100 seeds each, per MC–temperature treatment combination. The incubation was performed at 27°C with a photoperiod of 12 h and intensity of 35 μmol m−2 s−1 provided by cool daylight fluorescent lamps (Osram Sylvania, Wilmington, MA). In all trials, the emergence of the radicle (± 2 mm) was the criterion for scoring germination. The germination rate was determined at 14 d after the seeds were imbibed by expressing the number of seeds germinated during that time interval as a percentage of the total number of seeds (Rao et al. 2007).
Accelerated aging test
The accelerated aging test was performed according to the methodology described by Perry (1984). Seeds were incubated at 14% relative humidity (saturated KCl solution) (P704, Phytotechnology Laboratories®, Beijing, China) within glass desiccators, which were placed at 43.0 ± 1.0°C. At 7-d intervals, 0.1-g samples were extracted, and the elapsed time (d) was calculated so that each sample reached 50% of the initial germination rate (T 50) (Thanos and Doussi 1995).
Electrolyte leakage test
The electrolyte leakage test was adapted from Bailly et al. (2001). For each sample, four replicates of 0.15 g of previously equilibrated fresh seeds (for each interval) were placed in 35 mL of deionized water at 30 ± 0.5°C. The leachates were collected after 24 h of imbibition. The conductivity was measured in a conductivity meter (model DDS-IIA, Shanghai Leici Instrument Inc., Shanghai, China) and expressed as μS cm−1 (g dry mass)−1.
Lipid peroxidation
The seeds (three replicates of 0.2 g per treatment) were homogenized in a mortar and pestle with LN until a fine powder was obtained and 1.5 mL of 50 mmol L−1 KH2PO4–KOH buffer, pH 7.8 containing 1 mmol L−1 ethylenediaminetetraacetic acid (EDTA), 1 mmol L−1 1,4-dithioltreitol (DTT), 1 mmol L−1phenylmethylsulfonylfluoride (PMSF), 0.05% (v/v) Triton X-100, and 1% (w/v) polyvinylpyrrolidone (PVP-40). Chemical suppliers were Phytotechnology Laboratories® (Beijing, China) for KOH, EDTA, DTT, Triton X-100, and PVP-40 and Sigma-Aldrich® Quimica SL (Madrid, Spain) for KH2PO4 and PMSF. After homogenization, the extracts were centrifuged at 27,000×g for 25 min at 4°C in a Heal force® refrigerated centrifuge (model Neofuge 15R, Shanghai, China). The supernatant was then used to measure lipid peroxidation in terms of the rate of formation of substances that react with 2-thiobarbituric acid (TBA) (Buege and Aust 1978), modified by Du and Bramlage (1992). The absorbance of the supernatant was measured at 600 and 532 nm on a Rayleigh spectrophotometer model VIS-7236 (Beijing Beifen-Ruili Analytical Instrument (Group) Co. Ltd., Beijing, China). The non-specific absorbance of the reaction product at 600 nm was subtracted from the maximum absorbance at 532 nm to measure malondialdehyde (MDA). The results were expressed in μmol (mg dry mass)−1.
Superoxide determination
The seeds (three replicates of 0.2 g per treatment) were homogenized in a mortar and pestle with LN until a fine powder was obtained to which 1 mL of cold KH2PO4–KOH buffer (200 mmol L−1, pH 7.2), containing 1 mmol L−1 N,N-di-ethyldithiocarbamate (DDC) was added. Chemical suppliers were Phytotechnology Laboratories® for KOH, and Sigma-Aldrich® Quimica SL for KH2PO4 and DDC. The mixture was centrifuged at 15,000×g for 5 min at 4°C. The supernatant was immediately used for the spectrophotometric determination of the rate of O2 ·− generation (Kumutha et al. 2009). The absorbance of the product was measured at 540 nm in a Rayleígh spectrophotometer (Analytical Instrument (Group) Co., Ltd.). The rate of O2 ·− generation was calculated using a molar extinction coefficient of 12.8 mmol L−1 cm−1 and expressed as nmol O2 ·− (mg dry mass)−1 min−1.
Hydrogen peroxide determination
The seeds (three replicates of 0.2 g per treatment) were homogenized in a mortar and pestle with LN until a fine powder was obtained to which 1 mL of 5% (w/v) trichloroacetic acid (TCA) was added. The mixture was centrifuged at 13,000×g for 15 min at 4°C. The supernatant was adjusted to pH 8.4 with 4 mol L−1 NaOH and centrifuged at 1000×g for 3 min at 4°C. Chemical supplier was Sigma-Aldrich® Quimica SL. The supernatant was immediately used for the spectrophotometric determination of H2O2 according to Zhou et al. (2006). The increase in absorbance was measured at 505 nm in Rayleígh spectrophotometer (Analytical Instrument (Group) Co., Ltd.), and the concentration was expressed in nmol of H2O2 (mg dry mass)−1.
Enzyme assays
The seeds (three replicates of 0.2 g per treatment) were prepared as described for the lipid peroxidation assay. Superoxide dismutase activity (SOD, EC 1.15.1.1) was determined by the method described by Marklund and Marklund (1974). A unit of SOD (U) activity was defined as the amount of enzyme required to cause 50% inhibition of pyrogallol autooxidation monitored at 420 nm. The reaction was started with the addition of pyrogallol, and the change in absorbance was measured at 420 nm in a Rayleígh spectrophotometer (Analytical Instrument (Group) Co., Ltd.). The enzymatic activity was expressed as units of enzyme per mg of soluble proteins U (mg protein)−1.
For the determination of catalase (CAT) activity (CAT, EC 1.11.1.6), the method described by Aebi (1984) was followed. The progress of the reaction was recorded for 2 min at 25°C in Rayleígh spectrophotometer (Analytical Instrument (Group) Co., Ltd.). One unit of CAT activity referred to the amount of enzyme needed to reduce 1 nmol of H2O2 in 1 min. The enzymatic activity was expressed as U (mg protein)−1.
Statistical analysis
Statistical processing of the data was performed using the Statistical Package for Social Sciences (version 11.5 for Windows, SPSS Inc., Chicago, IL). Data were analyzed for normality using a Kolmogorov–Smirnov test and for homogeneity of variance using a Levene test. Parametric analyses were performed using bifactorial ANOVA, and the difference between means was estimated through Tukey’s post-hoc test. For statistical processing only, the germination rate data were arcsine transformed.
Results
Germination and vigor
Both the cryopreserved seeds (+ LN) and those stored at 5°C (− LN) exhibited high germination rate at (~ 90%) at seed MCs equal to or lower than 8.0%. However, at MCs > 8.0%, the germination rate decreased significantly and more drastically in cryostorage (Fig. 1). While germination of + LN seeds decreased from 83.3 to 63.5% when MC was increased from 13.0 to 18.0%, at a MC of 13 and 18.0%, only 43.3 and 0.8% of − LN seeds germinated, respectively.
Germination rate of Nicotiana tabacum L. cv. Sancti Spíritus 96 seeds collected 35 d after anthesis, dried to different moisture contents, and stored for 30 d in liquid nitrogen (+ LN) or at 5°C (− LN). Values indicated by different letters are statistically different (bifactorial ANOVA, Tukey, p ≤ 0.05, n = 4). ET total standard error.
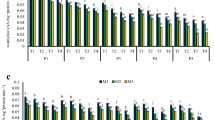
The rate of aging was measured in terms of T 50 (time taken to reach 50% of initial germination), which was lowest at MCs between 1.0 and 4.2% moisture (Fig. 2 A). However, at both storage temperatures as seed MC increased, T 50 decreased significantly. For example, + LN seeds at 2.1% MC exhibited a T 50 of 27.4 d, while T 50 for + LN seeds at 18.0% MC was just 5.9 d. However, it should be noted that aging of − LN seeds was more rapid than that of + LN seeds over the 30-d storage period.
Time to decrease to 50% of the initial germination rate (T 50) after accelerated aging (A), electrolyte leakage (B), and malondialdehyde content (C) of Nicotiana tabacum L. cv. Sancti Spíritus 96 seeds collected 35 d after anthesis, dried to different moisture contents, and stored for 30 d in liquid nitrogen (+ LN) or at 5°C (− LN). Values indicated by different letters in each graph are statistically different (bifactorial ANOVA, Tukey, p ≤ 0.05, n = 4). ET total standard error.
Electrolyte leakage, used as an indication of membrane damage, increased progressively as seed MC increased beyond 2.1% at both storage temperatures (Fig. 2 B). However, at MCs beyond 4.2%, this damage was more pronounced in − LN seeds than + LN seeds (at comparable MCs). Electrolyte leakage increased significantly from 26.3 μS cm−1 g−1 to values of 64.1, 107.4, and 188.8 μS cm−1 g−1 at 8.0, 13.0, and 18.0% MC, respectively, for − LN seeds. In comparison, leakage values for + LN seeds were 49.1, 80.4, and 143.3 μS cm−1 g−1 at 8.0, 13.0, and 18.0% MC, respectively, in − LN seeds.
Lipid peroxidation
Levels of lipid peroxidation, which were measured by MDA production, were at a minimum in seeds at 2.1% MC at both storage temperatures (Fig. 2 C). Dehydration beyond this MC did not reduce levels of lipid peroxidation, while seed MC increased lipid peroxidation, irrespective of the storage temperature. Although MDA values were comparable between + LN and − LN seeds at MCs up to 4.2%, above this value, the MDA levels in − LN seeds were significantly higher than + LN seeds at comparable MCs.
ROS production
At MCs lower than 8.0%, there were no significant differences in the rate of O2 ·− generation, between + LN and − LN seeds (Fig. 3 A). However, at both storage temperatures, a significant increase in O2 ·− formation was observed when dehydrating the seed to MC below 2.1% and above 8%. This increase in O2 ·− generation with increasing MC was comparatively more pronounced in − LN seeds. For example, radical generation in − LN seeds increased by 1.8 times when hydrated from 8.0 to 18.0% MC, and this parameter increased by 1.5 times with a similar increase in MC in + LN seeds.
Generation rate of O2 ·− (A), H2O2 content (B) and enzymatic activity superoxide dismutase (SOD) (C) and catalase (CAT) (D) in Nicotiana tabacum L. cv. Sancti Spíritus 96 seeds collected 35 d after anthesis, dried to different moisture contents, and stored for 30 d in liquid nitrogen (+ LN) or at 5°C (− LN). Values indicated by different letters in each graph are statistically different (bifactorial ANOVA, Tukey, p ≤ 0.05, n = 9). ET total standard error.
H2O2 production was at a minimum and comparable between storage temperatures at MCs below 2.1% (Fig. 3 B). Increasing MCs above 4.2% significantly increased H2O2 content in both + LN and − LN seeds; however, this increase was greater in the latter. For example, while H2O2 production in + LN seeds at 18.0% MC was 0.09 nmol mg−1, in − LN seeds, this parameter was 1.5 times higher (0.13 nmol mg−1).
Enzymatic activity
Except for seeds at 18.0%, which showed relatively lower SOD activity, the activity of this enzyme was comparable across MCs in + LN seeds (Fig. 3 C). Although, SOD activity in − LN seeds showed a similar pattern to + LN up to 8.0% MC and hydration up to 13.0% resulted in increased SOD activity from 5.24 U (mg protein)−1 at 8.0% MC to 6.68 U (mg protein)−1 at 18.0%. However, at 13.0% MC, SOD activity in − LN seeds reached a maximum level and thereafter decreased, even though the soluble protein content increased (data not shown).
Changes in CAT activity with MC were similar to those manifested by SOD (Fig. 3 D). In the + LN seeds, at MCs above 2.1%, the CAT activity increased, although this increase was significant only when the seed MC reached 13.0%. Meanwhile, in the − LN seeds, at 13% MC or less than 4.2%, the CAT activities were comparable in + LN seeds. However, in the 8.0–13.0% MC range, the CAT activity increased significantly, from 31.8 to 39.2 U (mg protein)−1. At 13.0% MC, the CAT activity observed in − LN seeds reached a maximum level 39.2 U (mg protein)−1, i.e., 18.2% higher than in + LN seeds at the same MC. However, CAT enzyme in activity − LN seeds decreased by 11.9% when the MC was increased to 18.0% moisture, despite a 7.8% increase in the soluble protein content (data not shown).
Discussion
Many processes can contribute to seed deterioration, although ROS and related chemical oxidation are likely to be the main culprits (Lee et al. 2010; Morscher et al. 2015; Nagel et al. 2016). Due to the absence of free water in dry seeds, non-enzymatic mechanisms are probably involved in the accumulation of ROS. However, with the progressive hydration of the seeds, the enzymatic mechanisms become more relevant (Bailly 2004; Morscher et al. 2015).
The measurements of O2 ·− (Fig. 3 A) and H2O2 (Fig. 3 B) production in these studies showed that there was a trend for ROS production to decrease as seed MC was reduced from 18.0 to 2.1% at both storage temperatures. This decline in MC was also accompanied by improved SOD activity and a decline in biomarkers of membrane damage, namely electrolyte leakage and MDA content. This oxidative imbalance is a sign of equilibrium displacement towards the increase of ROS content, perhaps due to depletion and damage of the antioxidant mechanisms (Xin et al. 2010; Zagorchev et al. 2013). At the two highest MCs (13.0 and 18.0%) this oxidative stress, which was more intense at comparable MCs in − LN seeds, appeared to be damaging enough to result in decreased germination rate, which was significantly more severe in − LN seeds.
Though not always significant, it should also be mentioned that dehydration below 1.0% moisture increased the ROS content and increased oxidative damage as manifested by an increase in MDA content and electrolyte leakage, but antioxidant activities were not compromised. Though this increase in ROS did not compromise the germination rate, it could reflect the potential damaging effects of ultra-dry storage reported for other desiccation-tolerant seeds (Vertucci et al. 1994). It is well established that the germination test is not sufficient enough to reflect differences in quality (vigor) between seed lots with similar germination rates (Navarro et al. 2015). This, together with the short conservation time that the seeds were subjected to in this study could explain the inter-treatment differences in the germination rate reflected at MCs ≤ 8.0% (Fig. 1).
For seeds stored at 5°C, progressively decreasing the MC from 18.0% could cause a gradual decrease in molecular mobility, resulting from the stabilization of the vitreous state. In this state, the kinetics of aging reactions should be reduced, particularly in terms of the formation of ROS, including the generation of O2 ·−, H2O2, and indirectly, OH-. In addition, the high viscosity of the cellular cytoplasm could affect the mobility of ROS and consequently decrease the damage to the macromolecules further away from the generation site (Buitink and Leprince 2008).
When seeds were stored at MCs ≥ 13.0%, there was a progressive decrease in the germination rate, regardless of the storage temperature (Fig. 1). The decrease was accompanied by increased electrolyte leakage (Fig. 2 B) and lipid peroxidation (Fig. 2 C). As alluded to earlier, it is possible that loss of viability as a response to stress during storage could be linked to increased accumulation of substances that react with ROS-mediated TBA (Benson and Bremner 2004). The strong negative correlation between germination rate and electrolyte leakage (+ LN r = − 0.94 and − LN r = − 0.97) and lipid peroxidation (+ LN r = 0.96 and − LN R = 0.998) supports this argument.
The aging rates of desiccation-tolerant seeds depend on water content and storage temperature, which are factors that determine the formation and stability of the vitreous state (Walters et al. 2005). The stabilizing effect of the vitreous state on macromolecular and structural components during storage is key to its essential role to ensure seed longevity (Sano et al. 2016). In addition, there is a linear relationship between the rate of aging and molecular mobility found for different tissues over a wide range of temperatures and MCs, which suggests that aging velocities are affected by intracellular viscosity (Golovina et al. 1997; Golovina et al. 2010; Mira et al. 2015). Generally, seed deterioration accelerates when tissues are not vitreous (Walters et al. 2005). If the longevity predictions based on the molecular mobility model are correct, then the longevity at cryogenic temperatures is higher than that estimated at 5°C (Walters et al. 2004), which is in complete agreement with the germination rate, accelerated aging, and redox metabolism results obtained in the present study (Figs. 1, 2, and 3).
However, the retention of seed viability during storage cannot be explained by vitreous state theory alone, whereby additional drying would reduce molecular mobility and prolong the useful life of the germplasm (Buitink and Leprince 2004). Rather, it has been determined that the characteristics of the intracellular vitreous matrix are modified below the MC corresponding to the optimum value, and ultra-dry storage may not be beneficial (Vertucci et al. 1994). While above this optimum MC the molecular mobility decreases with the loss of water, it increases again with drying beyond that value (Leprince and Walters-Vertucci 1995; Buitink et al. 1998; Buitink et al. 1999; Buitink and Leprince 2008). The formation of spaces, in which molecules such as oxygen and free radicals diffuse more freely despite the presence of the vitreous state, may be the cause of the increased mobility at MCs below the optimum (Buitink and Leprince 2004; Berjak 2006). An increase in molecular mobility, at MCs < 2.1%, could therefore be the cause of the increased levels of oxidative stress observed at the lowest MCs in this study. The increased rigidity of membranes due to ROS-mediated peroxidation could also explain the increased electrolyte leakage at these MCs. On the other hand, it is assumed that exposure of SS-96 seeds to LN caused vitrification (Walters et al. 2004). Therefore, the lower molecular mobility in the seeds conserved in LN could be the cause of the decrease in the reduced levels of oxidative stress in seeds stored at cryogenic temperatures relative to those stored at 5°C, significant at MCs > 8.0% (Fig. 3 A, B).
Exposure of seeds with MC ≥ 13.0% to LN caused damage to membrane structural integrity, as indicated by the significant increase in electrolyte leakage (Fig. 2 B). Pritchard (1995) and Hor et al. (2005) found a significant negative correlation between the high moisture freezing limit (HMFL) and seed lipid content. If seeds of N. tabacum L. are considered to contain 35–38% lipids (fresh weight basis) (Fuchs et al. 2013), then the HMFL for SS-96 seeds from the equation proposed by these authors would be in the vicinity of 13.0% MC. Therefore, the formation of ice crystals could be the primary cause of the loss of the integrity + LN seeds at MCs ≥ 13.0%. In addition, according to Benson and Bremner (2004), the loss of physical compartmentalization and metabolic decoupling has a negative effect on both primary metabolism and antioxidant defenses. When this loss occurs, such as during freezing, the metabolic imbalance leads to the production of toxic free radicals and their reaction products. There is experimental evidence that mitochondrial enzymes and adenosine triphosphate synthesis are dissociated with freeze–thaw cycles (Tappell 1966).
At the same MC range, − LN seeds suffered greater damage during the 30 d of storage than those conserved in LN (Figs. 1 and 2). High relative humidity and temperature conditions favor the formation of intermediate products related to aging and seed degradation (Leprince and Hoekstra 1998; Walters et al. 2005; Mira et al. 2010). In the present study, the presumed higher velocity of aging reactions at 5°C, relative to cryogenic temperatures, could have led to higher levels of metabolic imbalance and seed degradation in the former, as indicated by the poorer germination rate retention and more rapid aging of − LN seed.
Seed longevity during storage is closely related to the activation of antioxidant systems (Bailly et al. 2001; Bailly et al. 2008; Varghese and Naithani 2008). Lee et al. (2010) investigated the effects of the simultaneous overexpression of two antioxidant enzymes on the longevity and germination of tobacco seeds under stressful conditions. Transgenic seeds showed an increase in the enzyme activities of Cu/Zn-SOD and ascorbate peroxidase (APX) during their development and maintained the enzymatic activity after 2 yr of dry storage at room temperature. Transgenic seeds after this storage period showed lower electrolyte loss and higher vigor under various abiotic stress conditions compared to untransformed seeds. Those authors concluded that the simultaneous overexpression of the Cu/Zn-SOD and APX genes increased longevity and seed germination by attenuating the effect of oxidative stress produced during prolonged storage conditions and severe environmental stress.
At MCs below 8.0%, there were no significant changes in SOD and CAT enzymatic activities in the present study (Fig. 3 C, D). Bailly (2004) is of the opinion that the low molecular mobility at these levels of hydration diminishes the accessibility of the enzymes to their substrates. This situation suggests that the prevention of oxidative damage at low MCs could be more related to the elimination of ROS by antioxidant compounds (Fernández-Marín et al. 2013; Morscher et al. 2015). Similar reasoning can be applied to seeds stored at − 196°C, regardless of the MC at which they were stored.
In the present experiments, in the seeds stored at 5°C, an increase in SOD and CAT activities was observed when seeds were hydrated from 8.0 to 13.0% (Fig. 3 C, D). However, this increase in antioxidant activity could not curb ROS production, which may explain why germination rate decreases at 13.0% MC and levels of oxidative stress increased. After reaching a maximum of 13.0% MC, SOD and CAT activities suddenly declined in the 5°C conserved seeds when they were hydrated to 18.0%, and there was a simultaneous increase in ROS production. MCs in this range are considered extremely hazardous for seed conservation but still associated with low fluidity of the aqueous phase in cells (Berjak et al. 2007), which probably reduced the efficiency of the antioxidant mechanisms in the seeds investigated in this study. Enzymes such as SOD and CAT could therefore become targets of ROS attack with the consequent loss of their activity.
Numerous studies support the fact that reduced MC, under certain conditions, increases the longevity of orthodox seeds in storage (Mira et al. 2010; Walters et al. 2010). In addition, the benefit of the optimal MC has been shown for species such as Cicer arietinum L., Gossypium hirsutum L., Sesamum indicum L., Zea maize L., and Lactuca sativa L. (Probert 2003; Singh et al. 2003; Mira et al. 2010). Likewise, MCs similar to those identified to be optimum in the present study have been reported to extend the storage of N. tabacum by years in other studies (Meillng and Xiuping 1997).
Visscher et al. (2016) noted that the examples of longer-term seed survival in the ultra-dry state are associated with air-tight conditions (particularly, heat-sealed glass vials), suggesting that the potentially pernicious effect of extreme drying may be less damaging in the absence of oxygen. Therefore, anoxia, like ultra-drying, not only is a characteristic of ‘novel’ environments, such as the vacuum of space, but can also be imposed artificially in ex situ seed banks by seed cryopreservation in LN. It follows that the viability of seeds stored under an oxygen-free (anoxic) environment, whether imposed artificially or naturally, may slow the rate of viability loss (Groot et al. 2015).
Conclusions
The present research validates the importance of reduced MC in the long-term conservation of seeds and reinforces previous findings that optimum MCs should not be considered independently of the storage temperature. The cryopreserved tobacco seeds of SS-96 with a MC of 2.1%, exhibited the highest viability due to greater cell membrane integrity, which was reflected by lower lipid peroxidation and reduced electrolyte leakage due to relatively lower ROS production and higher antioxidant protection. It is therefore recommended that SS-96 seeds be cryopreserved at 2.1%, because drying the seeds below this MC did not improve storage longevity.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107
Bailly C, Audigier C, Ladonne F, Wagner MH, Coste F, Corbineau F, Côme D (2001) Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J Exp Bot 52:701–708
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biologies 331:806–814
Benson EE, Bremner D (2004) Oxidative stress in the frozen plant: a free radical point of view. In: Fuller BJ, Lane N, Benson EE (eds) Life in the frozen state. CRC Press, Boca Raton, Florida, USA, pp 205–242
Berjak P (2006) Unifying perspectives of some mechanisms basic to desiccation tolerance across life forms. Seed Sci Res 16:1–15
Berjak P, Farrant JM, Pammenter NW (2007) Seed desiccation-tolerance mechanisms. In: Jenks MA, Wood AJ (eds) Plant desiccation tolerance, 1st edn. Blackwell Publishing, Iowa, USA, pp 151–192
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Buitink J, Claessens MMAE, Hemminga MA, Hoekstra FA (1998) Influence of water content and temperature on molecular mobility and intracellular glasses in seed and pollen. Plant Physiol 118:531–541
Buitink J, Hemminga MA, Hoekstra FA (1999) Characterization of molecular mobility in seed tissues: an electron paramagnetic resonance spin probe study. Biophys J 76:3315–3322
Buitink J, Leprince O (2004) Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology 48:215–228
Buitink J, Leprince O (2008) Intracellular glasses and seed survival in the dry state. C R Biologies 331:788–795
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570
Fernández-Marín B, Kranner I, Sebastián MS, Artetxe U, Laza JM, Vilas JL, Pritchard HW, Nadajaran J, Míguez F, Becerril JM, García-Plazaola JI (2013) Evidence for the absence of enzymatic reactions in the glassy state. A case study of xanthophyll cycle pigments in the desiccation-tolerant moss Syntrichia ruralis. J Exp Bot 64:3033–3043
Fuchs J, Neuberger T, Rolletschek H, Schiebold S, Nguyen TH, Borisjuk N, Börner A, Melkus G, Jakob P, Borisjuk L (2013) A noninvasive platform for imaging and quantifying oil storage in submillimeter tobacco seed. Plant Physiol 161:583–593
Golovina EA, As HV, Hoekstra FA (2010) Membrane chemical stability and seed longevity. Eur Biophys J 39:657–668
Golovina EA, Wolkers WF, Hoekstra FA (1997) Long-term stability of protein secondary structure in dry seeds. Comp Biochem Physiol 117:343–348
Gonzalez-Arnao M, Martinez-Montero M, Cruz-Cruz C, Engelmann F (2014) Advances in cryogenic techniques for the long-term preservation of plant biodiversity. In: Ahuja M, Ramawat K (eds) Biotechnology and biodiversity, sustainable development and biodiversity. Springer International Publishing, Switzerland, pp 129–170
González-Benito ME, Iriondo JM, Pérez-García F (1998) Seed cryopreservation: an alternative method for the conservation of Spanish endemics. Seed Sci Technol 26:257–262
Groot SP, de Groot L, Kodde J, van Treuren R (2015) Prolonging the longevity of ex situ conserved seeds by storage under anoxia. Plant Genet Resour 13:18–26
Groot SP, Surki AA, de Vos RCH, Kodde J (2012) Seed storage at elevated partial pressure of oxygen, a fast method for analysing seed ageing under dry conditions. Ann Bot 110:1149–1159
Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K (2010) Chemistry and biochemistry of lipid peroxidation products. Free Radic Res 44:1098–1124
Hor YL, Kim YJ, Ugap A, Chabrillange N, Sinniah UR, Engelmann F, Dussert S (2005) Optimal hydration status for cryopreservation of intermediate oily seeds: citrus as a case study. Ann Bot 95:1153–1161
ISTA (2005) International rules for seed testing. International Seed Testing Association, Bassersdorf, Suiza
Kumutha D, Ezhilmathi K, Sairam RK, Srivastava GC, Deshmukh PS, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol Plant 53:75–84
Lee YP, Baek KH, Lee HS, Kwak SS, Bang JW, Kwon SY (2010) Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J Exp Bot 61:2499–2506
Leprince O, Hoekstra FA (1998) The responses of cytochrome redox state and energy metabolism to dehydration support a role for cytoplasmic viscosity in desiccation tolerance. Plant Physiol 118:1253–1264
Leprince O, Walters-Vertucci C (1995) A calorimetric study of the glass transition behaviors in axes of Phaseolus vulgaris L. seeds with relevance to storage stability. Plant Physiol 109:1471–1481
Li DZ, Pritchard HW (2009) The science and economics of ex situ plant conservation. Trends Plant Sci 14:614–621
Liu K, Li Y, Chen F, Yong F (2016) Lipid oxidation of brown rice stored at different temperatures. Int J Food Sci Technol 52(1):188–195
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Meillng X, Xiuping L (1997) Report of ultra dry preservation technology for tobacco seeds. Seed Industry and AGPD, Cultural Development, Beijlng, China 4
Michalak M, Plitta-Mchalak BP, Chmielarz P (2015) A new insight in desiccation tolerance and cryopreservation of mazzard cherry (Prunus avium L.) seeds. Open Life Sci 10:354–364
Mira S, Estrelles E, González-Benito ME (2015) Effect of water content and temperature on seed longevity of seven Brassicaceae species after 5 years of storage. Plant Biol 17:153–162
Mira S, González ME, Hill LM, Walters C (2010) Characterization of volatile production during storage of lettuce (Lactuca sativa) seed. J Exp Bot 61(14):3915–3924
Morscher F, Kranner I, Arc E, Bailly C, Roach T (2015) Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann Bot 116:669–678
Nagel M, Kodde J, Pistrick S, Mascher M, Börner A, Groot SPC (2016) Barley seed aging: genetics behind the dry elevated pressure of oxygen aging and moist controlled deterioration. Frontiers in Plant Sci 7:1–11
Navarro M, Febles G, Herrera RS (2015) Vigor: essential element for seed quality. Cuban J Agri Sci 49:447–458
Perry DA (1984) Manual de métodos de ensayos de vigor. Instituto Nacional de Semillas y Plantas de Vivero. Ministerio de Agricultura, Pesca y Alimentación, Madrid, España
Pritchard HW (1995) Cryopreservation of seeds. In: Day JG, McLellan MR (eds) Methods in molecular biology, vol 38. Humana Press Inc., Totowa, NJ, pp 133–144
Pritchard HW (2007) Cryopreservation of desiccation-tolerant seeds. In: Day JG, Stacey GN (eds) Methods in molecular biology: cryopreservation and freeze-drying protocols, 2nd edn. Humana Press Inc., Totowa, NJ, pp 185–201
Probert RJ (2003) Seed viability under ambient conditions, and the importance of drying. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ (eds) Seed conservation: turning science into practice. Kew, UK, Royal Botanic Gardens, pp 725–743
Rao NK, Hanson J, Dulloo ME, Ghosh K, Novell D, Larinde M (2007) Manual para el manejo de semillas en bancos de germoplasma. Manuales para Bancos de Germoplasma No. 8. Bioversity International, Roma, Italia
Sano N, Rajjou L, North HM, Debeaujon I, Marion-Poll A, Seo M (2016) Staying alive: molecular aspects of seed longevity. Plant Cell Physiol 57:660–674
Singh N, Singh AK, Dhillon BS (2003) Effect of ultra-drying on ex situ seed conservation. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ (eds) Seed conservation: turning science into practice. Royal Botanic Gardens, Kew, Reino Unido, pp 797–805
Stanwood PC, Bass LN (1981) Seed germplasm preservation using liquid nitrogen. Seed Sci Technol 9:423–437
Tappell AL (1966) Effects of low temperatures and freezing on enzymes and enzyme systems. In: Meryman HT (ed) Cryobiology. Academic Press, New York, pp 163–177
Thanos CA, Doussi MA (1995) Ecophysiology of seed germination in endemic labiates of Crete. Israel J Plant Sci 43:227–237
Touchell DH, Dixon KW (1994) Cryopreservation for seedbanking of Australian species. Ann Bot 74:541–546
Varghese B, Naithani SC (2008) Oxidative metabolism-related changes in cryogenically stored neem (Azadirachta indica A. Juss) seeds. J Plant Physiol 165:755–765
Veiga-Barbosa L, Mira S, González-Benito ME, Souza MM, Meletti LMM, Pérez-García F (2013) Seed germination, desiccation tolerance and cryopreservation of Passiflora species. Seed Sci Technol 41:89–97
Verdier J, Lalanne D, Pelletier S, Torres-Jerez I, Righetti K, Bandyopadhyay K, Leprince O, Chatelain E, Vu BL, Gouzy J, Gamas P, Udvardi MK, Buitink J (2013) A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of Medicago truncatula seeds. Plant Physiol 163:757–774
Vertucci CW, Roos EE, Crane J (1994) Theoretical basis of protocols for seed storage III. Optimum moisture contents for pea seeds stored at different temperatures. Ann Bot 74:531–540
Veselovsky VA, Veselova TV (2012) Lipid peroxidation, carbohydrate hydrolysis, and Amadori–Maillard reaction at early stages of dry seed aging. Russ J Plant Physiol 59:763–770
Visscher AM, Seal CE, Newton RJ, Frances AL, Pritchard HW (2016) Dry seeds and environmental extremes: consequences for seed lifespan and germination. Funct Plant Biol 43:656–668
Volk GM, Crane J, Caspersen AM, Hill LM, Gardner C, Walters C (2006) Massive cellular disruption occurs during early imbibition of Cuphea seeds containing crystallized triacylglycerols. Planta 224:1415–1426
Walters C, Ballesteros D, Vertucci VA (2010) Structural mechanics of seed deterioration: standing the test of time. Plant Sci 179:565–573
Walters C, Hill LM, Wheeler LJ (2005) Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integr Comp Biol 45:751–758
Walters C, Wheeler L, Stanwood PC (2004) Longevity of cryogenically stored seeds. Cryobiology 48:229–244
Xin X, Jing X-M, Liu Y, Song S-Q (2010) Viability loss pattern under rapid dehydration of Antiaris toxicaria axes and its relation to oxidative damage. J Integr Plant Biol 52(5):434–441
Zagorchev L, Seal CE, Kranner I, Odjakova M (2013) A central role for thiols in plant tolerance to abiotic stress. Int J Mol Sci 14:7405–7432
Zhou B, Wang J, Guo Z, Tan H, Zhu X (2006) A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul 49:113–118
Acknowledgements
We thank Magister Gilberto Torrecilla Guerra and Magister Otilio Ruiz Padrón for advising and editing at initial steps of the work and the manuscript. We thank also the participation of Dr. Sershen at the end to help edit the manuscript for language and improved aspects related to data presentation, interpretation, and discussion. This study was part of JLPR’s Magister Thesis from University of Ciego de Avila (Cuba).
Funding
This research was funded by the Cuban Ministry for Agriculture.
Author information
Authors and Affiliations
Contributions
Magister Juan Luis Pérez-Rodríguez (JLPR) was heavily involved in sourcing funding to support the project. Moreover, JLPR was involved in the design of all experiments, acquisition, analysis, and interpretation (statistical assessment) for the study. JLPR was also the primary person responsible for the initial drafting of the work (including the writing of manuscript and construction of figures). JLPR was also a major contributor providing detailed comments, additions, and major text modifications for drafts as the manuscript developed. This study was part of JLPR’s Magister Thesis from the University of Ciego de Avila (Cuba). Magister René Carlos Rodríguez Escriba (RCRE) and Magister Gustavo Lorente González (GLG) were heavily involved in the planning, design, analysis, and interpretation of the accelerated aging test experiment. Moreover, RCRE and GLG contributed to the determination of generation rate of O2 ·−, H2O2 content and enzymatic activities of superoxide dismutase (SOD) and catalase (CAT) of Nicotiana tabacum L. cv. Sancti Spíritus 96 seeds collected. RCRE and GLG were also involved in the interpretation of data and contributed for the initial drafting of the manuscript. Dr. Justo González-Olmedo (JGO) was involved in the design of the all experiments and the acquisition of data and data management to align with the original concept of the work. Dr. JGO was also involved in the writing of manuscript. Dr. Marcos Martinez-Montero (MMM) was heavily involved in sourcing funding to support the project and the conception and design of all experiments. MMM was also heavily involved in the acquisition of the data sets and the interpretation of data and its presentation in the manuscript. MMM was also a major contributor providing detailed comments, additions, and text modifications for different drafts of the manuscript. Finally, all authors approve the submission of this manuscript to be published and agree to be accountable for all aspects of the work.
Corresponding author
Additional information
Editor: Barbara Reed
Rights and permissions
About this article
Cite this article
Pérez-Rodríguez, J.L., Rodríguez Escriba, R.C., Lorente González, G.Y. et al. Effect of desiccation on physiological and biochemical indicators associated with the germination and vigor of cryopreserved seeds of Nicotiana tabacum L. cv. Sancti Spíritus 96. In Vitro Cell.Dev.Biol.-Plant 53, 440–448 (2017). https://doi.org/10.1007/s11627-017-9857-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9857-y