Abstract
Red fluorescent protein (DsRed) from reef coral was evaluated in comparison with green fluorescent protein (GFP) as a reporter gene for cassava transformation. Cassava friable embryogenic callus (FEC) was transformed with ER-targeted versions of DsRed and GFP constructs driven by the 35S cauliflower mosaic virus promoter. Efficiency of transformation was comparable for both visual marker genes at averages of 119 and 163 expressing plants recovered per cc of settled cell volume FEC for GFP and DsRed, respectively. High and uniform DsRed expression was observed at the single cell and proliferating callus stages, in somatic embryos and within organs of whole in vitro and greenhouse-grown plants in a manner similar to GFP. Plants expressing GFP and DsRed were robust and phenotypically normal with regard to growth, vigor, and formation of storage roots when grown in the greenhouse. Expression of marker genes within cross sections of petiole, woody stem, and storage roots from greenhouse-grown plants was determined. The interference of phenolic compounds and chlorotic tissues characteristic of the signal from GFP-expressing tissues was not observed within tissues transgenic for DsRed. Tissues and plants co-expressing DsRed and GFP were produced by co-culturing FEC with a mixed Agrobacterium suspension carrying GFP and DsRed gene constructs or by re-transformation of an existing GFP transgenic line with DsRed. Re-transformation of GFP-expressing tissues was the more efficient method for production of GFP/DsRed stacked plants. Co-expression of both marker genes within the same transformation unit was easily visualized at their respective wavelength with the aid of appropriate filters thus validating their potential for co-expression studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic transformation of the outcrossing, heterozygous, tropical root crop, cassava (Manihot esculenta Crantz) offers significant potential for integration of traits for enhanced nutritional content, elevated yield, pest and disease resistance, modified starch quality, reduced cyanogenic glycoside content, and improved post-harvest qualities (Fregene and Puonti-Kaerlas 2002; Taylor et al. 2004; Liu et al. 2011). Development of efficient transformation systems therefore remains an important research activity in cassava. Such efforts are empowered by access to suitable visual marker systems to allow assessment of the gene transfer process and subsequent steps of cell division, selection, and plant regeneration. Reporter genes also act as valuable tools in the study of gene function, promoter actions, and location of gene products within biological systems (Patterson 2007). Visual marker genes employed for cassava transformation systems to date include β-glucuronidase (GUS), (Zhang and Puonti-Kaerlas 2000), firefly luciferase (LUC) (Schrott 1995; Petersen et al. 2005), and green fluorescent protein (GFP) (Cubitt et al. 1995; Taylor et al. 2004; Nyaboga et al. 2013). While use of GUS and LUC are effective, their detection and monitoring are lethal to the tissues under study. Fluorescent proteins carry the benefit of being non-destructive, allowing continuous monitoring of the same putative transgenic tissues over time and across developmental process within tissue culture and transformation systems (Jach et al. 2001; Finer et al. 2006).
GFP, originally isolated from the jellyfish Aequorea victoria, is the best-understood and most widely applied fluorescent protein. Although GFP is commonly used in plants, its detection can be problematic due to the presence of endogenous compounds such as phenols that fluoresce in the green to yellow range with lower excitation wavelengths (Wenck 2006). Detection of GFP in chlorotic tissues can also be compromised due to masking from autofluorescence emanating from chlorophyll (Chiu et al. 1996; Wenck 2006).
The need for a red-emitting fluorescent protein that does not suffer these limitations resulted in the development of a fluorescent protein derived from the coral Discosoma striata, commonly referred to as DsRed, which possesses excitation and emission maxima at 558 and 583 nm, respectively (Patterson et al. 2001). Use of wild-type DsRed was limited due to its slow maturation rate and intermediate green state that can be problematic due to spectral overlap when co-expressed with other fluorescent proteins (Terskikh et al. 2002). Mutagenesis of wild-type DsRed drFP583 has yielded numerous variants such DsRed2, DsRedT1 (DsRed Express), and DsRedT3, all of which are designed to overcome limitations inherent in the original version (Baird et al. 2000). The first use of DsRed, as a reporter of plant gene expression, was demonstrated by Mas et al. (2000), by showing nuclear localization in tobacco BY2 protoplast cells. Since that time, effective expression and visualization of DsRed has been reported in several monocotyledonous and dicotyledonous species (Wenck et al. 2003).
Genetic transformation systems have been developed for cassava since the first reports in 1996 (Raemakers et al. 1996; Schopke et al. 1996; Gonzalez et al. 1998). Utilization of friable embryogenic callus developed from somatic embryogenic structures is now the most widely adopted method for use as target tissues for Agrobacterium-mediated transformation (Liu et al. 2011; Taylor et al. 2012). We report here genetic transformation of FEC and plant regeneration with DsRed and its evaluation as an alternative and additional fluorescent protein for use in cassava genetic transformation systems. Its efficacy is compared with GFP, as well as the ability to co-express and co-visualize both fluorescent proteins within the same organs.
Materials and Methods
Clone construction and Agrobacterium transformation.
A DsRed endoplasmic reticulum (ER)-targeted expression cassette driven by cauliflower mosaic virus promoter (35S) was PCR-amplified from pDsRed (Clontech, Mountain View, CA) and fused to the nopaline synthase terminator (NOS) using high fidelity Platinum(R) Taq Polymerase (Invitrogen, Waltham, MA) and the following primer sequences: CTGCAGCAAGCTTCTGGCCATCAT (forward) and TCTAGACCGATCTAGTAACATAGATGACACC (reverse).
The 1489-bp PCR fragment was digested with Pst1 and Xba1 (New England Biolabs, Ipswich, MA), the restricted fragments fractionated by agarose gel electrophoresis, purified using a Qiagen gel purification kit (Qiagen, Valencia, CA) and subcloned into plasmid vector p2300 (www.pCambia.org) to derive p900 (pCambia 2300:35S:DsRed-er T-nos). The 35S-promoter-driven DsRed cassette was excised from p900 as an Xba1/Pst1 fragment and cloned into pCambia1300 (www.pCambia.org) to generate p908 (pCambia 1300:35S: DsRed-er T-nos) in which the selectable marker gene was hpt. Plasmid p908 and a modified GFP ER-targeted expression cassette driven by the e35S promoter (p8116) fused to nptII selectable marker driven by a double 35S promoter (Taylor et al. 2012) were independently transformed into Agrobacterium tumefaciens strain LBA4404 by electroporation using Gene pulser®II (Bio-Rad, Irvine, CA).
Production of transgenic plants.
-
1.
Production of GFP and DsRed transgenic plants. Genetic transformation of cassava was achieved using friable embryogenic callus (FEC) of cultivar 60444 as described by Taylor et al. (2012) with minor modifications. In vitro shoot cuttings were maintained on MS basal medium (Murashige and Skoog 1962) supplemented with 2% (w/v) sucrose (MS2) and used as the source of leaf explants. Organized embryogenic structures (OES) were induced from immature leaves 2–5 mm in length by culturing for 4 wk on DKW/Juglans basal medium (Vahdati et al. 2004) supplemented with 2% (w/v) sucrose, MS vitamins, and 50 μM picloram. OES was excised and used to establish FEC target tissues through three successive culture cycles on Gresshoff and Doy basal medium (Gresshoff and Doy 1972) supplemented with 2% (w/v) sucrose and 50 μM picloram (GD2 50P) (Taylor et al. 2001, 2012). Eighteen to 21 d after subculture to the third cycle on GD2 50P, FEC was transformed via Agrobacterium strain LBA40444 and callus, embryo, and plant lines recovered as described by Taylor et al. (2012).
Transformation efficiency was determined by calculating the number of callus, embryo, and plant lines derived per settled cell volume (SCV) of the starting FEC material. The SCV was measured by transferring the FEC target tissues at the end of the 3-d co-culture period to a 15-mL Falcon tube, allowing the tissue to settle for a period of 30 min, and the graduated scale used to determine the volume of the embryogenic tissues (Taylor et al. 2012).
-
2.
Production of GFP/DsRed stacked transgenic plants by re-transformation of GFP expressing FEC. GFP-expressing lines were assessed for T-DNA copy number by Southern blot analysis. A single copy line designated Line 36A was selected for induction of OES and FEC target tissues as described above for re-transformation with DsRed. GFP-expressing callus was co-cultured with Agrobacterium carrying construct p908 harboring DsRed and the hygromycin selectable (hpt) marker. Transgenic tissues were recovered on medium containing 40 μg/mL hygromycin to regenerate GFP/DsRed co-expressing plants following the subculture regimen described by Taylor et al. (2012).
-
3.
Co-transformation with DsRed and GFP. Overnight cultures of Agrobacterium independently harboring p908 and p8116 were adjusted to an A600 of 0.05 using GD2 50P medium. Suspensions were combined to generate a 10-mL total volume with combinations of 5:5 mL, 2:8 mL, 1:9 mL, and 9:1 mL for 1:1, 1:5, 1:10, and 10:1 ratios of p900 and p8116, respectively. FEC was co-cultured with the Agrobacteria and co-transformed transgenic tissues selected and recovered as described.
Microscopy and visual assessment of transgenic plants and organs.
GFP, DsRed, and GFP/DsRed co-expressing transgenic plant lines were transferred to soil in 7-cm Landmark square pots and established in the greenhouse as previously described by Taylor et al. (2012) (Yadav et al. 2011). The plants were grown for a period of 3 mo to allow development of storage roots.
A Nikon SMZ 1500 dissection microscope was used to visualize GFP and DsRed fluorescing tissues by means of fluorescence illumination. For GFP, a blue GFP/DAPI filter set with excitation wavelength range of 379–401 nm, dichroic mirror of 420 nm, and emission wavelength range of 435–485 nm was used, while a TRITC filter set with excitation wavelength range of HQ 530–560 nm, dichroic mirror Q 570 (LP), and emission wavelength range of 590–650 nm was employed for detection of DsRed.
Determination of GFP/DsRed co-expressing events was analyzed by sliding between the GFP and DsRed filter sets while viewing the same tissue. Transient expression of the two visual marker genes was determined at the end of the 3-d co-culture period using a visual scale of 1 to 5, where a score of 1 corresponds to 1 to 249 visible fluorescent units per SCV of FEC sample, 2 = 50 to 500, 3 = 501 to 1000, and 4 = 1001 to 3000, and 5 is greater than 3000 expressing units per SCV of FEC sample. Ten days later, the cultures growing on GD2 50P medium containing 250 mg/L carbenicillin were removed from the 28°C growth chamber and visualized using the fluorescence dissecting microscope at either GFP or DsRed excitation wavelength. Fluorescence units were counted as the plate was moved through the light source and were scored accordingly. Stably expressing GFP and DsRed callus lines growing under antibiotic selection pressure (27.5 μg/mL paramomycin for DsRed and GFP transgenics as well as the co-transformed events, 40 μg/mL hygromycin for the retransformed events) were subsequently transferred to stage 1 regeneration medium (MS2 media supplemented with 5 μM NAA plus 45 μM paramomycin or 40 μg/mL hygromycin). Stably expressing viable tissues were counted and recorded as independent events 21 d after transfer to stage 1 regeneration medium.
Cross sections of petioles, stems, and storage roots from 3-mo-old greenhouse-grown plants were produced by hand sectioning using a sharp single edge razor. Tissue sections were immersed in sterile water to prevent desiccation and then transferred to a microscope slide in water and covered with a cover slip. Images were captured by means of Q imaging software and a RETIGA 1300 camera attached to the Nikon SMZ 1500 fluorescence dissecting microscope. The images were adjusted for brightness and contrast using Adobe Photoshop.
Southern blot analysis.
DNA samples were isolated from in vitro propagated GFP- and DsRed-expressing plantlets using the Dellaporta DNA extraction protocol (Dellaporta et al. 1983). DNA quality was determined by agarose gel electrophoresis and quantified by NanoDrop 2000 UV-Vis Spectrophotometer (Thermo Scientific, Pittsburgh, PA). A total of 10 μg was digested overnight with HindIII and then run overnight in a 0.8% (w/v) agarose gel at 35 V before blotting onto Amersham Hybond-N+nylon membrane (GE Healthcare, Piscataway, NJ).
Probes were prepared using PCR DIG synthesis kit from Roche and the corresponding primers, DsRed (TTTGGAGTCAACATAGTAGTACCCTGGTAG sense primer and ATGTCAAGCACCCTGCCGACA antisense primer) and GFP (TTGTTTGTCTGCCGTGATGT sense primer and GGTGATGTTAATGGGCACAA antisense primer) at 40°C overnight. CDP-star (Roche Applied Science, Indianapolis, IN) was used according to manufacturer’s instructions for the detection of bands and viewed using a developed X-ray film.
Results
Production of DsRed- and GFP-expressing plants.
Tissues were scored for presence of GFP and DsRed at 3 and 13 d after inoculation with Agrobacterium. Numbers of visually detectable, transiently expressing single cells seen at the end of the co-culture period (3 d after Agrobacterium inoculation) were similar for both marker genes. Tissues transformed with GFP produced an average of 140 fluorescing cells per cc SCV of FEC sample and DsRed an average of 180 fluorescing cells per cc SCV FEC sample (Fig. 1a ). Ten days later, the average number of stably fluorescing cells for both marker genes had decreased to 100 and 150 fluorescing cells per cc SCV FEC starting material for GFP and DsRed, respectively (Fig. 1a ).
Production and recovery of DsRed- and GFP-expressing tissues in cv. 60444. (a) Assessment of transformation at three (transient) and 10 d (stable) after co-culture. Average scores for transient and stable DsRed and GFP expression were evaluated by counting expressing cells. (b) Evaluation of GFP and DsRed expression at different stages (callus, embryo, or plantlets) during the regeneration process. Transformation efficiency with DsRed was greater in comparison to GFP giving higher values of callus, embryo, and plant lines recovered per cc SCV of starting target material.
Independent viable lines expressing both marker genes were determined by scoring for GFP- and DsRed-expressing callus lines after 21-d culture on stage 1 regeneration medium. Agrobacterium-mediated genetic transformation of FEC with p8116 (e35S-ERGFP) generated an average of 119 GFP-expressing callus lines per cc SCV, from which an average of 54 cotyledon-stage embryos lines were generated on BAP-containing maturation medium. Subsequent transfer to germination medium resulted in regeneration of 41 GFP-expressing plant lines per cc SCV starting tissue. Transformation with DsRed was more efficient in this study such that FEC transformed with p900 (35S-DsRed) generated an average of 163 DsRed expressing callus lines per cc SCV from which an average of 78 and 68 DsRed-expressing embryo and plant lines were recovered per cc SCV respectively (Fig. 1b ).
Recovery of DsRed/GFP stacked transgenic plants.
Transgenic events co-expressing DsRed and GFP were generated by two methods. In the first, a GFP-expressing plant line produced as described above, transgenic for a single copy of the T-DNA, was re-transformed with DsRed. In the second method, Agrobacterium suspensions carrying plasmid p900 or p8116 were prepared and mixed and used for inoculation and co-culture of non-transgenic FEC target tissues.
GFP-expressing plant lines obtained by transformation with p8116 were analyzed by Southern blotting to identify those carrying a single integration of the T-DNA (Fig. 7a ). One such transgenic line, designated Line 36, was chosen. FEC target tissues were generated from Line 36 and re-transformed with p908, harboring the hygromycin selection marker and DsRed visual marker gene. Hygromycin killing curves were determined by treating non-transgenic FEC tissues with GD2 50P medium supplemented with concentrations of hygromycin ranging from 0, 10, 20, 30, to 40 μg/mL and mortality rates determined every 7 d for a period of 4 wk. The experiment was repeated three times. Mortality was evaluated visually by comparing the viability of tissues on various concentrations of hygromycin with the control on zero concentration. White pale tissues were scored as dead and yellow lustrous tissues scored as viable. The number of dead to viable tissue was used to determine percentage survival of the tissues. Preliminary results indicated that mortality rates of 42, 81, 88, and 100% were recorded at 7, 14, 21, and 28 d, respectively, when tissues were cultured on medium containing 40 μg/mL hygromycin (data not shown). This level of antibiotics was therefore adopted for subsequent selection of transgenic events through the callus dividing and maturation stages. Utilizing this method, an average of 11 GFP/DsRed co-expressing plant lines were recovered per cc SCV from re-transforming FEC tissues derived from Line 36 (Fig. 2). As a control, FEC derived from Line 36A target were regenerated alongside the transformation experiment (without hygromycin selection) to evaluate the efficiency of retransforming transgenic GFP-expressing tissues.
Assessment of either GFP or GFP/DsRed stacked events at various developmental stages (callus, embryo, or plantlets) of the transformation system. Transformation and recovery of GFP/DsRed stacked events at various transformation stages was compared with GFP transgenic control. Numbers of GFP/DsRed stacked callus lines recovered were less compared to the GFP control. At the embryo and plant stages, more stacked events were recovered compared to the control. Re-transformation of GFP transgenic tissues with DsRed had no adverse effect on tissue and plant recovery.
Production of transgenic events co-expressing DsRed and GFP was also achieved by simultaneous co-transformation with Agrobacterium carrying p908 and p8116. Averages of 20, 26, 7, and 11 independent lines per cc SCV co-expressing both marker genes were recovered to the mature embryo stage from 1:1, 1:5, 1:10, and 10:1 p908:p8116 ratios used in the inoculation and co-culture stages, respectively (Fig. 3). These embryo lines were not transferred to MS2 medium for further regeneration in this experiment because plant lines are recoverable at about 70% from these tissues in cv. 60444, as reported by Taylor et al. (2012). The average number of GFP-expressing transgenic events recovered increased as the ratio of GFP to DsRed was increased in the mixed Agrobacterium suspension used for FEC co-culture. Conversely, transformation with Agrobacterium suspensions composed of a higher ratio of DsRed to GFP resulted in a higher proportion of the recovered events seen to be expressing DsRed. Co-expression efficiency for both marker genes within the same transgenic event was found to be 17, 24, 7, and 8% for DsRed/GFP ratios of 1:1, 1:5, 1:10, and 10:1, respectively.
Expression of transgenic events recovered after co-culture with various ratios of A. tumefaciens carrying gene constructs for the visual markers DsRed and GFP. Values are shown for lines expressing GFP only (GFP) and DsRed only (DsRed) and co-expressing DsRed and GFP (GFP/DsRED). Data is shown for independent transgenic lines recovered and scored at the end of the stage 2, embryo maturation step.
Visual assessment of DsRed and GFP expression.
Tissues were assessed for expression of DsRed and GFP at multiple stages after co-culture with Agrobacterium, to regeneration of whole plantlets in tissue culture and within 3-mo-old greenhouse-grown plants. Plants expressing GFP and DsRed singly, and plants stacked to co-express both visual marker genes were robust and appeared phenotypically normal with regard to growth and vigor when compared to non-transgenic controls (Fig. 4).
Transient expression of both fluorescent marker genes was visible shortly after Agrobacterium co-culture. In both cases, strongly expressing single cells could be easily visualized against the background signal from the non-transgenic FEC. The GFP signal matured rapidly with single expressing cells and was detectable as soon as 2 d after application of Agrobacterium. Similar expression of DsRed was not visible until 5 d after co-culture. Stable transformation was determined 10 d after co-culture when actively dividing multicellular expressing units could be seen. Expression of both reporter marker genes continued to be evaluated as they progressed through the transformation stages.
High and uniform DsRed expression was observed in the callus, somatic embryo, and organs of whole in vitro and greenhouse-generated plants in a manner similar to GFP (Fig. 5). Both marker genes showed strong signals when expressed independently at the callus and embryo stage (Fig. 5). DsRed however showed a brighter and distinct expression on chlorophyll-containing tissues compared to GFP due to interference with chlorophyll autofluorescence at this wavelength (Fig. 5 A3, B3). Visualization of DsRed on the co-expressing events was seen without any interference contrarily to the bleed-through experienced with GFP visualization under the GFP longpass filters, producing a characteristic orange color for co-expressing tissues. This did not constitute a problem as this distinctive color aided identification and isolation of cells and tissues transgenic for both marker genes.
Visual assessments of DsRed and GFP expression and co-expression of GFP and DsRed in the same plant at different stages in culture system: A1 to A3, expression of DsRed at callus, embryo and plantlet stages, respectively; B1 to B3, expression of GFP at callus, embryo, and plantlet stages, respectively; C1 to C3, co-expression of DsRed and GFP within the same tissues at callus, embryo, and plantlet stages, respectively, visualized by means of Nikon SMZ 1500 dissection microscope using blue GFP/DAP filter set with 379–401-nm excitation wavelength, 435–485-nm emission wavelength, TRITC filter with HQ 530–560-nm excitation wavelength and 590–650-nm emission wavelength for DsRed. D1 to D3, images of callus, embryo, and in vitro plantlets respectively under white light.
Visual assessment of sectioned cassava organs.
Expression of the marker genes within organs of greenhouse grown cassava plants was analyzed 34 mo after transfer to soil. Patterns of GFP and DsRed expression in cross sections of the petiole, semi-woody stem, and storage roots were similar for both marker genes, showing uniform expression across the sectioned organs, with good resolution of the vascular tissues seen in both cases (Fig. 6). Chlorophyll interference was observed within GFP-expressing leaves such that only the veins and midrib showed green fluorescence. Conversely, DsRed maintained its characteristic red fluorescence throughout the surface of the leaf (Fig. 6 A1, B1) allowing detailed expression of the marker gene to be observed in both the vascular and lamina tissues.
Visual assessment of DsRed and GFP expression and of co-expression of GFP and DsRed in cross sections of 12-wk-old soil-grown transgenic plants. A1–A4: constitutive expression of DsRed in a leaf and in cross sections of the petiole, semi-woody stem, and storage root, respectively. B1–B4: constitutive expression of GFP in a leaf and in cross sections of the petiole, semi-woody stem, and storage root, respectively. C1–C4: co-expression of DsRed and GFP within the same organs, leaf, and in cross sections of the petiole, semi-woody stem, and storage root, respectively.
No apparent interference was seen in the co-expression of both marker genes in the stacked DsRed/GFP events when visualizing cross sections of the petiole, woody stem, and roots (Fig. 6 C1–4).
Molecular analysis.
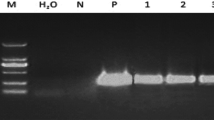
Transgene integration into cassava genome for both marker genes was confirmed by Southern blot analysis (Fig. 7). Nine GFP-expressing events were evaluated with six lines showing single copy transgene integration and two as multicopy integrations (Fig. 7a ). For DsRed integration and copy number, one plant line was seen to carry a single copy T-DNA while the other eight events showed integrations of between two and four copies (Fig. 7b ). Figure 7c confirms integration of the DsRed transgene into the genome of transgenic GFP line 2.
Southern blot analysis confirming GFP and DsRed integration into the cassava genome of cv. 60444. (a) GFP integration in p8116 recovered events using HindIII restriction enzyme with GFP probe showing samples 4 to 6 as single T-DNA integration events. Sample 4 representing Line 36A was chosen as target plant for gene targeting. (b) DsRed integration in p900 recovered events using Nhe1 restriction enzyme with DsRed probe. (c) DsRed integration in GFP/DsRed stacked events using Xba1 restriction with DsRed probe.
Discussion
A comparative evaluation of DsRed expression with GFP was carried out to determine the possible application of DsRed as an additional visual marker system in cassava transformation. Previous biochemical studies carried out by Baird et al. (2000) revealed the potential of DsRed as an alternative marker system to GFP. The number of plant lines recovered in any given transformation experiment is determined by numerous factors including the cultivar transformed, target tissue used, constructs carrying transgenes for the desired trait, and type of selection marker employed. The transformation efficiency for cassava cv. 60444 used in this study has been extensively optimized as compared to other farmer-preferred cultivars. The transformation efficiencies reported in the present studies were approximately twice that obtained by Taylor et al. (2012). An average of 22 transgenic plants per cc SCV FEC was obtained from cassava FEC transformed with siRNA constructs for resistance to CMD and CBSV compared to 68 and 41 expressing DsRed and GFP plant lines recovered per cc SCV recovered in the present study. Data from transformation efficiencies of cassava reported here therefore illustrate that recovery and expression of DsRed in cassava is equal to, or more efficient, than that of GFP. In both cases, transgenic plants were robust and appeared phenotypically normal with regard to growth and vigor when established in the greenhouse (Fig. 4).
DsRed showed similar, and to some extent, enhanced expression patterns at various stages of the transformation and regeneration process compared to GFP (Fig. 5A, B ). Distinct bright red fluorescence was observed from the single cell stage and throughout the callus maturation, somatic embryo regeneration, and plant recovery phases. This confirms the effectiveness of DsRed as a tool for tracking transgenic cells and tissues through the transformation and tissue culture system in a manner similar to GFP. At the whole plant level, minimal interference by phenolic compounds and chlorotic tissues was seen for DsRed when compared with GFP. Chlorotic tissues fluoresce red with lower excitation wavelengths such as used for green fluorescent proteins, thus masking expression of GFP in these tissues, whereas at the higher wavelength used for red fluorescent proteins, chlorophyll does not autofluoresce in the same manner (Wenck 2006). This was evident in our study where GFP expression was visible only at the veins and midrib of chlorophyll-containing tissues while pale red fluorescence emanated from the leaf surfaces as a result of chlorophyll autofluorescence. Expression of both marker genes was clearly visible within cross sections of the petiole, woody stem, and storage roots (Fig. 6), with high resolution of the cortex, the vascular bundles and pith within these organs.
In this study, the compatibility of DsRed and GFP for co-expression within the same transgenic cassava tissues was evaluated. The successful implementation of this system will enhance the stacking of multiple traits in cassava, providing information with regard to gene synergy and interference, and allow labeling of different transgenically expressed proteins and viral pathogens (Goodin et al. 2002). Both strategies employed to develop DsRed/GFP stacked co-expressing plants were successful. In the first, we report for the first time stacking of transgenes in cassava by re-transformation to integrate T-DNA carrying DsRed and hpt expression cassettes into a plant already transgenic for GFP and nptII. Hygromycin selective pressure was used to recover the stacked DsRed/GFP transgenic tissues and plants. In our hands selection and recovery of transgenic events with nptII proved more effective than hygromycin and differed from that reported by Zainuddin et al. (2012).
In a second gene stacking strategy, FEC target tissues were inoculated and co-cultured with Agrobacterium lines carrying either p900 (35S-DsRed-er) or p8116 (e35S-GFP-er) mixed at various ratios. Comparison of the two methods employed to produce the stacked DsRed/GFP events provides interesting contrast. Assessed for efficiency, re-transformation of the existing GFP-expressing line with DsRed was more efficient than simultaneous co-transformation with both marker genes. A total of 11 transgenic events per cc SCV of starting FEC material were recovered from the re-transformation experiment and nine of these events were co-expressing DsRed and GFP. In the co-transformation experiment, a total of 109 transgenic events per cc SCV were recovered from the 1:5 DsRed/GFP combining ratio and only 26 of these events co-expressed DsRed and GFP. Both methods have value, especially if given expression cassettes that are not compatible within the same T-DNA. However, although more efficient, stacking by re-transformation into an already transgenic line is less attractive if time is important, as 16–18 mo are required to recover the co-expressing events. Simultaneous co-transformation will produce co-expressing plant lines within 6 mo.
Co-expression of DsRed and GFP was evaluated by sliding between both filter sets on the microscope. Both marker genes showed similar localization and expression patterns at the cross sections of the petiole, woody stem, and storage roots (Fig. 6 C2–4). For the leaf surface, DsRed and GFP showed similar localization patterns but differed in their expression patterns. GFP expression was only detected at the mid-rib and veins but was masked by chlorophyll on the leaf blade, whereas DsRed exhibited clear and distinct expression at various parts of the leaf (Fig. 6 C1). No masking or silencing effect was observed between both marker genes during co-expression within the same cells or tissues. Goodin et al. (2002) conducted an extensive expression experiment to determine the practicality of using DsRed and GFP in co-localization studies in Nicotiana bethamiana and showed that DsRed and GFP fusion proteins can be distinguished in co-localization studies.
In this study, we have demonstrated the potential of DsRed as an alternative, non-destructive visual fluorescent marker for cassava transformation, thereby expanding the range of visual marker systems that could effectively be applied in cassava transgenic studies. Also, we were able to confirm the feasibility of co-expressing DsRed and GFP within a single transformation unit without cross-spectral interference. This study has provided a tool for studying multiple gene expression in cassava and describes simple methods for stacking multiple transgenes into cassava.
References
Baird GS, Zacharias DA, Tsien RY (2000) Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci U S A 97:11984–11989
Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY (1995) Understanding, improving and using green fluorescent proteins. Trends Biochem Sci 20:448–455
Dellaporta S, Wood J, Hicks JB (1983) A plant DNA minipreparation: Version II. Plant Mol Biol Rep 1:19–21
Finer JJ, Beck SL, Buenrostro-Nava MT, Chi Y, Ling PP (2006) Monitoring gene expression in plant tissues. In: Dutta Gupta S, Ibaraki Y (eds) Plant Tissue Culture Engineering, 6th edn. Springer, Dordrecht, pp 31–46
Fregene M, Puonti-Kaerlas J (2002) Cassava biotechnology. In: Hillocks RJ, Thresh JM (eds) Cassava: biology, production and utilization. CABI, Wallingford, pp 179–208
Gonzalez AE, Schopke C, Taylor NJ, Beachy RN, Fauquet CM (1998) Regeneration of transgenic cassava plants (Manihot esculenta Crantz) through Agrobacterium-mediated transformation of embryogenic suspension cultures. Plant Cell Rep 17:827–831
Goodin MM, Dietzgen RG, Schichnes D, Ruzin S, Jackson AO (2002) pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J 31:375–383
Gresshoff PM, Doy CH (1972) Development and differentiation of haploid Lycopersicon esculentum (tomato). Planta 107:161–170
Jach G, Binot E, Frings S, Luxa K, Schell J (2001) Use of red fluorescent protein from Discosoma sp. (dsRED) as a reporter for plant gene expression. Plant J 28:483–491
Liu J, Zheng Q, Ma Q, Gadidasu KK, Zhang P (2011) Cassava genetic transformation and its application in breeding. J Integr Plant Biol 53:552–569
Mas P, Devlin PF, Panda S, Kay SA (2000) Functional interaction of phytochrome B and cryptochrome 2. Nature 408:207–211
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nyaboga E, Njiru J, Nguu E, Gruissem W, Vanderschuren H, Tripathi L (2013) Unlocking the potential of tropical root crop biotechnology in east Africa by establishing a genetic transformation platform for local farmer-preferred cassava cultivars. Front Plant Sci 4:526
Patterson G (2007) Fluorescent proteins for cell biology. In: Anson DA (ed) Reporter Genes: A Practical Guide, 411th edn. Humana Press, Totowa, NJ, pp 47–80
Patterson G, Day RN, Piston D (2001) Fluorescent protein spectra. J Cell Sci 114:837–838
Petersen W, Umbeck P, Hokanson K, Halsey M (2005) Biosafety considerations for selectable and scorable markers used in cassava (Manihot esculenta Crantz) biotechnology. Environ Biosaf Res 4:89–102
Raemakers CJJM, Sofiari E, Taylor N, Henshaw G, Jacobsen E, Visser RGF (1996) Production of transgenic cassava (Manihot esculenta Crantz) plants by particle bombardment using luciferase activity as selection marker. Mol Breeding 2:339–349
Schopke C, Taylor N, Carcamo R, Konan NK, Marmey P, Henshaw GG, Beachy RN, Fauquet C (1996) Regeneration of transgenic cassava plants (Manihot esculenta Crantz) from microbombarded embryogenic suspension cultures. Nat Biotechnol 14:731–735
Schrott M (1995) Selectable marker and reporter genes. In: Potrykus I, Spangenberg G (eds) Gene transfer to plants. Springer-Verlag, Berlin, pp 325–336
Taylor N, Chavarriaga P, Raemakers K, Siritunga D, Zhang P (2004) Development and application of transgenic technologies in cassava. Plant Mol Biol 56:671–688
Taylor N, Gaitan-Solis E, Moll T, Trauterman B, Jones T, Pranjal A, Trembley C, Abernathy V, Corbin D, Fauquet CM (2012) A High-throughput platform for the production and analysis of transgenic cassava (Manihot esculenta) plants. Trop Plant Biol 5:127–139
Taylor NJ, Masona MV, Carcamo R, Ho T, Schöpke C, Fauquet CM (2001) Production of embryogenic tissues and regeneration of transgenic plants in cassava (Manihot esculenta Crantz). Euphytica 120:25–34
Terskikh AV, Fradkov AF, Zaraisky AG, Kajava AV, Angres B (2002) Analysis of DsRed Mutants Space around the fluorophore accelerates fluorescence development. J Biol Chem 277:7633–7636
Vahdati K, Leslie C, Zamani Z, McGranahan G (2004) Rooting and acclimatization of in vitro-grown shoots from mature trees of three Persian walnut cultivars. Hortic Sci 39:324–327
Wenck A, Pugieux C, Turner M, Dunn M, Stacy C, Tiozzo A, Dunder E, van Grinsven E, Khan R, Sigareva M, Wang WC, Reed J, Drayton P, Oliver D, Trafford H, Legris G, Rushton H, Tayab S, Launis K, Chang YF, Chen DF, Melchers L (2003) Reef-coral proteins as visual, non-destructive reporters for plant transformation. Plant Cell Rep 22:244–251
Wenck AR (2006) “Application of reef coral fluorescent proteins to plant biotechnology. http://www.clontech.com/xxclt_ibcGetAttachment.jsp?cItemId=15754&minisite=10020&secItmId=19741. Cited 13 August 2012”
Yadav JS, Ogwok E, Wagaba H, Patil BL, Bagewadi B, Alicai T, Gaitan-Solis E, Taylor NJ, Fauquet CM (2011) RNAi-mediated resistance to Cassava brown streak Uganda virus in transgenic cassava. Mol Plant Pathol 12:677–687
Zainuddin IM, Schlegel K, Gruissem W, Vanderschuren H (2012) Robust transformation procedure for the production of transgenic farmer-preferred cassava landraces. Plant Meth 8:24
Zhang P, Puonti-Kaerlas J (2000) PIG-mediated cassava transformation using positive and negative selection. Plant Cell Rep 19:1041–1048
Acknowledgment
This work was supported by the BioCassava Plus Project funded by the Bill & Melinda Gates Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Okwuonu, I.C., Achi, O.K., Egesi, C.N. et al. Evaluation of red fluorescent protein (DsRed) as alternative visual marker of genetic transformation in cassava (Manihot esculenta Crantz) . In Vitro Cell.Dev.Biol.-Plant 51, 571–579 (2015). https://doi.org/10.1007/s11627-015-9718-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9718-5