Abstract
An efficient protocol for the in vitro micrpropagation of Saussurea involucrata Kar. et Kir, an endangered Chinese medicinal plant, was developed. Shoot organogenesis was obtained following culture of leaf explants on Murashige and Skoog (MS) medium supplemented with thidiazuron (TDZ). After 28 d of culture, 15.6 ± 1.4 shoots were regenerated per leaf explant on MS medium containing 0.5 μM TDZ. After transfer of shoots to a medium containing 5.0 μM indole-3-acetic acid, approximately 80% of the regenerated shoots formed roots and whole plantlets. After transfer of rooted shoots to the greenhouse, 83% of the regenerated plantlets survived and grew vigorously. The regeneration protocol developed in this study provides a basis for germplasm conservation and for the production of plant material necessary to study the medicinally active components of S. involucrata.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Saussurea involucrata Kar. et Kir., one of the most well-known Chinese medicinal plants, is commonly used for treating rheumatoid arthritis, gynopathy, and high-altitude diseases (Li and Zhao 1989). S. involucrata extracts also show anti-inflammatory, anti-tumor, and analgesic activities (Liu et al. 1985; Jia et al. 2005). The overexploitation of native plants for commercial purposes has resulted in the near extinction of S. involucrata in China, and the species is listed as a nationally protected wild plant (Fu 1992).
As demand has increased for S. involucrata, there is an urgent need to develop methods for the efficient propagation and conservation of this plant. Conventional propagation methods using seeds are ineffective due to the high mortality rate of the seedlings in the early stages of growth, while the use of rhizomes for vegetative propagation may destroy the already endangered mother plants. In vitro propagation techniques provide useful systems for the mass multiplication and germplasm conservation of many threatened plant species (Liu et al. 2004a, b) and offer great potential for the propagation of plant species such as S. involucrata.
In plant tissue culture systems, the balance between auxin and cytokinin plays an important role in determining the morphogenetic development of an explant (Skoog and Miller 1957; Gaspar et al. 1996). A high cytokinin to auxin ratio generally favors the formation of shoots, while a low cytokinin to auxin ratio induces root formation. A balance between the two growth regulators promotes callus formation. Manipulation of the composition and ratio of these plant growth regulators (PGRs) is often the primary empirical approach used for optimization of in vitro micropropagation methods (Shukla et al. 2012).
Thidiazuron (N-phenyl-N-(1, 2, 3-thidiazol-5-yl) urea; TDZ), a phenylurea derivative with cytokinin-like activity, is effective in a wide variety of plant species for the induction of both somatic embryogenesis (Malik and Saxena 1992; Murthy et al. 1998; Akasaka et al. 2000; Jones et al. 2007) and shoot organogenesis (Li et al. 2000; Murch et al. 2000; Liu et al. 2003). The objectives of the current study were to test the effectiveness of TDZ for the induction of S. involucrata shoot organogenesis, compare the TDZ-induced morphogenesis with the response stimulated by balanced auxin/cytokinin treatments, and develop an effective protocol for the regeneration of S. involucrata from leaf explants.
Materials and Methods
S. involucrata Kar. et Kir. seeds were obtained from Tianshan mountain, Xinjiang, China. Seeds were surface sterilized by placement in 70% ethanol for 30 s, followed by immersion in 5.4% sodium hypochlorite for 20 min, and then rinsing three times with sterile distilled water. Surface-sterilized seeds were germinated and maintained on Murashige and Skoog (1962; MS) solid medium for 30 d in a growth chamber, with a 16-h photoperiod under cool-white light (30–40 μmol m−2 s−1) at 25°C. Preliminary results comparing callus induced from leaf, stem, and root material indicated that the callus from leaf explant was compact, green, and readily formed shoots, while callus from stems and root explants was less compact, white–green, and shoot formation was less reliable. Therefore, leaf explants were used throughout the study. Leaf explants (approximately 0.5 × 0.5 cm in size) were sectioned from the 30-d old seedlings (approximately 4.0 cm height) and incubated on MS medium supplemented with 0, 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, 10, 15, or 20 μM TDZ. The effect of exposure time to TDZ was evaluated by cultivating the leaf explants on MS medium with 0.5 μM TDZ (identified as the optimal concentration in the previous experiment) for a specific period (0, 7, 14, 21, 28, 35, 42, and 49 d) followed by subculturing onto fresh TDZ-free medium. The frequency of shoot regeneration and the number of shoots per leaf explant were recorded after 49 d of culture (starting from the initial day of inoculation).
To induce root organogenesis, green regenerated shoots larger than 30 mm were excised from the explant tissue and cultured on half-strength MS medium supplemented with 1, 5, or 10 μM indole-3-acetic acid (IAA). All media were adjusted to pH 5.8 and supplemented with 0.6% agar (Bacterialogical grade, Sanland International Inc, Shanghai, China) and 3% sucrose before autoclaving at 121°C for 18 min. The 450 ml plastic culture boxes (Gentel Co. Ltd., Beijing, China) were sealed with Parafilm® and incubated for 28 d under a 16/8 h (light/dark) photoperiod with a light intensity of 30–40 μmol m−2 s−1 provided by cool-white fluorescent lamps.
Rooted plantlets were removed from the media, rinsed in water, and transferred to a potting soil mixture containing south nutrition soil/perlite/vermiculite (3:1:1, v/v/v; Hebei, China) in the greenhouse. Each plantlet was covered with a polyethylene bag in order to maintain a high humidity (~90%). After 21 d, the polyethylene covers were removed and the plants were gradually exposed to ambient greenhouse conditions. Supplemental lighting was not supplied and the average light level on the benches over the course of the experiment was 244 μmol m−2 s−1.
All experiments were conducted using a completely randomized design and each experiment consisted of five explants per culture dish and 10 replicate dishes per treatment. Each experiment was repeated twice. All data are presented as the mean ± standard error. The data were subjected to a one-way analysis of variance and the Tukey’s honest significant difference multiple range test was used to calculate significant differences. SPSS for windows (SPSS Inc., version 7.5.1, Chicago, USA) was used for all statistical analyses and a value of P < 0.05 was considered significant.
Results and Discussion
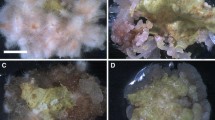
Leaf explants from S. involucrata seedlings (Fig. 1A ) were incubated on solid MS medium supplemented with varying levels of TDZ for the induction of shoot regeneration. After 14 d culture, compact, light green calli developed from the cut margins of the leaf explants, and after 21 d culture, regenerated shoots appeared (Fig 1B ). After 49 d, significantly more adventitious shoots were observed on leaf explants cultured on media containing 0.5 μM TDZ compared to the other TDZ levels, with an average of 8.5 ± 0.6 shoots per leaf explant and a frequency of shoot regeneration of 69.0 ± 2.0% (Figs. 1C and 2). The number of regenerated shoots decreased at TDZ concentrations higher than 0.5 μM.
TDZ-induced plant regeneration from S. involucrata leaf explants. (A) An intact S. involucrata seedling germinated in MS medium, (B) Callus formation from a leaf explant (B1; bar 1.0 cm) and shoot primordia on the surface of the callus after 14 d on MS medium containing 0.5 μM TDZ (B2; bar 5.0 mm). (C) Regenerated shoots cultivated for 49 d on MS medium containing 0.5 μM TDZ. (D) Proliferation and elongation of regenerated shoots cultivated on 0.5 μM TDZ-supplemented medium for 28 d followed by subculture on MS medium without PGRs for 21 d. (E) Rooting of regenerated shoots on half-strength MS medium supplemented with 5 μM IAA after 28 d. (F) Micropropagated plants transplanted to soil after 60 d.
The duration of exposure to TDZ also affected the shoot regeneration from the leaf explants of S. involucrata. The maximum average number of shoots per leaf explant (15.6 ± 1.4) was produced in cultures grown on 0.5 μM TDZ-supplemented medium for 28 d followed by subculturing on MS medium without PGRs for 21 d (Fig. 1D ). Exposure times longer or shorter than 28 d resulted in significantly fewer shoots per explant (Fig. 3).
The number of regenerated shoots per leaf explant using TDZ obtained here higher than that was obtained in previous studies where benzylaminopurine (BAP) and naphthaleneacetic acid (NAA) were used (Guo et al. 2007). In the previous study, the most effective combination (10 μM BAP and 2.5 μM NAA) produced an average of 5.2 ± 0.4 shoots per leaf explant and a shoot regeneration frequency of 66.0 ± 9.2%. Although the shoot regeneration frequency was similar using 0.5 μM TDZ (69.0 ± 2.0%), exposing the shoot explants to 0.5 μM TDZ increased the average number of regenerated shoots per explant.
Regenerated shoots larger than 30 mm were separated and used for rooting media evaluation. All of the media-induced rooting, including media without PGRs, however, in the absence of PGRs, fewer roots were induced, and those that were produced were much shorter compared to those induced using IAA treatments (Table 1). The optimal rooting was observed on the medium containing 5 μM IAA (Fig. 1E ). The frequency of root formation on this medium was 81.0 ± 7.0%, the regenerated shoots developed an average of 9.2 ± 0.5 roots per shoot and an average root length of 12 ± 0.8 mm after 28 d culture (Table 1). Increasing the concentration of IAA above 5 μM decreased the rooting percentage, the number of roots per regenerated shoot, and the average length of the roots. The plantlets were transferred to soil after 45 d and cultivated to maturity with a survival rate of 83.0% (Fig. 1F ).
TDZ was surprisingly effective for shoot regeneration from leaf explants of S. involucrata. At the optimum exposure time of 28 d, a relatively low level of TDZ (0.5 μM) induced more than 15 regenerated shoots per leaf explant, representing a threefold increase over the optimized BAP–NAA combination previously reported (Guo et al. 2007). Similar results were recorded for other medicinal plants, including Scutellaria baicalensis and Artemisia judaica, in which large numbers of de novo shoots were regenerated in response to TDZ exposure (Liu et al. 2003; Li et al. 2000).
This study has resulted in a protocol which can be utilized for the regeneration and mass propagation of S. involucrata. This method may also be used to select and clone superior individual genotypes which could be further improved using genetic engineering approaches. In addition, mass propagation may be used to produce the large quantities of plant tissue needed for the biochemical characterization of the medicinally active constituents of S. involucrata. Lastly, this protocol may be used for the generation of the large number of viable plantlets needed to meet commercial demand and potentially replenish natural populations.
References
Akasaka Y, Daimon H, Mii M (2000) Improved plant regeneration from cultured leaf segments in peanut (Arachis hypogaea L.) by limited exposure to thidiazuron. Plant Sci 156:169–175
Fu LG (1992) China plant red data book-rare and endangered plants. Chinese Science Press, Beijing, pp 234–235
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol-Plant 32:272–289
Guo B, Gao M, Liu CZ (2007) In vitro propagation of an endangered medicinal plant Saussurea involucrata Kar. et Kir. Plant Cell Rep 26:261–265
Jia JM, Wu CF, Liu W, Yu H, Hao Y, Zheng JH, Ji YR (2005) Anti-inflammatory and analgesic activities of the tissue culture of Saussurea involucrata. Biol Pharm Bull 28:1612–1614
Jones MPA, Yi Z, Murch SJ, Saxena PK (2007) Thidiazuron-induced regeneration of Echinacea purpurea L.: micropropagation in solid and liquid culture systems. Plant Cell Rep 26:13–19
Li GH, Zhao RC (1989) Studies on pharmacological actions of Saussurea involucrata Kar. et Kir. Acta Pharm Sin 15:368–369
Li H, Murch SJ, Saxena PK (2000) Thidiazuron-induced de novo shoot organogenesis on seedlings, etiolated hypocotyls and stem segments of Huang-qin. Plant Cell Tiss Org Cult 62:169–173
Liu CZ, Murch SJ, EL-Demerdash M, Saxena PK (2003) Regeneration of the Egyptian medicinal plant Artemisia judaica L. Plant Cell Rep 21:525–530
Liu CZ, Murch SJ, El-Demerdash M, Saxena PK (2004b) Artemisia judaica L.: mass propagation and antioxidant potential. J Biotechnol 110:63–71
Liu CZ, Murch SJ, Jain JC, Saxena PK (2004a) Goldenseal (Hydrastis canadensis L.): in vitro regeneration for germplasm conservation and elimination of heavy metal contamination. In Vitro Cell Dev Biol-Plant 40:75–79
Liu L, Xiao X, Zhang L (1985) Effect of the flavonoids from Saussurea involucrata on DNA synthesis of cancer cells. Lanzhou Univ Nat Sci 21:80–83
Malik KA, Saxena PK (1992) Thidiazuron induces high frequency shoot regeneration in intact seedlings of pea (Pisum sativum), chickpea (Cicer arietinum) and lentil (Lens culinaris). Aust J Plant Physiol 19:731–740
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Murch SJ, Choffe KL, Victor JMR, Slimmon TY, KrishnaRaj S, Saxena PK (2000) Thiazuron-induced plant regeneration from hypocotyl cultures of St. John’s wort (Hypericum perforatum L. cv ‘Anthos’). Plant Cell Rep 19:576–581
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol-Plant 34:267–275
Shukla MR, Jones AMP, Sullivan JA, Liu CZ, Gosling S, Saxena PK (2012) In vitro conservation of American elm (Ulmus americana): potential role of auxin metabolism in sustained plant proliferation. Can J For Res 42:686–697
Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11:118–131
Acknowledgments
This work was funded by the National Natural Science Foundation of China (no. 21150110459), the Knowledge Innovation Program of the Chinese Academy of Sciences (nos. YZ- 20606-03 & Y227051304), and the Chinese Academy of Sciences Fellowship for Young International Scientists (no. 2011Y1GA01).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Praveen Saxena
Rights and permissions
About this article
Cite this article
Guo, B., Stiles, A.R. & Liu, CZ. Thidiazuron enhances shoot organogenesis from leaf explants of Saussurea involucrata Kar. et Kir. In Vitro Cell.Dev.Biol.-Plant 48, 609–612 (2012). https://doi.org/10.1007/s11627-012-9468-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-012-9468-6