Abstract
Enhanced numbers of multiple shoots were induced from shoot tip explants of cucumber. The effects of amino acids (leucine, isoleucine, methionine, threonine, and tryptophan) and polyamines (spermidine, spermine, and putrescine) along with benzyladenine (BA) on multiple shoot induction were investigated. A Murashige and Skoog (MS) medium containing a combination of BA (4.44 μM), leucine (88 μM), and spermidine (68 μM) induced the maximum number of shoots (36.6 shoots per explant) compared to BA (4.44 μM) alone or BA (4.44 μM) with leucine (88 μM). The regenerated shoots were elongated on the same medium. Elongated shoots were transferred to the MS medium fortified with BA (4.44 μM), leucine (88 μM), and putrescine (62 μM) for root induction. Rooted plants were hardened and successfully established in soil with a 90% survival rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyamines (PAs), particularly spermidine, spermine, and putrescine, are present in all plant cells (Galston 1983; Bais and Ravishankar 2002). PAs play an important role in cell division and differentiation (Yamada et al. 1986; Basu et al. 1989). PAs have been implicated in a variety of morphogenic processes such as somatic embryogenesis in Daucus carota cells (Bastola and Minocha 1995) and Dactylis glomerata L. (Li and Burritt 2003), bud formation in thin-layer explants of Nicotiana tobacum (Kaur-Sawhney et al. 1986; Tiburcio et al. 1988; Scaramagli et al. 1999), shoot regeneration from Passiflora leaves (Desai and Mehta 1985), and cotyledonary explants of Brassica campestris (Chi et al. 1994) and Cucumis melo (Tian et al. 1994). Bagni and Torrigiani (1982), Smith (1985), and Galston and Kaur-Sawhney (1987) postulated that PAs may act as endogenous growth regulators or secondary hormonal messengers (Galston 1983; Davies 1987). Recently, Zhu and Chen (2005) reported enhanced shoot morphogenesis from cotyledons of Cucumis sativus (cucumber) in vitro in the presence of PAs.
Similarly, the role of amino acids in plant regeneration and somatic embryogenesis is known to a considerable extent (Ronchi et al. 1984; Chowdhry et al. 1993), and small changes in amino acid content evoke various morphogenic responses in an in vitro culture (Dey et al. 1998). Muyuan et al. (1990) reported increased frequency of differentiation and the percentage of green plant regeneration in Hordeum vulgare callus in a Murashige and Skoog (MS) medium supplemented with amino acids such as alanine, asparagines, and glutamine. Similarly, asparagine and proline increased the production of embryogenic callus and the frequency of plant regeneration in the embryo-derived callus cultures of Sorghum bicolor (Rao et al. 1995). Hare et al. (2001) reported proline-stimulated in vitro shoot organogenesis from Arabidopsis hypocotyls explants. Amino acid conjugates, along with suitable auxin in the culture medium, brought a high frequency of plant regeneration from leaf disk explants of Arachis hypogaea and Cajanus cajan (Eapen and George 1993).
It is clear from the aforesaid reports that PAs and amino acids play a role in morphogenic processes in tissue culture. Therefore, in the present study, we have undertaken to examine the effects of amino acids and PAs on shoot differentiation from shoot tip explants of cucumber.
Materials and Methods
Seed Collection and Germination.
Seeds of cucumber cv. Poinsett 76 (Indo-American hybrid seeds [Bangalore, India]) were soaked in tap water for 15 min before surface sterilization with 70% alcohol for 1 min and 2.5% (v/v) Teepol (commercial detergent, Reckitt and Benckiser, Kolkatta, India) for 15 min, followed by three rinses with sterile distilled water. Seeds were further surface sterilized by soaking in a 0.1% (w/v) aqueous mercuric chloride (HgCl2) solution for 10 min and rinsing four times with sterile distilled water. Surface-sterilized seeds were germinated in darkness for 48 h in 25 × 150-mm test tubes (Borosil, India) containing sterile moist cotton and plugged tightly with nonabsorbent cotton, then grown under light at an intensity of 30 μmol m-2 s−1 cool white fluorescent light in a 16-h photoperiod at 25 ± 2°C.
Explant Preparation, Inoculation, and Culture.
Shoot tips (5 mm length) from 5-d-old in vitro-grown seedlings were excised, and they were inoculated in 25 × 150-mm test tubes plugged with nonabsorbent cotton (Borosil, New Delhi, India; one shoot tip per tube) containing 20 ml MS medium (Murashige and Skoog 1962) consisting of 87.6 mM sucrose and 0.8% (w/v) agar (Himedia, Mumbai, India) with different concentrations of benzyladenine (BA; 0, 0.88, 1.78, 2.64, 3.52, 4.44, 5.55, and 6.66 μM). Separate experiments were carried out using the optimal concentration of BA (4.44 μM) with different amino acids (leucine [44, 88, 132, and 176 μM], isoleucine [44, 88, 132, and 176 μM], methionine [38, 76, 114, and 152 μM], threonine [49, 98, 147, and 196 μM], and tryptophan [26.5, 53, 79.5, and 106 μM]; Sisco Research Laboratories, Mumbai, India) and PAs (spermidine [34, 68, 102, and 136 μM], spermine [24.5, 49, 73.5, and 98 μM], and putrescine [31, 62, 93, and 124 μM]; Sisco Research Laboratories). Appropriate controls were maintained (without BA, BA 4.44 μM, BA 4.44 μM + leucine 88 μM) for each experiment. The pH of the medium was adjusted to 5.8 before autoclaving at 1.06 kg cm−2 (121° C) for 20 min. Individual shoots (>1 cm length) were excised from shoot clusters arising from the explants and subcultured on the MS medium containing BA (4.44 μM) with leucine (88 μM) and spermidine (68 μM) for further shoot development. After 3 wk, shoots longer than 4 cm were selected and transferred to the MS medium containing BA (4.44 μM) with leucine (88 μM) and putrescine (62 μM) for root induction. All cultures were maintained under cool white fluorescent light at 30 µmol m−2 s−1 for a 16-h photoperiod at 25 ± 2°C.
Transplantation and Acclimatization.
Rooted plantlets (>4 cm in length with a mean number of 9.2 roots per shoot) were washed thoroughly with tap water to remove agar and transplanted to plastic pots containing a mixture of autoclaved sand, soil, and vermiculite (1:1:1 v/v/v). Potted plants were grown in a growth chamber (Sanyo, Tokyo, Japan) at 85% relative humidity for 3–4 wk, and then moved to greenhouse for 4 wk before transfer to the field. Initially, plants were covered with polyethene bags to maintain high humidity (80%) and supplied daily with Hoagland nutrient solution (Hoagland and Arnon 1950). The polyethene bags were gradually removed when the plants had shown acclimatization. After 4-wk acclimatization, plants were transplanted to terra cotta and grown in the garden.
Statistical Analysis.
Each treatment consisted of 20 explants and each experiment was repeated three times. The data from the three separate experiments were pooled, and the mean and standard error was then derived from this. The mean number of shoots regenerated was calculated per responding explant. Comparisons between the mean values of treatments were carried out using Duncan’s multiple-range test with significance determined at 5% level (Gomez and Gomez 1976).
Results and Discussion
Effect of Amino Acids on Shoot Differentiation.
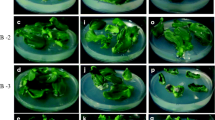
Shoot tip explants have already been used successfully for shoot production in cucumber (Vasudevan et al. 2001). When BA alone was used in the MS medium, 62% of shoot tip explants produced shoots with an average of eight shoots per explant at the optimal concentration tested (4.44 μM; Table 1). During this culture, basal callusing and rooting did not occur. BA is known to induce multiple shoots from axillary buds and leaf explants of cucumber (Aziz and McCown 1985; Misra and Bhatnagar 1995). There is evidence to show that amino acids act as an efficient morphogenic regulators in a number of species (Tupy et al. 1983; John and Guha-Mukherjee 1997). Media enriched with amino acids or hydrolyzed proteins enhanced regeneration of explants in vitro (Sen et al. 2002). Therefore, different amino acids namely, leucine, isoleucine, methionine, threonine, and tryptophan, were employed in the MS medium independently to study their effect on multiple shoot induction from shoot tips of cucumber along with BA at 4.44 μM (Table 2). At 88 μM leucine and 4.44 μM BA, 82% of explants responded in producing multiple shoots with an average of 21.4 shoots per explant. At the same concentration, isoleucine evoked 78% culture response and produced 20.4 shoots per explant. The other amino acids viz., methionine at 76 μM, threonine at 98 μM, and tryptophan at 53 μM, produced 18.6, 13.6, and 9.2 shoots per shoot tip explant, respectively. Though their response was less than those to leucine and isoleucine treatments, their effects on shoot induction were significantly higher than with BA alone (Table 2). The effects of methionine, threonine, and tryptophan were studied here for the first time in shoot differentiation from the axillary buds of the shoot tip explant in cucumber. Basu et al. (1989) reported that the exogenous application of leucine and isoleucine resulted in an improved shoot formation in Brassica juncea, whereas amino acids like methionine and threonine enhanced cell proliferation. Basu et al. (1989) speculated that increased threonine deaminase activity due to an exogenous supply of amino acids both in vivo and in vitro affected morphogenic responses and concluded that amino acids played a regulatory role in Brassica morphogenesis. It is clear from our present investigation that a BA and leucine combination stimulated axillary bud production and enhanced multiple shoots by more than twofold compared to BA alone, using shoot tip explants of cucumber.
Effect of PAs on Shoot Differentiation.
Our next aim was to assess the effect of PAs on multiple shoot induction with the optimal concentrations of BA (4.44 μM) and leucine (88 μM). At the optimal level of 68 μM spermidine, 92% of shoot tip explants produced an average of 36.6 shoots per explant (Table 3) accompanied by a higher number of nodes per shoot and shoot length (6.2 cm). Although the addition of spermine (49 μM) and putrescine (62 μM) produced a greater number of shoots per explant when compared to a BA and leucine combination, this difference was not statistically significant. Desai and Mehta (1985), Kaur-Sawhney et al. (1986), Galston and Kaur-Sawhney (1990), Chi et al. (1994), and Walden et al. (1997) suggested that PAs are important for cell growth, somatic embryogenesis, and shoot morphogenesis. Zhu and Chen (2005) reported the effect of putrescine, spermidine, and spermine for enhancing adventitious shoot formation from cotyledons of cucumber in vitro and concluded that spermine was indispensable for adventitious shoot formation in cucumber. Similar results were observed in Brassica rapa (Chi et al. 1994). However, in the present study, spermidine rather than putrescine or spermine was most effective in multiple shoot induction from the shoot tips of cucumber. A similar observation was made by Tanimoto et al. (1994) for adventitious shoot formation from stem segments of Torenia in vitro. Tian et al. (1994) had shown that putrescine, spermidine, and spermine were all involved in adventitious shoot formation from cotyledons of melon in vitro. This evidence shows that putrescine, spermidine, and spermine may play dissimilar roles in different species or in different explants, as observed by Zhu and Chen (2005). Martin-Tanguy (2001) suggested that spermidine and spermine play significant roles in adventitious shoot formation, which can be promoted by exogenous PA treatment. In the present study, exogenous application of BA (4.44 μM), leucine (88 μM), and spermidine (68 μM) in the MS medium promoted the highest shoot induction frequency accompanied by shoot elongation. PAs have been shown to interact with phytohormones (Altman 1982; Alabadi et al. 1996; Tonon et al. 2001). PAs have been regarded as a new class of plant growth regulators or hormonal secondary messengers and as one of the reserves of carbon and nitrogen at least in cultured tissues (Flores and Filner 1985; Altman and Levin 1993). Scholten (1998) suggested that regeneration and differentiation in a series of plant species could be drastically improved by the application of PAs. In our present study, it is presumed that spermidine and leucine, which provided an amine reserve and nitrogen source, respectively, and along with BA triggered enhanced shoot differentiation from axillary buds of shoot tips of cucumber.
Effect of PAs on Rooting.
When the elongated shoots were transferred to the MS medium containing a combination of putrescine (62 μM) along with BA (4.44 μM) and leucine (88 μM), 98% of shoots produced well-developed roots with an average of 9.2 roots per shoot (Table 4). Treatments with the other two PAs, spermidine and spermine, caused no response except at the highest tested concentration and produced a lower number of roots. Tarenghi et al. (1995), Nag et al. (1999), and Tang and Newton (2005) reported the promotive role of spermidine and putrescine on root elongation and growth from stem cuttings of Vigna radiata, Fragaria microcuttings, and regenerated Virginia pine plantlets, respectively. A number of unrelated dicotyledonous species, such as Nicotiana tabacum, Phaseolus vulgaris, Vitis vinifera, Arabidopsis thaliana, and Pringlea antiscorbutica also share common features of PA involvement in root development (Couee et al. 2004). In an earlier study, Selvaraj et al. (2002) reported the role of indole butyric acid along with BA on the root induction of cucumber shoots in vitro and recorded the production of 5.6 roots per shoot with a mean root length of 3.2 cm. In general, cytokinins are known to inhibit rooting of in vitro-raised shoots. In the present study, when BA was used along with leucine in the root induction medium, no rooting of elongated shoots was recorded. When putrescine was added along with BA and leucine, the elongated shoots produced a maximum number of roots. De Klerk et al. (2001) observed that at low concentration, certain cytokinins like isopentenyladenine and isopentenyladenosine enhanced rooting in Malus domestica root stock. De Klerk et al. (2001) opined that cytokinin is essential during root formation as it induces cell division in stem cuttings, which is a necessary initial step in adventitious root formation. Geneve and Hackett (1990) reported that putrescine was found to be associated with root development and elongation in Hedera helix. A positive correlation between PA contents and adventitious root formation has been observed in leaf explants of Cucumis melo (Tian et al. 1994). We presume that a combination of BA and putrescine was responsible for better rooting of in vitro-raised cucumber shoots as BA was necessary for initial cell division at the base of the elongated shoots and putrescine for the production of higher number of adventitious roots. As PAs play an important role in the interactions between environment and development of root architecture (Couee et al. 2004; Galston et al. 1997), the impact of PA, particularly putrescine, on higher root development may suggest novel improvement strategies for successful transplantation and acclimatization programs involving cucumber cultivars raised in the tissue cultures.
Transplantation and Acclimatization.
Efficient hardening and acclimatization could be obtained when plantlets were at least 4 cm long with an average of 9.2 roots. Ninety percent of hardened plants ultimately survived. A correlation between plant height and better acclimatization and subsequent survival was already reported for Citrullus lanatus (Compton et al. 1993) and Cucumis hystrix (Compton et al. 2001). With the use of this protocol, approximately 36 plants per shoot tip explant were obtained within a short culture period of 70–80 d.
Conclusions
The shoot tip explants of cucumber offer the production of shoots from axillary buds at a high frequency in a short culture period. Since the explant preparation is more convenient than the use of other explants, this technique may provide technical simplicity in cucumber micropropagation and tissue culture. Our experiment suggests that exogenous application of spermidine and leucine along with BA plays a synergistic role in enhancing the multiple shoot formation from shoot tip explants of cucumber in vitro, followed by the use of putrescine for increased root development, which ultimately resulted in better acclimatization and survival of regenerated shoots.
References
Alabadi A.; Aguero M. S.; Perez-Amador M. A.; Carbonell J. Arginase, arginine decarboxylase, ornithine decarboxylase and polyamines in tomato ovaries. Changes in unpollinated ovaries and parthenocarpic fruits induced by auxin or gibberellin. Plant Physiol. 112: 1237–1244; 1996.
Altman A. Retardation of radish leaf senescence by polyamines. Physiol. Plant. 54: 189–193; 1982.
Altman A.; Levin N. Interaction of polyamines and nitrogen nutrition in plants. Physiol. Plant. 89: 653–658; 1993.
Aziz H. A.; McCown B. H. Hormonal response of shoot and callus cultures of cucumber (Cucumis sativus L.). Sci. Hortic. 20: 540; 1985.
Bagni N.; Torrigiani P. Polyamines: A new class of growth substances. In: KarssenC. M.; Van LoonL. C.; VreugdenhilD. (eds) Progress in plant growth regulation. Kluwer, Dordrecht, The Netherlands, pp 264–275; 1982.
Bais H. P.; Ravishankar G. A. Role of polyamines in the ontogeny and their biotechnological applications. Plant Cell Tiss. Org. Cult. 69: 1–34; 2002.
Bastola D. R.; Minocha S. C. Increased putrescine biosynthesis through transfer of mouse ornithine cDNA in carrot promotes somatic embryogenesis. Plant Physiol. 109: 63–71; 1995.
Basu A.; Sethi U.; Guha-Mukherjee S. Regulation of cell proliferation and morphogenesis by aminoacids in Brassica tissue cultures and its correlation with threonine deaminase. Plant Cell Rep. 8: 333–335; 1989.
Chi G. L.; Lin W. S.; Lee J. E. E.; Pua E. C. Role of polyamines on de novo shoot morphogenesis from cotyledons of Brassica campestris spp. Pekinensis (Lour) Olsson in vitro. Plant Cell Rep. 13: 323–329; 1994.
Chowdhry N.; Tyagi A. K.; Maheshwari N.; Maheshwari S. C. Effect of L-proline and L-tryptophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativa L. cv. Pusa 169). Plant Cell Tiss. Org. Cult. 32: 357–361; 1993.
Compton M. E.; Gray D. J.; Elmstrom G. W. A simple protocol for micropropagating diploid and tetraploid watermelon using shoot tip explants. Plant Cell Tiss. Org. Cult. 3: 211–217; 1993.
Compton M. E.; Pierson B.; Staub J. E. Micropropagation for recovery of Cucumis hystrix. Plant Cell Tiss. Org. Cult. 64: 63–67; 2001.
Couee I.; Hummel I.; Sulmon C.; Gouesbet G.; Amrani A. E. Involvement of polyamines in root development. Plant Cell Tiss. Org. Cult. 76: 1–10; 2004.
Davies P. J. The plant hormones: Their nature, occurrence, and functions. In: DaviesP. J. (ed) Plant hormones and their role in plant growth and development. Martinus Nijhoff, Boston, MA, pp 1–12; 1987.
De Klerk G. J.; Hanecakova J.; Jasik J. The role of cytokinins in rooting of stem slices cut from apple microcuttings. Plant Biosys. 135: 79–84; 2001.
Desai H. V.; Mehta A. R. Changes in polyamine levels during shoot formation, root formation and callus induction in cultured Passiflora leaf discs. J. Plant. Physiol. 119: 45–53; 1985.
Dey O. K. S. M.; Kalia S.; Ghose S.; Guha-Mukherjee S. Biochemical basis of differentiation in plant tissue culture. Curr. Sci. 74: 591–596; 1998.
Eapen S.; George L. Plant regeneration from leaf discs of peanut and pigeonpea: Influence of benzyladenine, indoleacetic acid and indoleacetic acid-amino acid conjugates. Plant Cell Tiss. Org. Cult. 353: 223–227; 1993.
Flores H. E.; Filner P. Polyamine catabolism in higher plants: Characterization of pyrroline dehydrogenase. Plant Growth Regul. 3: 277–291; 1985.
Galston A. W. Polyamines as modulators of plant development. Bioscience. 33: 382–388; 1983.
Galston A. W.; Kaur-Sawhney R. Polyamines as endogenous growth regulators. In: DaviesP. J. (ed) Plant hormones and their role in plant growth and development. Martinus Nijhoff, Dordrecht, pp 280–295; 1987.
Galston A. W.; Kaur-Sawhney R. Polyamines in plant physiology. Plant Physiol. 94: 406–410; 1990.
Galston A. W.; Kaur-Sawhney R.; Altabella T.; Tiburcio A. F. Plant polyamines in reproductive activity and response to abiotic stress. Bot. Acta. 110: 197–207; 1997.
Geneve R. L.; Hackett W. P. Ethylene evolution and endogenous polyamine levels during adventitious root formation in English ivy. In: FloresH. E.; ArtecaR. N.; ShanonJ. C. (eds) Polyamine and ethylene: Biochemistry, physiology and interactions. Amer. Soc. Plant Physiologists, Rockville, pp 332–334; 1990.
Gomez K. A.; Gomez K. A. Statistical procedures for agricultural research with emphasis of rice. International Rice Research Institute, Los Banos, Phillipines; 1976.
Hare P. D.; Cress W. A.; van Staden J. The effects of exogenous proline and proline analogues on in vitro shoot organogenesis in Arabidopsis. Plant Growth Reg. 342: 203–207; 2001.
Hoagland, D. R.; Arnon, D. I. The water culture method for growing plants without soil. California Agric. Exp. Sta. Bull., no. 347; 1950.
John S. J.; Guha-Mukherjee S. In: TewaryK. K.; SinghalG. S. (eds) Plant molecular biology and biotechnology. Narosa, New Delhi, pp 17–28; 1997.
Kaur-Sawhney R.; Tiburcio A. F.; Galston A. W. Polyamine-mediated control of organogenesis in thin layer explants of tobacco. Plant Physiol. 80: 37; 1986.
Li Z. L.; Burritt D. J. Changes in endogenous polyamines during the formation of somatic embryos from isogenic lines of Dactylis glomerata L. with different regenerative capacities. Plant Growth Regul. 40: 65–74; 2003.
Martin-Tanguy J. Metabolism and function of polyamines in plants: Recent development (new approaches). Plant Growth Regul. 34: 135–148; 2001.
Misra A. K.; Bhatnagar S. P. Direct shoot regeneration from the leaf explant of cucumber (Cucumis sativus L.). Phytomorphology. 45: 47–55; 1995.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 15: 473–497; 1962.
Muyuan Z.; Abing X.; Miaobao Y.; Chunnong H.; Zhilong Y.; Linji W.; Jianjun Y. Effects of amino acids on callus differentiation in barley anther culture. Plant Cell Tiss. Org. Cult. 22: 201–204; 1990.
Nag S.; Saha K.; Chowdhuri M. Role of auxin and polyamine in adventitious root formation at the base of mung bean cuttings. Indian J. Plant Physiol. 4: 247–255; 1999.
Rao A. M.; Padma Sree K.; Kavi Kishor P. B. Enhanced plant regeneration in grain and sweet sorghum by asparagine, proline and cefotaxime. Plant Cell Rep. 15: 72–75; 1995.
Ronchi V. N.; Caligo M. A.; Nizzolini M.; Luccarini G. Stimulation of carrot somatic embryogenesis by proline and serine. Plant Cell Rep. 3: 210–214; 1984.
Scaramagli S.; Biondi S.; Capitani P.; Gerola P.; Altamura M. M.; Torrigiani P. T. Polyamines conjugate levels and ethylene biosynthesis: Inverse relationship with vegetative bud formation in tobacco thin layers. Physiol. Plant. 105: 367–376; 1999.
Scholten H. J. Effect of polyamines on the growth and development of some horticultural crops in micropropagation. Sci. Hortic. 77: 83–88; 1998.
Selvaraj N.; Vengadesan G.; Vasudevan A.; Prem Anand R.; Ramesh Anbazhagan V.; Ganapathi A. Micropropagation of Cucumis sativus L. from field grown plants. In: MaynardD. N. (ed) Proceedings of the Cucurbitaceae Conference. Acta Hortic., Belgium, pp 149–156; 2002.
Sen J.; Kalia S.; Guha-Mukherjee S. Level of endogenous free amino acids during various stages of culture of Vigna mungo (L.) Hepper—somatic embryogenesis, organogenesis and plant regeneration. Curr. Sci. 82: 429–433; 2002.
Smith T. A. Polyamines. Ann. Rev. Plant Physiol. 36: 117–143; 1985.
Tang W.; Newton R. Polyamines promote root elongation and growth by increasing root cell division in regenerated Virginia pine (Pinus virginiana Mill.). Plant Cell Rep. 24: 581–589; 2005.
Tanimoto S.; Matsubara Y.; Ishioka N. Significance of spermidine in the initiation of adventitious buds in stem segments of Torenia. Plant Cell Physiol. 35: 1071–1077; 1994.
Tarenghi E.; Carre M.; Martin-Tanguy J. Effects of inhibitors of polyamine biosynthesis and of polyamines on strawberry microcutting growth and development. Plant Cell Tiss. Org. Cult. 42: 47–55; 1995.
Tian C. E.; Li R. G.; Guan H. Relationship between polyamines and morphogenesis in cotyledons of Cucumis melo L. cultured in vitro. Acta Bot. Sin. 36: 219–222; 1994.
Tiburcio A. F.; Kaur-Sawhney R.; Galston A. W. Polyamine biosynthesis during vegetative and floral bud differentiation in thin layer tobacco tissue cultures. Plant Cell Physiol. 29: 1241–1249; 1988.
Tonon G.; Kevers C.; Gaspar T. Changes in polyamines, auxins and peroxidase activity during in vitro rooting of Fraxinus angustifolia shoots: An auxin independent rooting model. Tree Physiol. 2110: 655–663; 2001.
Tupy J.; Hrabetova E.; Capkova V. Amino acids and bivalent cations in the growth of tobacco pollen in mass culture. Plant Sci. Lett. 30: 91–98; 1983.
Vasudevan A.; Selvaraj N.; Suresh Kumar S.; Ganapathi A. Multiple shoot induction from shoot tip explants of Cucumber (Cucumis sativus L.). Cucurbit Genet. Coop. Rep. 24: 8–12; 2001.
Walden R.; Cordeiro A.; Tiburcio A. F. Polyamines: Small molecules triggering pathways in plant growth and development. Plant Physiol. 113: 1009–1013; 1997.
Yamada Y.; Kumpaisal R.; Hashimoto T.; Sugimoto Y. Growth and aspartate kinase activity in wheat cell suspension culture: Effects of lysine analogs and aspartate-derived amino acids. Plant Cell Physiol. 27: 607–617; 1986.
Zhu C.; Chen Z. Role of polyamines in adventitious shoot morphogenesis from cotyledons of cucumber in vitro. Plant Cell Tiss. Org. Cult. 81: 45–53; 2005.
Acknowledgment
The authors are grateful to Department of Science and Technology (DST), Government of India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Gregory C. Phillips
Rights and permissions
About this article
Cite this article
Vasudevan, A., Selvaraj, N., Ganapathi, A. et al. Leucine and spermidine enhance shoot differentiation in cucumber (Cucumis sativus L.). In Vitro Cell.Dev.Biol.-Plant 44, 300–306 (2008). https://doi.org/10.1007/s11627-008-9135-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-008-9135-0