Abstract
Crocin has plentiful pharmacological effects, but its role in osteogenesis differentiation of bone marrow mesenchymal stem cells (BMSCs) is unexplored. This study explored the effect of crocin on osteogenesis differentiation, in order to provide evidence for its clinical application. In cell experiments, human BMSCs (hBMSCs) were induced by osteogenesis differentiation medium or crocin. In animal experiments, steroid-induced osteonecrosis of the femoral head (SANFH) rat models was established using lipopolysaccharide (LPS) plus methylprednisolone (MPS), and then treated with crocin. The osteogenesis differentiation capacity of hBMSCs was analyzed by alkaline phosphatase (ALP) and alizarin red S staining. Histopathological changes in rat femoral head tissues were observed by hematoxylin and eosin (H&E) staining. The expression levels of RUNX2, COL1A1, OCN, and GSK-3β in hBMSCs and rat femoral head tissues were measured by quantitative real-time polymerase chain reaction (qRT-PCR) or western blot (WB) analysis. ALP and alizarin red S staining demonstrated that LAP activity and calcium nodules were increased in hBMSCs treated with crocin. From H&E staining results, femoral head tissues of SANFH models showed typical osteonecrosis, which could be ameliorated by crocin. WB and qRT-PCR assays detected that the expression levels of RUNX2, COL1A1, and OCN in hBMSCs and femoral head tissues of models were obviously increased after crocin treatment, while GSK-3β phosphorylation was reduced. In general, the action of crocin was concentration-dependent. Crocin might be beneficial to the recovery of SANFH through accelerating osteogenesis differentiation of BMSCs, which might be a novel therapy for related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroid-induced osteonecrosis of the femoral head (SANFH) is a serious complication caused by long-term treatment with high-dose corticosteroids, such as systemic lupus erythematosus, rheumatoid arthritis, multiple myeloma, and other inflammatory and autoimmune diseases (Wang et al. 2018a). With the progress of the disease, the lesions gradually involved myeloid joint and myeloid white, and then developed into femoral head collapse and myeloid joint osteoarthritis in the late stage, leading to unbearable pain, broken, and even disability, which seriously affects the patient s’ life quality (Kubo et al. 2016). When the disease is serious to a certain extent, artificial joint replacement surgery is often needed to ensure normal joint function and alleviate symptoms, but the operation is generally expensive and the risk of secondary surgery is high, so many patients can hardly bear the financial pressure (Rahman et al. 2013). Therefore, in-depth analysis of the pathogenesis of SANFH and development of corresponding new drugs are important to release the stress of patients.
Up to now, although the pathogenesis of SANFH has not been clearly explained, the main mechanism theory focuses on the osteogenesis differentiation imbalance of bone marrow mesenchymal stem cells (BMSCs) (Hao et al. 2016). BMSCs are pluripotent stem cells, which can be used as seed cells to differentiate into bone cells, cartilage cells, fat cells, muscle (Li et al. 2017; Dai et al. 2018; He et al. 2018) cells, and so on. There are increasing evidences have shown that the osteogenesis differentiation ability of BMSCs in SANFH patients is decreased, while the adipogenesis differentiation ability is enhanced, which leads to the insufficiency of blood supply and oxygen supply of cell tissues, promotes cell dysfunction and apoptosis, and then results in the vicious circle of femoral head osteonecrosis (Sun et al. 2015; Wang et al. 2018b). It can be seen that improving the osteogenesis differentiation ability of BMSCs is expected to be a new breakthrough point in the treatment of SANFH.
Saffron is a perennial flower of crocus in Iridaceae, and it is also a kind of traditional precious Chinese medicine (Christodoulou et al. 2015). Crocin is one of the main components of saffron, which has been proved to have a variety of pharmacological effects, including tumor treatment, anti-depression, hypolipidemia, reducing inflammation, and oxidative stress (Moshiri et al. 2015). A recent report pointed out that crocin can inhibit adipogenesis differentiation of preadipocytes and facilitate fat decomposition, which was hopeful to be a clinical therapy for obesity and related metabolic diseases (Gu et al. 2018). Nevertheless, the effect of crocin on the osteogenesis differentiation ability of BMSCs remains unknown. Based on this, this study analyzed the role of crocin in osteogenesis differentiation of human BMSCs (hBMSCs) in vitro, and verified it through in vivo animal models, so as to provide basic research for the clinical application of crocin and search possible treatment for SANFH.
Materials and Methods
Statement
In this study, experiments on animals were supported by the Animal Ethical Committee of Affiliated Zhongshan Hospital of Dalian University, and the experimental processes complied with the Guidelines of The Use of Laboratory Animals in China.
Cell incubation
hBMSCs were obtained from American Type Culture Collection (ATCC; #PCS-500-012, Manassas, VA), and sustained in α-minimum essential medium (α-MEM; Gibco BRL, Grand Island, NY) containing 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and 1% penicillin/streptomycin in a humidified circumstance at 37°C with 5% CO2. hBMSCs were passaged when adherent cells reached a density of 80–90%. hBMSCs at passages three to six were used in all experiments. hBMSCs were digested and passaged when the density reached 80–90%, and cells at passage 3–6 were used in the following experiments.
Cell viability assay
The effect of crocin on hBMSCs viability was firstly tested by Cell Counting Kit-8 (CCK-8) assay. Briefly, hBMSCs were cultured in 96-well plates (1 × 104 cells/well) and treated with different concentrations of crocin (0, 10, 25, 50, 100, 1000 μmol/L; #42553-65-1, purity: > 98%, Nanjing DASF Biotechnology Co. LTD, Nanjing, China) for 14 d. Afterwards, 10 μL CCK-8 reagent (Dojindo Laboratories, Kumamoto, Japan) was instilled into each well for another 2 h. The optical density (OD) value was measured in each well under a microplate reader (Omega Bio-Tek, Inc., Norcross, GA) at a wavelength of 490 nm.
Osteogenesis differentiation induction
To detect the osteogenesis differentiation ability of the hBMSCs, cells were seeded in 24-well plates (5 × 103 cells/well), and distributed into three groups: control group, positive control group, and crocin treatment group. As a contrast, hBMSCs cultured in α-MEM medium alone were used as control group. hBMSCs in positive control group were induced by osteogenesis differentiation medium (α-MEM medium containing 100 nmol/L dexamethasone, 10 nmol/L β-glycerophosphate and 50 μg/mL L-ascorbic acids (Sigma-Aldrich, St. Louis, MO)) for 21 d. In crocin treatment group, crocin of 10, 25, and 50 μmol/L were selected as the concentrations for hBMSCs treatment in following cell experiments.
Alkaline phosphatase staining
Cells were seeded in 24-well plates (5 × 104 cells/well). Alkaline phosphatase (ALP) staining was performed to measure the osteogenesis differentiation ability of the hBMSCs in different groups. For the staining, hBMSCs were rinsed twice by phosphate buffer saline (PBS) solution at the end of osteogenesis differentiation, fixed with 4% polyformaldehyde for 30 s, followed by staining with the BCIP/NBT ALP staining kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. The images were obtained from the AxioVision4Ac microscope (Carl Zeiss, Jena, Germany). The final estimation was based on the absorbance at 405 nm as previously described (Nie et al. 2016). The BCA assay kit was used to determine the protein concentration, and the ALP activity was then normalized to the protein levels. ALP activity was quantified at a wavelength of 405 nm using Type354 microplate reader (Thermo Fisher Scientific, Waltham, MA), and calculated as OD values/mg total protein.
Alizarin red S staining
Cells were seeded in 24-well plates (5 × 104 cells/well). After osteogenesis differentiation, alizarin red S staining was performed to observe the calcified nodules formation in hBMSCs with different disposition. Briefly, hBMSCs were rinsed three times with PBS and fixed with 4% paraformaldehyde. The calcified nodules in cells were dyed by alizarin red S staining solution (Sigma-Aldrich) for 30 min on the basis of the manufacturer’s instructions. Then, the experimental results were observed under the AxioVision4Ac microscope (Carl Zeiss). For further quantitative analysis of calcified nodules (Liu et al. 2018), the absorbance of the solution was measured at 620 nm using Type354 microplate reader (Thermo Fisher Scientific).
Animal experiment
A total of 30 8-week-old Sprague-Dawley rats (half male and half female; weight: 250–300 g) used in this study were provided by Guangzhou Medical Laboratory Animal Center (Guangzhou, China). All rats were fed in a set environment relative with relative humidity of 60–70%, temperature at (21 ± 2)°C, light/dark cycle every 12 h, and the water and food were supplied freely. After feeding for 7 days, rats were randomly divided into five groups with equal amount: control group, model group, high-concentration crocin treatment (crocin-H) group, medium-concentration crocin treatment (crocin-M) group, and low-concentration crocin treatment (crocin-L) group. In model group, rats were intravenously injected with lipopolysaccharide (LPS, 10 μg/kg; #SMB00610, Sigma-Aldrich); after 24 h, rats received intramuscular injection of methylprednisolone (MPS, 20 mg/kg; Meilunbio, Dalian, China) three times a day for 5 consecutive days to establish SANFH models. On the basis of treatment in the model group, after 4 wk of MPS injection, rats in the crocin-treated group were given crocin solution (#P5620; Solaibio, Beijing, China) (crocin-H: 40 mg/kg; crocin-M: 20 mg/kg; crocin-L: 10 mg/kg) orally per day for 14 d. For comparison, rats in control were given the same amount of saline. In the end, 8 wk after MPS injection, all rats were sacrificed by overdose anesthesia (pentobarbital sodium of 150 mg/kg), and femoral heads were collected. The bone samples were crushed by liquid nitrogen conditions for protein and RNA detection.
Hematoxylin and eosin staining
Segregated femoral heads were fixed with 4% formalin, decalcified by 10% ethylenediaminetetraacetic acid (EDTA), and neutralized with sodium sulfate buffer for approximately 4 wk. Then, the tissues were routinely embedded in paraffin and sliced into sections (4 μm), followed by staining with hematoxylin and eosin (H&E) staining kit (Beyotime) according to the manufacturer’s specification. The experimental images were observed under the LEICA DM 4000 microscope (Leica, Wetzlar, Germany). Rates of empty lacunae (%) were blindly assessed by two independent authors according to previous studies (Qin et al. 2006).
Western blot analysis
In hBMSCs and rat femoral head tissues of each group, the total protein was extracted by RIPA buffer (Beyotime). The concentration of lysed protein was measured using the Bicinchoninic Protein Assay kit (BCA, Pierce, Rockford, IL). Forty-microgram protein samples were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, Beyotime), followed by shifting onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA). Then, skim milk was used to block the membranes at room temper (Abcam, Cambridge, MA) overnight at 4°C, including runt-related transcription factor 2 (RUNX2; 1 μg/mL, ab32518, Abcam), collagen type I alpha1 (COL1A1; 1:1000, #84336, Cell Signaling Technology), osteocalcin (OCN; 5 μg/mL, ab13420, Abcam), phosphorylated glycogen synthase kinase-3β (p-GSK-3β; 1:1000, ab129068, Abcam), GSK-3β (1:1000, ab93926, Abcam), and using GAPDH (1:1000, ab181602, Abcam) as the internal reference. Subsequently, the homologous secondary antibodies (goat anti-rabbit IgG H&L (HRP; 1:7000, ab97051) and goat anti-mouse IgG H&L (HRP; 1:1000, ab150113)) were added for another 1 h at room temperature. The blots were developed with the enhanced chemiluminescence -detecting kit (ECL; Thermo Fisher Scientific).
Quantitative real-time polymerase chain reaction assay
For in-depth determination, quantitative real-time polymerase chain reaction (qRT-PCR) assay was proceeded to measure the relevant mRNA expression in hBMSCs and rat femoral head tissues of each group. Total RNA was isolated from cells and tissues using TRIzol reagent (Invitrogen). The quality and integrity of total RNA were detected by the NanoDrop-2000c spectrophotometer (Thermo Fisher Scientific) and 1% agarose modified gel electrophoresis, respectively. The first-strand cDNA was compounded from the isolated RNA (1 μg) though the PrimeScript RT Master Mix Perfect Real Time (TaKaRa, Shiga, Japan) according to the manufacturer’s instructions. qRT-PCR assay was implemented by the ABI Prism 7500 Fast Real-time PCR System (Applied Biosystems, Foster City, CA), and the reaction conditions were set as follows: 30 s at 95°C, 50 cycles of 5 s at 95°C and 30 s at 60°C, 15 s at 95°C, and followed by 30 s at 60°C. The corresponding mRNA expression levels were normalized to β-actin, and the data were evaluated by the comparative 2-ΔΔCt method (Rao et al. 2013). The sequences of primers were shown in Table 1 and synthesized by Gene Pharma (Shanghai, China).
Statistical analysis
Statistical Package of the Social Sciences 20.0 software (SPSS, Inc., Chicago, IL) was used for data analysis. The measurement data were presented as mean ± standard deviation (SD). The difference between groups was performed by Student’s t test or one-way analysis of variance (ANOVA). All experiments in vivo and in vitro were performed in triplicate. P < 0.05 was considered as statistically significant.
Results
Crocin effectively promoted osteogenesis differentiation of hBMSCs
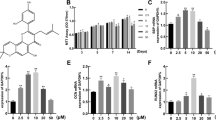
After 14 days of culture with different concentrations of crocin, CCK-8 experimental results showed that 100 and 1000 μmol/L of crocin availably inhibited hBMSCs viability (P < 0.05), whereas 10, 25, and 50 μmol/L of crocin had no evident effect on cell viability (Fig. 1a). In subsequent experiments, the concentrations of 10, 25, and 50 μmol/L were selected as therapeutic concentrations to explore the effect of crocin on osteogenesis differentiation of hBMSCs. In ALP staining experiment, it was demonstrated that ALP activity was slightly increased in hBMSCs induced by osteogenesis differentiation medium, but notably elevated in cells treated with crocin at 10, 25, and 50 μmol/L compared with control and OS groups (P < 0.01, Fig. 1b). Consistently, alizarin red S staining also revealed that orange calcium nodules in hBMSCs were increased after osteogenesis differentiation induction, which in crocin-treated cells were largely formed compared with control and OS groups (P < 0.01, Fig. 1c). Overall, the promoting capacity of crocin on ALP activity and calcium nodule formation increased with the augmentation of concentration.
Crocin effectively promoted osteogenesis differentiation of hBMSCs. (a) The viability of hBMSCs after treatment with different concentrations of crocin (0, 10, 25, 50, 100, 1000 μmol/L) was tested by Cell Counting Kit-8 (CCK-8) assay. In subsequent experiments, cells were distributed into following groups: control group, positive control group (osteogenesis differentiation medium (OS)), and crocin treatment group (10, 25, 50 μmol/L). (b) Alkaline phosphatase (ALP) staining was performed to measure the osteogenesis differentiation ability of the hBMSCs in different groups, and ALP activity was compared among different groups. (c) Alizarin red S staining was performed to observe the calcified nodules formation in hBMSCs with different disposition, and compared by OD values. *P < 0.05, **P < 0.01, vs. crocin of 0 μmol/L. ^^^P < 0.001, vs. control; ##P < 0.01, ###P < 0.001, vs. OS.
Crocin facilitated the phosphorylation of GSK-3β in hBMSCs
As shown in Fig. 2, both western blot (WB) and qRT-PCR assays detected that the expression levels of RUNX2, COL1A1, and OCN proteins and genes in hBMSCs were obviously increased after induction by osteogenesis differentiation medium and crocin (P < 0.01). Interestingly, GSK-3β phosphorylation was observably reduced by osteogenesis differentiation induction medium and crocin (P < 0.001), but the expression levels of total GSK-3β proteins and mRNA in cells were not significant. With the increase of crocin concentration, its effect on genes and proteins related to osteogenesis differentiation became more obvious.
Crocin facilitated the phosphorylation of GSK-3β in hBMSCs. Cells were distributed into following groups: control group, positive control group (osteogenesis differentiation medium (OS)), and crocin treatment group (10, 25, 50 μmol/L). (a–d) Western blot (WB) and (e) quantitative real-time polymerase chain reaction (qRT-PCR) assays were used to determine the expression levels of RUNX2, COL1A1, OCN, and GSK-3 β in hBMSCs in different groups. ^^P < 0.01, ^^^P < 0.001, vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. OS.
Crocin improved femoral head regeneration in SANFH rat models
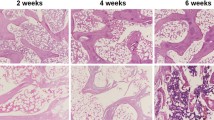
In animal experiments, we further analyzed the effect of crocin on SANFH rat models. According to the H&E staining (Fig. 3a), compared with the normal control group, the femoral head of SANFH rat models showed typical osteonecrosis, with diffuse voids in the bone trabecular and bone marrow cell necrosis in surrounding. After treatment with crocin of 10 and 25 μmol/L, the diffuse voids in the bone trabecular and bone marrow cell necrosis in surrounding were improved (P < 0.01). When the therapeutic concentration reached 50 μmol/L, the diffuse voids were virtually invisible and no significant pathological changes were observed in the femoral head.
Crocin improved femoral head regeneration in steroid-induced osteonecrosis of the femoral head (SANFH) rats models (A). A total of 30 rats were randomly divided into five groups with equal amount: control group, SANFH model group, high-concentration crocin treatment (crocin-H) group, medium-concentration crocin treatment (crocin-M) group, and low-concentration crocin treatment (crocin-L) group. The pathological changes of femoral head tissues of rats in different groups were observed through hematoxylin and eosin (H&E) staining, rate of empty lacunae was compared among different groups. ^^^P < 0.001, vs. control; &&P < 0.01, &&&P < 0.001, vs. model.
Crocin facilitated the phosphorylation of GSK-3β in femoral head tissues of SANFH rat models
Similar to the cell experiments, we again examined the expression levels of genes and proteins related to osteogenesis differentiation in the femoral head tissues of SANFH rat models. In experimental results of WB and qRT-PCR assays (Fig. 4), it could be seen that the expression levels of RUNX2, COL1A1, and OCN proteins and genes were prominently decreasing in femoral head tissues of SANFH rat models, while the GSK-3β phosphorylation was observably increased (P < 0.001). In reverse, crocin promoted the expression of RUNX2, COL1A1, and OCN and inhibited the phosphorylation of GSK-3β in a concentration-dependent manner (P < 0.05).
Crocin facilitated the phosphorylation of GSK-3β in femoral head tissues of steroid-induced osteonecrosis of the femoral head (SANFH) rat models. A total of 30 rats were randomly divided into five groups with equal amount: control group, SANFH model group, high-concentration crocin treatment (crocin-H) group, medium-concentration crocin treatment (crocin-M) group, and low-concentration crocin treatment (crocin-L) group. (a–d) Western blot (WB) and (e) quantitative real-time polymerase chain reaction (qRT-PCR) assays were used to determine the expression levels of RUNX2, COL1A1, OCN, and GSK-3 β in femoral head tissues of rats in different groups. ^^^P < 0.001, vs. control; &P < 0.05, &&P < 0.01, &&&P < 0.001, vs. model.
Discussion
Because glucocorticoid is the first-line therapeutic drug for the treatment of asthma, systemic lupus erythematosus, organ transplantation, and many other clinical diseases, there are a large number of new cases of SANFH every year (Wang et al. 2013; Faienza et al. 2014). The clinical treatment progress of SANFH is slow with the high possibility of joint disability, which seriously affects the normal life and work of patients (Arai et al. 2017). Numerous findings support that the decreased osteogenesis differentiation ability of BMSCs is closely related to the pathogenesis of SANFH (Wang et al. 2016; Pei et al. 2017). A recent research has reported that crocin promotes bone resorption and has protective effects against bone diseases (Suh et al. 2019). Long-term treatment with crocin enhances survival selectively in female rats with colon cancer without major toxic effects (García-Olmo et al. 1999). Crocin attenuates osteoarthritis symptoms through alleviating oxidative stress and inflammation, suggesting that crocin is a potential medicine for osteoarthritis therapy (Lei et al. 2017). In this present study, hBMSCs were cultured with different concentrations of crocin, and it was found that crocin of 0, 25, and 50 μmol/L had no significant inhibitory effect on hBMSCs viability, which were used as experimental concentrations in subsequent cell experiments to inquire the effect of crocin on osteogenesis differentiation of hBMSCs.
In this study, alizarin red S staining and ALP staining were selected as the indicators to evaluate the osteogenesis differentiation ability of hBMSCs. Among them, ALP is a membrane bound enzyme, which can hydrolyze organic phosphate to release inorganic phosphorus and start the process of calcification, marking the beginning of osteogenesis differentiation (Siller and Whyte 2018). Alizarin red can chelate with calcium ions to form complexes that can identify calcium salt components in cells and can be used to mark calcium nodules formed by osteogenesis differentiation of hBMSCs (Lei et al. 2018). According to the staining results, both alizarin red S staining and ALP staining in crocin-induced hBMSCs were positive, and the staining intensities were stronger than the positive control with concentration dependence. Consistent with our results, the findings of Kalalinia F et al. (Kalalinia et al. 2018) also revealed that crocin could enhance the alizarin red S staining intensity and increase the ALP activity of rat BMSCs, thus effectively promoting the osteogenesis differentiation of rat BMSCs. These experimental phenomena indicated that crocin can induce osteogenesis differentiation of hBMSCs,
It is currently thought that the differentiation of stem cells into specific cell lines is mainly mediated by specific transcription factors (Rauch et al. 2019). RUNX2 is a key transcription factor necessary for mesenchymal stem cells to differentiate into osteoblastic cell lines, which can directly promote the synthesis and secretion of genes needed for osteogenesis differentiation, such as OCN and COL1A1 (Chava et al. 2018). COL1A1 is the main organic component of bone matrix; its expression can control the morphology, differentiation, and other biological functions of bone cells, which plays an important role in maintaining the integrity and biomechanical properties of bone structure (Bardai et al. 2016). OCN is an essential factor for bone calcification, which plays a key role in maintaining normal mineralization and is one of the signs of late osteogenesis differentiation (Ching et al. 2017). In general, decreased expression of RUNX2, OCN, and COL1A1 indicates decreased osteogenesis differentiation of cells, while increased expression of these indicators prompts increased osteogenesis differentiation capacity (Zhang et al. 2019).
In the correlated pathway, it was reported that the activation of Wnt signaling pathway could trigger RUNX2 expression and involve in the osteogenesis differentiation (Cai et al. 2016). GSK-3β is a fundamental negative regulator in Wnt signaling pathway, which loses its activity after phosphorylation and regulates the status of Wnt signaling pathway, thus participating in osteogenesis differentiation (Arioka et al. 2013). There are some evidences sustained that the high level of GSK-3β can inhibit the osteogenesis differentiation of hBMSCs, whereas the suppression of GSK-3β exerted auxo-action in osteogenesis differentiation (Huang et al. 2018; Zhao et al. 2016). Through WB and qRT-PCR experiments, we found that crocin upregulated the expression levels of RUNX2, COL1A1, and OCN and inhibited the phosphorylation of GSK-3β in hBMSCs in a concentration-dependent manner. These laboratorial results further supported the promoting role of crocin in osteogenesis differentiation of hBMSCs.
In addition, animal experiments were carried out in this paper to verify the outcomes of cell experiments and improve the credibility of our study. Based on past experiences (Pei et al. 2017), we constructed SANFH rat models using LPS and MPS and then treated them with crocin solution at various concentrations. On the basis of H&E staining results, the femoral head tissues of the rat models showed typical osteonecrosis, while these changes were effectively ameliorated after crocin treatment. In addition, with the increase of crocin concentration, the diffuse voids in the bone trabecular in the femoral head tissues of SANFH rat models gradually decreased, and the pathological changes further disappeared. Downregulation of miR-124 expression enhanced GSK-3β expression, weakened Wnt/β-catenin pathway activity, and inhibited the differentiation of ligament fibroblasts into osteoblasts (Tang et al. 2018). As for the expression levels of factors related to osteogenesis differentiation, this study detected that the expression levels of RUNX2, COL1A1, and OCN in femoral head tissues of SANFH rat models were observably reduced, while the phosphorylated GSK-3β was significantly increased to inhibit osteogenesis differentiation. Interestingly, these transformations were reversed under crocin treatment, which were consistent with the results of cell experiments. Thus, it could be speculated that crocin could alleviate the development of SANFH by increasing the expression levels of RUNX2, COL1A1, and OCN, as well as decreasing phosphorylated GSK-3β, to promote osteogenesis differentiation of BMSCs.
Conclusion
In conclusion, we found though in vitro experiments that crocin effectively enhanced the osteogenesis differentiation of hBMSCs and alleviated osteonecrosis in SANFH rat models in vivo, accompanied by inhibition of GSK-3β phosphorylation. These findings manifested that crocin might be beneficial to the recovery of SANFH though accelerating osteogenesis differentiation of BMSCs, which was expected to be a novel therapy for related diseases. Whether crocin had the same effect on WT rodents was an interesting topic and would be studied in the future.
References
Arai R, Takahashi D, Inoue M, Irie T, Iwasaki N (2017) Efficacy of teriparatide in the treatment of nontraumatic osteonecrosis of the femoral head: a retrospective comparative study with alendronate Bmc Musculoskeletal Disorders 18
Arioka M et al. (2013) Acceleration of bone development and regeneration through the Wnt/beta-catenin signaling pathway in mice heterozygously deficient for GSK-3beta biochemical and biophysical research communications 440:677-682 https://doi.org/10.1016/j.bbrc.2013.09.126
Bardai G et al. (2016) Osteogenesis imperfecta type I caused by COL1A1 deletions calcified tissue international 98:76-84 https://doi.org/10.1007/s00223-015-0066-6
Cai T, Sun D, Duan Y, Wen P, Dai C, Yang J, He W (2016) WNT/beta-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp Cell Res 345:206–217. https://doi.org/10.1016/j.yexcr.2016.06.007
Chava S, Chennakesavulu S, Gayatri BM, Reddy ABM (2018) A novel phosphorylation by AMP-activated kinase regulates RUNX2 from ubiquitination in osteogenesis over adipogenesis cell death & disease 9:754 https://doi.org/10.1038/s41419-018-0791-7
Ching HS, Luddin N, Rahman IA, Ponnuraj KT (2017) Expression of odontogenic and osteogenic markers in DPSCs and SHED: a review current stem cell research & therapy 12:71–79 https://doi.org/10.2174/1574888x11666160815095733
Christodoulou E, Kadoglou NP, Kostomitsopoulos N, Valsami G (2015) Saffron: a natural product with potential pharmaceutical applications. J Pharm Pharmacol 67:1634–1649. https://doi.org/10.1111/jphp.12456
Dai F, Du P, Chang Y, Ji E, Xu Y, Wei C, Li J (2018) Downregulation of MiR-199b-5p inducing differentiation of bone-marrow mesenchymal stem cells (BMSCs) toward cardiomyocyte-like cells via HSF1/HSP70 pathway medical science monitor : international medical journal of experimental and clinical research 24:2700-2710 https://doi.org/10.12659/msm.907441
Faienza MF et al. (2014) Treatment of osteoporosis in children with glucocorticoid-treated diseases expert review of endocrinology & metabolism 9:525-534 https://doi.org/10.1586/17446651.2014.936384
García-Olmo DC, Riese HH, Escribano J, Ontañón J, Fernandez JA, Atiénzar M, García-Olmo D (1999) Effects of long-term treatment of colon adenocarcinoma with crocin, a carotenoid from saffron (Crocus sativus L.): an experimental study in the rat. Nutr Cancer 35:120–126. https://doi.org/10.1207/s15327914nc352_4
Gu M, Luo L, Fang K (2018) Crocin inhibits obesity via AMPK-dependent inhibition of adipocyte differentiation and promotion of lipolysis. Biosci Trends 12:587–594. https://doi.org/10.5582/bst.2018.01240
Hao C et al (2016) MiR-708 promotes steroid-induced osteonecrosis of femoral head, suppresses osteogenic differentiation by targeting SMAD3. Sci Rep 6:22599. https://doi.org/10.1038/srep22599
He Y, Zhou L, Fan Z, Liu S, Fang W (2018) Palmitic acid, but not high-glucose, induced myocardial apoptosis is alleviated by Nacetylcysteine due to attenuated mitochondrial-derived ROS accumulation-induced endoplasmic reticulum stress. Cell Death Dis 9:568. https://doi.org/10.1038/s41419-018-0593-y
Huang L, Wang Y, Jiang Y, Wu Y, Hu C, Ouyang H (2018) High levels of GSK-3beta signalling reduce osteogenic differentiation of stem cells in osteonecrosis of femoral head. J Biochem 163:243–251. https://doi.org/10.1093/jb/mvx076
Kalalinia F, Ghasim H, Amel Farzad S, Pishavar E, Ramezani M, Hashemi M (2018) Comparison of the effect of crocin and crocetin, two major compounds extracted from saffron, on osteogenic differentiation of mesenchymal stem cells. Life Sci 208:262–267. https://doi.org/10.1016/j.lfs.2018.07.043
Kubo T, Ueshima K, Saito M, Ishida M, Arai Y, Fujiwara H (2016) Clinical and basic research on steroid-induced osteonecrosis of the femoral head in Japan Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association 21:407–413 https://doi.org/10.1016/j.jos.2016.03.008
Lei M, Guo C, Hua L, Xue S, Yu D, Zhang C, Wang D (2017) Crocin attenuates joint pain and muscle dysfunction in osteoarthritis rat inflammation 40:2086–2093 https://doi.org/10.1007/s10753-017-0648-8
Lei Q, Lin D, Huang WX, Wu D, Chen J (2018) [Effects of calcium ion on the migration and osteogenic differentiation of human osteoblasts] Hua xi kou qiang yi xue za zhi = Huaxi kouqiang yixue zazhi = West China journal of stomatology 36:602-608 https://doi.org/10.7518/hxkq.2018.06.004
Li C, Wei GJ, Xu L, Rong JS, Tao SQ, Wang YS (2017) The involvement of senescence induced by the telomere shortness in the decline of osteogenic differentiation in BMSCs European review for medical and pharmacological sciences 21:1117–1124
Liu FF, Zhao S, Liu P, Huo SP (2018) Influence of mTOR signaling pathway on ketamine-induced injuries in the hippocampal neurons of rats Neurol Res:1-10 https://doi.org/10.1080/01616412.2018.1531203
Moshiri M, Vahabzadeh M, Hosseinzadeh H (2015) Clinical applications of saffron (Crocus sativus) and its constituents: a review. Drug Research 65:287–295. https://doi.org/10.1055/s-0034-1375681
Nie B, Ao H, Zhou J, Tang T, Yue B (2016) Biofunctionalization of titanium with bacitracin immobilization shows potential for anti-bacteria, osteogenesis and reduction of macrophage inflammation. Colloids Surf B: Biointerfaces 145:728–739. https://doi.org/10.1016/j.colsurfb.2016.05.089
Pei J, Fan L, Nan K, Li J, Shi Z, Dang X, Wang K (2017) Excessive activation of TLR4/NF-kappaB interactively suppresses the canonical Wnt/beta-catenin pathway and induces SANFH in SD. Rats Scientific Reports 7:11928. https://doi.org/10.1038/s41598-017-12196-8
Qin L et al (2006) Multiple bioimaging modalities in evaluation of an experimental osteonecrosis induced by a combination of lipopolysaccharide and methylprednisolone. Bone 39:863–871. https://doi.org/10.1016/j.bone.2006.04.018
Rahman WA, Garbuz DS, Masri BA (2013) Total hip arthroplasty in steroid-induced osteonecrosis: early functional and radiological outcomes. Can J Surg J canadien de chirurgie 56:41–46. https://doi.org/10.1503/cjs.032510
Rao X, Huang X, Zhou Z, Lin X (2013) An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis Biostatistics, bioinformatics and biomathematics 3:71-85
Rauch A et al (2019) Osteogenesis depends on commissioning of a network of stem cell transcription factors that act as repressors of adipogenesis. Nat Genet 51:716–727. https://doi.org/10.1038/s41588-019-0359-1
Siller AF, Whyte MP (2018) Alkaline phosphatase: discovery and naming of our favorite enzyme. J Bone Miner Res 33:362–364. https://doi.org/10.1002/jbmr.3225
Suh KS, Chon S, Jung WW, Choi EM (2019) Crocin attenuates methylglyoxal-induced osteoclast dysfunction by regulating glyoxalase, oxidative stress, and mitochondrial function Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 124:367-373 https://doi.org/10.1016/j.fct.2018.12.031
Sun ZB et al. (2015) Icariin may benefit the mesenchymal stem cells of patients with steroid-associated osteonecrosis by ABCB1-promoter demethylation: a preliminary study Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 26:187-197 https://doi.org/10.1007/s00198-014-2809-z
Tang SL, Huang QH, Wu LG, Liu C, Cai AL (2018) MiR-124 regulates osteoblast differentiation through GSK-3β in ankylosing spondylitis European review for medical and pharmacological sciences 22:6616–6624 https://doi.org/10.26355/eurrev_201810_16136
Wang A, Ren M, Wang J (2018a) The pathogenesis of steroid-induced osteonecrosis of the femoral head: a systematic review of the literature. Gene 671:103–109. https://doi.org/10.1016/j.gene.2018.05.091
Wang P et al. (2016) Effect of Colla Cornus Cervi combined with LV-mediated BMP7 transfected BMSCS on ANFH in rats Acta poloniae pharmaceutica 73:1521-1530
Wang Q, Yang Q., Chen G., du Z., Ren M., Wang A., Zhao H., Li Z., Zhang G., Song Y. (2018b) LncRNA expression profiling of BMSCs in osteonecrosis of the femoral head associated with increased adipogenic and decreased osteogenic differentiation Scientific reports 8:9127 https://doi.org/10.1038/s41598-018-27501-2
Wang XS, Zhuang QY, Weng XS, Lin J, Jin J, Qian WW (2013) Etiological and clinical analysis of osteonecrosis of the femoral head in Chinese patients. Chin Med J 126:290–295
Zhang K et al. (2019) The PERK-EIF2alpha-ATF4 signaling branch regulates osteoblast differentiation and proliferation by PTH American journal of physiology endocrinology and metabolism 316:E590-e604 https://doi.org/10.1152/ajpendo.00371.2018
Zhao R, Li Y, Lin Z, Wan J, Xu C, Zeng Y, Zhu Y (2016) miR-199b-5p modulates BMSC osteogenesis via suppressing GSK-3beta/beta-catenin signaling pathway Biochemical and biophysical research communications 477:749-754 https://doi.org/10.1016/j.bbrc.2016.06.130
Funding
This manuscript was supported by the funding of Health industry research project (No. 201402016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Tetsuji Okamoto
Rights and permissions
About this article
Cite this article
Li, B., Qin, K., Wang, B. et al. Crocin promotes osteogenesis differentiation of bone marrow mesenchymal stem cells. In Vitro Cell.Dev.Biol.-Animal 56, 680–688 (2020). https://doi.org/10.1007/s11626-020-00487-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-020-00487-w