Abstract
Hypoxia-inducible factor 1α (HIF-1α) is one of the master regulators of hypoxia reactions, playing an important role in bone modeling, remodeling, and homeostasis. And overexpression of HIF-1α in mature osteoblasts through conditional deletion of the von Hippel-Lindau (VHL) gene profoundly increases angiogenesis and osteogenesis. Studies showed that mice with osteoblasts lacking Vhl had a high level of Hif-1α and increased bone mass and density. On the contrary, Hif-1α conditional knockout mice had decreased bone mass and density. Our in vitro study showed that osteoprotegerin (OPG), an essential regulator of osteoclastic activity, can be upregulated by HIF-1α and in turn downregulate the resorption activity of osteoclasts. We showed that HIF-1α may directly bind to the upstream site of OPG and enhance its expression. Our study suggested that a novel mechanism, which works via OPG signaling, may mediate the function of HIF-1α in bone remodeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mature bone maintains structural integrity and mechanical stability through bone remodeling process (bone formation and resorption are always balanced at the same site simultaneously) (Boughner et al. 2007; Yang 2009). Osteoblasts and osteoclasts both participate in the process of bone remodeling and are regulated by many molecular factors. In view of the structure, micro-environment, and biomechanical characteristics of bone, the regulatory mechanism of hypoxia-inducible factors (HIFs) on osteoblasts and osteoclasts draws more and more attention (Martinez et al. 2010).
HIFs have three sub-types, HIF-1, HIF-2, and HIF-3. Present researches are mainly focused on HIF-1 and its oxygen-sensing element hypoxia-inducible factor 1α (HIF-1α). HIF-1 is the core transcription factor regulating cellular internal environment and homeostasis under hypoxia conditions, and it has been the hotspot of biology since it was identified in 1992. Under normoxia, the prolines in the oxygen-dependent degradation domain of HIF-1α are hydroxided, and then, HIF-1α is degraded after combining with protein von Hippel-Lindau (pVHL). While in hypoxia or anoxia, accumulated HIF-1α is transported to nucleus and combines with HIF-1β, activating the sensitive target genes of HIF-1 and causing the oxygen-adaptive response of tissue and cell (Ratcliffe 2007). More than 100 genes regulated by HIF-1 have been found, such as erythropoietin (EPO), glucose transporter (Glut), and vascular endothelial growth factor (VEGF), the products of which play a vital role in various biological behavior, including erythropoiesis, glucose and energy metabolism, vascular remodeling, cell proliferation, and apoptosis (Semenza 2001a, b, 2009, 2010). Thus, HIF-1 is the core transcription factor regulating cellular internal environment and homeostasis under hypoxia (Majmundar et al. 2010).
Recent studies demonstrated mesenchyme-derived marrow stroma cell, chondrocyte, and osteoblast are all oxygen-sensing cells, which express HIF-1α and regulate its functional phenotype adaptation (Wan et al. 2010; Tamama et al. 2011). Mice with conditional Vhl knockout (Hif-1α over-expressed) osteoblasts showed a similar “osteopetrosis” phenotype due to imbalance of bone remodeling, which means the activity and function of osteoblasts are increased, while those of osteoclasts remain unchanged, or even be reduced (Wang et al. 2007). In addition, the results from conditional Vhl knockout in vivo model showed that the upregulation of osteoblastic marker genes and the increase of osteoblastic function are independent on the direct promotion of angiogenesis (Zhang et al. 2010). On the contrary, Mice lacking Hif-1α in osteoblasts were viable and developed normally, but had decreases in bone volume and vascularity (Wang et al. 2007). Interestingly, Hif-2α would be upregulated in mice without Hif-1α and may compensate some function of Hif-1α (Wang et al. 2007).
Thus, besides promoting bone development directly by increasing angiogenesis or osteoblastic activity, activating hypoxia/HIF-1 pathway might also disturb osteoblasts and osteoclasts coupling to inhibit osteoclast generation.
In this study, we used an in vitro co-cultured model of calvarial-derived osteoblasts and bone marrow-derived osteoclastic precursor cells to explore the regulatory mechanism of hypoxia/HIF pathway in osteoblasts and osteoclasts coupling, in order to deepen the understanding about the cellular and molecular mechanisms of oxygen sensing, especially the role which hypoxia/HIF pathway plays in the bone remodeling process.
Materials and Methods
Osteoblasts/osteoclasts isolation and co-culture.
The procedures about primary osteoblasts isolation and culture as well as adenovirus infection were introduced before (Wang et al. 2007; Wan et al. 2010). Briefly, calvaria of newborn mice were digested in 200 U/ml collagenase type I (Worthington Biochemical Corp., Lakewood, NJ) solution for 15 min at 37°C under constant agitation. Then, the solution was collected, and the digestion was repeated again for four times. The digestion solution 3–5 containing the osteoblasts were pooled together and filtrated by 70-μm filter. After centrifugation, osteoblasts were cultured in α-minimum essential medium (α-MEM) (Invitrogen, Waltham, MA) containing 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2. The adenoviruses used here were purchased from Agilent Technologies (Santa Clara, CA), and the infection was carried out according to the manufacturer’s instructions. Briefly, adenoviruses were amplified by infecting HEK-293 cells. Cells were harvested when cytopathic effects became apparent. After lysis by five freeze/thaw cycles, cell debris was pelleted by centrifugation and subjected to further cesium chloride purification procedures. Concentration of purified virus was calculated by measuring the value of OD260. For deletion of the Hif-1α or/and Vhl, osteoblasts containing floxed Hif-1α or/and Vhl alleles were cultured to 70% confluence and then, in the absence of serum, were infected with Ad-Cre or, as a control, an Ad-GFP, at 800 MOI, unless otherwise noted. After 1 h, culture medium containing 10% FBS was added, and the cells were allowed to recover for 48 h before stimulation.
Bilateral femurs and tibias of 1-mo-old mice were isolated, and the metaphyses were cut off, put into ice-cold α-MEM, and washed by the solution repeatedly (3~5 times) until diaphysis turned white. Then, the solution was collected and centrifugated at 1500 rpm for 6 min. The supernatant was abandoned, and the precipitation was resuspended by 2 ml culture medium. After stabilization on ice for 1 min, 1.7 ml solution without precipitation was transferred into a new tube, and 0.3 ml culture medium was added into the tube. The tube was put on ice for stabilization again; then, 1.7 ml solution containing bone marrow-derived osteoclastic precursor cells was transferred into a new tube and used for counting and seeding.
The osteoclastic precursor cells (5 × 106/cell) were co-cultured with the control osteoblasts and ∆HIF1α/∆VHL/∆HIF1α∆VHL osteoblasts (105/cell), respectively, in α-MEM containing 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO2 for 5, 9, and 13 d. The culture medium was half changed every 2 d until the time points.
We carried out the experiment in accordance with The Code of Ethics of the World Medical Association.
TRAP staining.
TRAP staining was carried out using the TRAP kit (387A-1KT, Sigma, Taufkirchen, Germany) according to the manufacturer’s instruction. The cells were grown on glass coverslips and rinsed briefly in phosphate-buffered saline (PBS) before fixation. The samples were fixed in ice-cold methanol, acetone (1–10 min) and washed twice with ice-cold PBS after that. Then, the samples were incubated for 10 min with PBS containing 0.25% Triton X-100 (or 100 μM digitonin or 0.5% saponin).
Ivory bone resorption experiment.
Elephant ivory slices (6 mm diameter, kindly donated by the Centre de conservation et d'étude des collections, Lyon, France) from 3-mo-old calves were used in these experiments. Slices (0.3-mm-thick) were cut with a low speed saw (Buehler, Lake Bluff, IL), cleaned by sonication, sterilized for 2 min in 70% ethanol, and stored dry at −20°C until it was used in in vitro resorption assay. Ivory and bone slices were incubated with 0.2 M d-ribose in 10 ml of phosphate-buffered saline (PBS) solution, containing 100 units/ml penicillin, 100 μg/ml streptomycin, and 1.5 mM phenyl-methylsulfonyl fluoride, for 45 and 60 d at 37°C, respectively.
Real-time PCR.
Total RNA was extracted from osteoblasts using the TRIzol method as recommended by Invitrogen. The yield and purity of RNA was estimated spectrophotometrically using the A260/A280 ratio. Three micrograms of RNA were reverse-transcribed into complementary DNA (cDNA) using SuperScript first-strand synthesis system (Invitrogen). One microliter of cDNA was subjected to PCR amplification using SYBR GREEN PCR Master Mix (Applied Biosystems, Life Technologies, Waltham, MA) and sequence-specific primers (Table 1). Then, mixture was denatured in 94°C for 5 min, then run in circle (94°C 30 s, Tm 30 s, 72°C 4 0 s, 90°C 10 s) for 35 times.
Western blotting.
We used 1 × SDS to prepare the lysate from cultured cells. Run the sodium dodecyl sulfate polyacrylamide gel (10%) for 3 h at 100 V, and then transfer the protein from the gel to the PVDF membrane with 100 mA for 2 h. The membrane was incubated with primary antibody at 4°C overnight, then washed by 0.1% Tween-20 for three times, and 10 min each. Then, the membrane was incubated in 1:3000 secondary antibody dilutions for 2 h and then washed by PBS three times, 10 min each. The results are analyzed by ImageQuant (LAS 4000 mini, GE, Fairfield, CT). Primary antibody: β-actin (ab8227, Abcam, Cambridge, MA), HIF-1α (NB100-105, Novus, Saint Charles, MO), HIF-2α (NB100-122, Novus), RANKL (NB100-56512, Novus), VHL (NB110-10160, Novus), osteoprotegerin (OPG) (NBP1-70810, Novus), VEGF (NB100-664, Novus); secondary antibody: immunopure antibody goat anti-rabbit IgG (H+L) (Thermo, Waltham, MA).
Chromatin immunoprecipitation.
In the upstream areas of Opg and Rankl there are hypoxia-responsive element (HRE) binding sequences 5′-CGTC-3′. PCR was used to get the sequence which contains the HRE binding site. Primers were designed by Primer Premier 5.0 (Table 2).
Statistics.
Data were presented as mean ± SD. Statistical analysis was conducted using SPSS 13.0 software, and comparison was made by Student t test. p < 0.05 was considered as statistically significant.
Results
Osteoblasts lacking Vhl highly express Hif-1α in vitro.
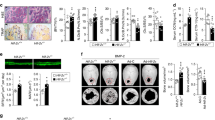
In order to study the function of Hif-1α in vitro, we co-cultured each different genetic osteoblasts (control, ∆HIF1α/∆VHL/∆HIF1α∆VHL) with osteoclasts. With this method, we could check the activity of osteoclasts in vitro. At first, we checked the expression level of Hif-1α in each group and got the result just as expected. Under normoxia only in the ∆VHL group, there was high expression of Hif-1α, while under hypoxia, both control and ∆VHL groups had high expression of Hif-1α (Fig. 1A ). Later, we also checked the protein level of Hif-1α in each test group (Fig. 1B ). These results proved that in the ∆VHL osteoblasts, the level of Hif-1α was high and suggested that the in vitro model could also be used to study the function of Hif-1α.
Osteoblasts lacking Vhl high express Hif-1α in vitro. a The gene expression of Hif-1α in co-culture of osteoblasts and osteoclasts under normoxia and hypoxia for 5 d. Under normoxia, Vhl −/− group has overexpressed level of Hif-1α, and under hypoxia, both control group and Vhl −/− group have a high level of Hif-1α expression. b The protein product of Hif-1α in co-culture of osteoblasts and osteoclasts under normoxia and hypoxia for 7, 10, and 13 d. △HIF1α: Hif-1α −/−, △VHL: Vhl −/−, △HIF1α△VHL: Hif-1α −/−& Vhl −/−. # p < 0.01, vs. normoxia.
High expression of Hif-1α in osteoblasts downregulates differentiation and activity of osteoclasts.
Studies in mice indicated that there were fewer osteoclasts in the Vhl knockout group (Wang et al. 2007). Then, we took in vitro experiments to insure the discovery in vivo. After co-culturing of osteoblasts and osteoclasts for 13 d, the number of TRAP-positive cells was at the least in Vhl knockout group and at the most in Hif-1α knockout group (Fig. 2A, B ). This result suggested that Hif-1α might suppress the generation of osteoclasts.
Hif-1α downregulates the generation and activity of osteoclasts. a TRAP staining of the co-cultured cells. After co-culture of osteoblasts and osteoclasts for 13 d, the number of TRAP-positive cells was the least in Vhl knockout group and the most in Hif-1α knockout group. b The gene expression of TRAP in co-culture of osteoblasts and osteoclasts under normoxia for 5, 9, and 13 d. After osteoblast and osteoclast co-culture for 5, 9, and 13 d, the expression of TRAP gene was the most in Hif-1α knockout group and the least in Vhl knockout group. c Co-cultured osteoblasts (control, Hif-1α −/−& Vhl −/− , Vhl −/−, Hif-1α −/−) with osteoclasts was used to test the resorption activity. The absorbed ivory area presents the activity of osteoclasts. d Quantification of absorption area. The absorbed area of ivory is the largest in Hif-1α −/− group and smallest in Vhl −/−group. △HIF1α: Hif-1α −/−, △VHL: Vhl −/−, △HIF1α△VHL: Hif-1α −/−& Vhl −/−. # p < 0.01, vs. control; *p < 0.05, vs. control.
It is rational to think that the bone resorption activity may also be suppressed by high expression of Hif-1α, so we used ivory bone resorption experiment to analyze the resorption activity of each group cells and found that the osteoclasts co-cultured with osteoblasts lacking Hif-1α showed the highest resorption activity. We noticed that there was no significant difference of bone resorption activity between wild-type and Hif-1α & Vhl double knockout groups. This indicated that there were some other factors contributing to the phenotype in the Vhl knockout group (Fig. 2C, D ). Indeed, resent studies showed that Hif-2α was also upregulated in the absence of Vhl (Wang et al. 2007).
All these indicated that Hif-1α could downregulate osteoclastic activity in order to suppress bone resorption.
High expression of Hif-1α upregulates the level of Opg.
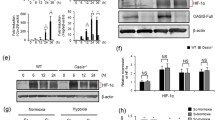
OPG regulates the differentiation and activity of osteoclasts together with receptor activator of nuclear factor-κ B ligand (RANKL) via receptor activator of nuclear factor-κ B (RANK) (Simonet et al. 1997; Knowles and Athanasou 2009; Pivonka et al. 2010; Tekkesin et al. 2011). In our study, we checked the expression level of Opg and Rankl using RT-PCR. Interestingly, the Vhl knockout osteoblasts showed highest Opg expression level and Hif-1α −/− group showed the lowest (Fig. 3A ), while the Rankl expression was not changed (Fig. 3B ). As a result, the Opg/Rankl ratio was greatly upregulated in Vhl −/− group and stayed the lowest in Hif-1α −/− group (Fig. 3C ). This result suggested that high level of Hif-1α could stimulate Opg expression and through this signaling pathway downregulate the activity of osteoclasts.
Hif-1α upregulates the level of Opg. a, b, c Relative expression of the genes. RT-PCR results showed that Opg transcription was obviously upregulated in Vhl −/− group from 5 to 9 d (a); however, there was no change in Rankl level (b). In the end, the Vhl −/− group had the highest Opg/Rankl ration (c). d, e ChIP results showed that Hif-1α could bind to Opg1; however, the other site upstream of Opg showed no such function (d). The two sites upstream Rankl showed no interaction with Hif-1α in vitro (e). White bars indicate anti-IgG; black bars indicate anti-Hif-1α. △HIF1α: Hif-1α −/−, △VHL: Vhl −/−, △HIF1α△VHL: Hif-1α −/−& Vhl −/−. # p < 0.01, vs. control; *p < 0.05, vs. control.
With the help of bioinformatics methods, we found that there were two hypoxia-responsive element (HRE) binding sites each in the upstream areas of both Opg and Rankl genes. So, we used chromatin immunoprecipitation (ChIP) to test whether Hif-1α could interact with them or not. Interestingly, we found that Hif-1α could only interact with Opg1 but not Opg2 binding site. Then, we checked the interaction between Hif-1α and Rankl with the same method and found no interaction between them (Fig. 3D, E ). This result suggested that Hif-1α could directly stimulate the transcription of Opg1 and in turn suppress the activity of osteoclasts.
Discussion
Previous studies showed that Hif-1α facilitates bone formation; however, the exact mechanism is still unknown. What we know is that Hif-1α could mediate angiogenesis to osteogenesis coupling during skeleton development (Wang et al. 2007; Nakahama 2010), but these studies also indicated that there must be some other mechanisms contributing to the phenotypes caused by deletion of Vhl.
In our study, we found that Hif-1α could suppress the number and activity of osteoclasts in vitro. This discovery suggested that a novel mechanism may be involved in mediating the function of Hif-1α. OPG and RANKL are essential regulators which couple bone formation and resorption (Boyce et al. 2005; Brzoska and Rogalska 2013; Liu et al. 2013). Further studies showed that Opg was upregulated while RANKL was unchanged in present of high level of Hif-1α, and Hif-1α may directly bind to the promoter site of Opg and stimulate its transcription.
In recent studies, overexpression of Hif-1α leads to robust bone formation (Wang et al. 2007). However, evidences also show that the formation of the flat bones of skull is not affected (Wang et al. 2007). One explanation is that skull formed through an intramembranous process involving condensing mesenchymal stem cells. These precursor cells can differentiate directly into osteoblasts. So, even though overexpression of Hif-1α suppresses angiogenesis and in turn decreases osteoblasts differentiation, the skull formation would not be affected (Chung et al. 2004). Interestingly, in our recent study, the formation of skull in Hif-1α conditional knockout mice is delayed (data not shown). Thus, we suppose that the change of OPG level mediates the delay of skull development. Osteoblast has been shown to express components of the HIF signaling pathway including prolyl hydroxylase enzymes 1, 2, and 3 (PHD1/2/3), VHL, HIF-1, and HIF-2 under both physiological and pathophysiological conditions indicating that HIF signaling is an important mediator of normal osteoblast function and the related disease. According to previous reports, the PHDs and VHL negatively regulate the pathway under normoxic conditions by cooperatively targeting hypoxia-inducible transcription factors HIF-1 and HIF-2 for proteasomal degradation, whereas HIF-1 and HIF-2 are stabilized and coordinate the cellular response to hypoxia by activating gene expression programs that facilitate oxygen delivery and cellular adaptation to oxygen deprivation under hypoxic conditions (Semenza 2001a, b). Wang et al. showed that osteoblast-specific knockout of Hif-1α mice does not have the same phenotype as Hif-1α & Vhl double knockout mice, and in fact, the defects in Hif-1α & Vhl double knockout is not so severe as in the Hif-1α single knockout mice (Wang et al. 2007), and they concluded that the overexpressed Hif-2α could compensate some function of Hif-1α in Hif-1α & Vhl double knockout mice. We here also observed that Hif-1α & Vhl double knockout showed more Trap mRNA expression and bone resorption. We attributed this effect to compensate the function exerted by Hif-2α. Though Hif-1α and Hif-2α are homologous and both contain the conserved ODD domain (Maxwell et al. 1999), some recent studies indicate that Hif-1α and HIF-2α may have different functions, and there are differences in expression levels of them in different cell types (Akeno et al. 2001; Bardos and Ashcroft 2005). Whether Hif-2α could have effects on RANKL/RANK/OPG pathway is unknown, further studies should be done to reveal the relationship between them.
Some studies also suggested that the OPG level was not affected in the Vhl knockout mice. However, in these studies the OPG level was only checked in serum (Wang et al. 2007). Mice, human, or other vertebrates may have some mechanisms which can maintain the OPG level in serum; otherwise, it is difficult to explain the different levels of resorption activity in the whole osseous system. We suggest that HIF-1α upregulates the level of OPG, and OPG acts directly on the nearby osteoclasts. However, in the circulation, OPG level may be regulated by some unknown mechanisms.
References
Akeno N, Czyzyk-Krzeska MF, Gross TS, Clemens TL (2001) Hypoxia induces vascular endothelial growth factor gene transcription in human osteoblast-like cells through the hypoxia-inducible factor-2alpha. Endocrinology 142(2):959–962
Bardos JI, Ashcroft M (2005) Negative and positive regulation of HIF-1: a complex network. Biochim Biophys Acta 1755(2):107–120
Boughner JC, Buchtova M, Fu K, Diewert V, Hallgrimsson B, Richman JM (2007) Embryonic development of Python sebae—I: staging criteria and macroscopic skeletal morphogenesis of the head and limbs. Zoology (Jena) 110(3):212–230
Boyce BF, Xing L, Chen D (2005) Osteoprotegerin, the bone protector, is a surprising target for beta-catenin signaling. Cell Metab 2(6):344–345
Brzoska MM, Rogalska J (2013) Protective effect of zinc supplementation against cadmium-induced oxidative stress and the RANK/RANKL/OPG system imbalance in the bone tissue of rats. Toxicol Appl Pharmacol 272(1):208–220
Chung UI, Kawaguchi H, Takato T, Nakamura K (2004) Distinct osteogenic mechanisms of bones of distinct origins. J Orthop Sci 9(4):410–414
Knowles HJ, Athanasou NA (2009) Canonical and non-canonical pathways of osteoclast formation. Histol Histopathol 24(3):337–346
Liu C, Zhang Y, Kong X, Zhu L, Pang J, Xu Y, Chen W, Zhan H, Lu A, Lin N (2013) Triptolide prevents bone destruction in the collagen-induced arthritis model of rheumatoid arthritis by targeting RANKL/RANK/OPG signal pathway. Evid Based Complement Alternat Med 2013:626038
Majmundar AJ, Wong WJ, Simon MC (2010) Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40(2):294–309
Martinez MD, Schmid GJ, McKenzie JA, Ornitz DM, Silva MJ (2010) Healing of non-displaced fractures produced by fatigue loading of the mouse ulna. Bone 46(6):1604–1612
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399(6733):271–275
Nakahama K (2010) Cellular communications in bone homeostasis and repair. Cell Mol Life Sci 67(23):4001–4009
Pivonka P, Zimak J, Smith DW, Gardiner BS, Dunstan CR, Sims NA, Martin TJ, Mundy GR (2010) Theoretical investigation of the role of the RANK-RANKL-OPG system in bone remodeling. J Theor Biol 262(2):306–316
Ratcliffe PJ (2007) HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest 117(4):862–865
Semenza GL (2001a) Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res 49(5):614–617
Semenza GL (2001b) Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med 7(8):345–350
Semenza GL (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24:97–106
Semenza GL (2010) Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med 2(3):336–361
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89(2):309–319
Tamama K, Kawasaki H, Kerpedjieva SS, Guan J, Ganju RK, Sen CK (2011) Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem 112(3):804–817
Tekkesin MS, Mutlu S, Olgac V (2011) The role of RANK/RANKL/OPG signalling pathways in osteoclastogenesis in odontogenic keratocysts, radicular cysts, and ameloblastomas. Head Neck Pathol 5(3):248–253
Wan C, Shao J, Gilbert SR, Riddle RC, Long F, Johnson RS, Schipani E, Clemens TL (2010) Role of HIF-1alpha in skeletal development. Ann N Y Acad Sci 1192:322–326
Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL (2007) The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117(6):1616–1626
Yang Y (2009) Skeletal morphogenesis during embryonic development. Crit Rev Eukaryot Gene Expr 19(3):197–218
Zhang YG, Yang Z, Zhang H, Wang C, Liu M, Guo X, Xu P (2010) Effect of negative pressure on human bone marrow mesenchymal stem cells in vitro. Connect Tissue Res 51(1):14–21
Acknowledgments
This work was supported by National Natural Science Foundation of China (81371958, 81201367), the Foundation of Science and Technology Commission of Shanghai Municipality (12JC1408200, 13431900702), Shanghai Municipal Natural Science Foundation (10ZR1427700), Key Discipline Construction Project of Pudong Health Bureau of Shanghai (PWZx2014-09), and Academic Leaders Training Program of Pudong Health Bureau of Shanghai (PWRd2012-16).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Editor: T. Okamoto
Jin Shao and Yan Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shao, J., Zhang, Y., Yang, T. et al. HIF-1α disturbs osteoblasts and osteoclasts coupling in bone remodeling by up-regulating OPG expression. In Vitro Cell.Dev.Biol.-Animal 51, 808–814 (2015). https://doi.org/10.1007/s11626-015-9895-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9895-x