Abstract
The objective of the study is to evaluate efficiency of in vitro isolation and myogenic differentiation of mesenchymal stem cells (MSCs) derived from adipose connective tissue (AD-MSCs), bone marrow (BM-MSCs), and skeletal muscle tissue (MC-MSCs). MSCs were isolated from adipose connective tissue, bone marrow, and skeletal muscle tissue of two adult 6-wk-old rats. Cultured MSCs were treated with 5-azacytidine (AZA) to induce myogenic differentiation. Isolated MSCs and differentiated cells were evaluated by immunocytochemistry (ICC), fluorescence-activated cell sorting (FACS), PCR, and RT-PCR. AD-MSCs showed the highest proliferation rate while BM-MSCs had the lowest one. In ICC, isolated MSCs had strong CD90- and CD44-positive expression and negative expression of CD45, CD31, and CD34, while AZA-treated MSCs had strong positive desmin expression. In FACS analysis, AD-MSCs had the highest percentage of CD90- and CD44-positive-expressing cells (99% and 96%) followed by BM-MSCs (97% and 94%) and MC-MSCs (92% and 91%).At 1 wk after incubation with AZA treatment, the peak of myogenin expression reached 93% in differentiated MC-MSCs, 83.3% in BM-MSCs, and 77% in AD-MSCs. MSCs isolated from adipose connective tissue, bone marrow, and skeletal muscle tissue have the same morphology and phenotype, but AD-MSCs were the most easily accessible and had the highest rate of growth on cultivation and the highest percentage of stem cell marker expression. Moreover, although MC-MSCs showed the highest rate of myogenic differentiation potential and expression of myoblast markers, AD-MSCs and BM-MSCs still can be valuable alternatives. The differentiated myoblastic cells could be an available new choice for myoblastic auto-transplantation in regeneration medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A great attention is directed toward the stem cells and its role in regenerative medicine and tissue engineering in the last decade. Although embryonic stem (ES) cells have been considered as candidates for transplantation therapy (Beauchamp et al. 2000), clinical application of these cells is currently making slow progress in views of ethical issues, the potential risks of carcinogenesis (Curzer 2004), as well as problems relating to histocompatibility (Dreyfus et al. 2004).
However, the postnatal adult stem cells, which were termed mesenchymal stem cells (MSCs), are immune compatible in nature, and there are no ethical issues related to their use (Pittenger et al. 1999). These MSCs are plastic-adherent cells and have the ability to proliferate and differentiate into many cell linages (Meirelles Lda and Nardi 2009). The MSCs can be collected by minimally invasive procedures and found in abundant quantities (Gimble 2003), can be safely and effectively transplanted to either an autologous or allogeneic host, and can be manufactured in accordance with current Good Manufacturing Practice guidelines (Gimble et al. 2007).
MSCs could be derived from bone marrow (Friedenstein et al. 1968) adipose tissue (Zuk et al. 2002), muscle (Asakura et al. 2001), synovium (De Bari et al. 2001b), periosteum (De Bari et al. 2001a), or other tissues. Recently, they attracted researcher’s attention as clinically applicable sources of auto-grafting cells because these cells are a heterogeneous population of stem/progenitor cells with pluripotent capacity to differentiate into mesodermal and non-mesodermal cell lineages, including osteocytes (Caplan 1991), adipocytes (Zuk et al. 2001), chondrocytes (Pittenger et al. 1999), myocytes (Wakitani et al. 1995), and neurons(Gage 2000).
Liu 2009 reported several characteristics of MSCs that making these cells very interesting targets for potential therapeutic use in regenerative medicine and tissue engineering, such as the potential to differentiate into multiple lineages and the ability to be expanded in vitro while retaining their original lineage differentiation commitment (Liu et al. 2009).
Skeletal muscle and striated sphincteric disorders caused by trauma or surgery that result in a loss of muscle tissue can be treated by tissue engineering techniques, which is a promising method to treat tissue defects (Mollmann et al. 2011). For skeletal muscle cell transplantation, satellite cells are the most commonly used cell source because of their muscular origin and ability to restore functional muscle tissue (Boonen and Post 2008). However, because of their lack of differentiation in higher passages, MSCs have some clear advantages for their use in skeletal muscle tissue engineering (Carlson and Conboy 2007).
Multinucleated myotubes can be generated by MSCs differentiation towards the myogenic lineage. Their myogenic potential was underlined by their ability to differentiate into the myogenic lineage and their expression of muscle-specific markers. Moreover, based on the immunomodulatory properties, they could provide a most promising cell source for skeletal muscle tissue replacement. Induction of myogenic differentiation in MSCs is now considered to be highly promising for skeletal muscle tissue engineering and reconstruction of fully functional skeletal muscle tissue (Beier et al. 2011).
In this study, we aimed to evaluate efficiency of isolation of MSCs from adipose connective tissue, bone marrow, and skeletal muscle tissue and also to differentiate these stem cells into skeletal myocytes and compare the efficiency of these differentiated cells in vitro.
Materials and Methods
Animals.
Two adult six-wk-old male Sprague–Dawley (SD) rats were used in this study. All procedures involving the rats were permitted by the Institutional Animal Care and Use Committee and performed according to the ethical principles, the Guidelines, laws and regulations of Animal Experimentation at Kobe University (Kobe 2011).
Isolation and culture of MSCs.
From the adipose tissue, rat adipose tissue from the subcutaneous inguinal and abdominal region was used. The adipose tissue was cut into fine pieces. These pieces were digested with 0.2% collagenase (Sigma Chemical, St Louis, MO) at 37°C for 30 min in a shaking water bath, and the resultant cell suspension was filtered through 100, 70, and 40 μm pore-diameter nylon mesh to remove tissue debris. Then, collagenase was removed by dilution with phosphate-buffered saline solution (PBS) and centrifuged twice for 5 min at 1,500×g each time. In this way, most of the matured adipocytes remained in the supernatant. The cell pellet was suspended in (0.83% NH4Cl) lyses buffer and incubated for 10 min at 4°C to remove the contaminating red blood cells. Cells were washed and centrifuged twice with PBS.
From muscle tissue.
Muscle-derived stem cells were harvested from the gastrocnemius or semimembranosus muscle that was cut into fine pieces. These pieces were enzymatically dissociated by adding 0.2% collagenase for 1 h at 37°C in a shaking water bath. The mixture was centrifuged at 1,500×g for 5 min and the supernatant discarded. The cell pellet was suspended in (0.83% NH4Cl) lyses buffer and incubated for 10 min at 4°C to remove contaminating red blood cells. Then, cells were washed with PBS and filtered through 100, 70, and 40 μm pore-diameter nylon mesh to allow separation of the MC-MSCs from the muscle debris.
From bone marrow.
Bone marrow was obtained from femurs and tibias through flushing method using a 1-ml injector with basal medium and was treated with (0.83% NH4Cl) lyses buffer and incubated for 10 min at 4°C to remove contaminating red blood cells. Cells were washed and centrifuged twice.
Culture and expansion of MSCs.
The isolated cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma Chemical) supplemented with10% fetal bovine serum, 2 ng/ml basic fibroblastic growth factor, and 1% penicillin/streptomycin in 100 mm culture dishes at 37°C in a 5% CO2 humidified incubator. After 24 h, the non-adherent cells were removed and adherent MSCs cells were cultured.
Cell proliferation assay.
The cells after the primary cultivation were seeded at a density of 500 per well in 96-well tissue culture plates. The cell number was assessed at 2, 4, 6, and 8 d after the seeding. The Alamar Blue (BioSource International Inc., Camarillo, CA) assay was performed with a fluorimetric method according to the previously described procedure (Ahmed et al. 1994). The emitted fluorescence was compared with a standard curve from a known number of cells to determine the cells number.
Myogenic differentiation.
For myogenic differentiation, MSCs from passage 2 (p2) were seeded in six-well plates at 50,000 cells per well. Twenty-four hours after seeding, the cells were washed with PBS twice. Then, the cells were treated with 5-azacytidine (Sigma Chemical) 10 mmol/l in DMEM with 10% FBS and 5% horse serum followed by incubation for 24 h (Drost et al. 2009). After the incubation period, the cells were washed and replaced with fresh DMEM medium and incubated in a CO2 incubator. Other group of cells was taken as control without adding the drug. The cells were observed daily, and the medium was changed once every 3 d until the experiment was terminated at 28 d.
These isolated stem cells and AZA-treated cells from all tissues are analyzed by microscopical examination, immunocytochemical staining, and flow cytometry to evaluate the expression of stem cell surface markers and myogenic markers.
Immunocytofluorescence staining.
Immunocytochemical staining of isolated stem cells and AZA-treated cells was performed as previously described (Adhim et al. 2011). Briefly, the cells were seeded in four-well Lab-Tek chamber slides at 37°C in a humidified CO2 incubator until they are 50–70% confluent. Then, the cells were fixed by 4% paraformaldehyde in PBS for 20 min at room temperature, rinsed three times with PBS and incubated in 0.1% Triton X-100 in PBS for 15 min at room temperature. After that, cells were rinsed three times in PBS and blocked by 1% BSA for 30 min at room temperature. The cells were then incubated overnight at 4°C with the following primary antibodies: mouse monoclonal antibodies against CD90, CD44 (HCAM), CD45, and CD34 (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1:200, rabbit polyclonal antibody against CD31 (PECAM-1) (Santa Cruz Biotechnology), diluted 1:200 and rabbit monoclonal antibody against Desmin (Abcam, Cambridge, UK) diluted 1:200, and followed by incubation with Alexa Fluor 647 conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature, away from light. Cells were co-stained with 4′,6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR) at 1:500 dilution in PBS, to visualize the nuclei. Stained cells were mounted with fluorescent mounting medium (Dako, Carpinteria, CA). Skeletal muscle from DS rats served as positive control for all ICC staining, and in each group, an isotope control was performed using mouse-IgG1 (Becton Dickinson, San Jose, CA) instead of the primary antibody. The fluorescent images were analyzed and obtained by a confocal laser scanning microscope (LSM 700; Carl Zeiss Meditec, Göttingen, Germany).
Flow cytometry.
Cultured isolated stem cells and AZA-treated cells were analyzed using FACS (cell analyzer) (FACScan, Becton Dickinson) (Yamamoto et al. 2007). In brief, the cells were harvested and incubated for 30 min on ice with 1% BSA containing the primary antibodies directed against the following cell surface markers: CD90, CD45, CD44, CD31, and CD34 (Santa Cruz Biotechnology), and myogenin (mouse monoclonal, Becton Dickinson). After washing, the cells were incubated for another 30 min away from light on ice with Alexa Fluor 647 (Santa Cruz Biotechnology) as a secondary antibody. Then, the cells were washed twice before the analysis.
Reverse transcription polymerase chain reaction.
Total RNA was extracted from cultured cells (treated and control groups) using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The extracted messenger RNA (mRNA) was reverse transcribed using the Taq-Man Reverse Transcription Reagents kit (Applied Biosystems, Foster City, CA). The first-strand complementary DNA (cDNA) was amplified using Taq-Man PCR kit (Applied Biosystems). The amplified (cDNA) were separated on 1% agarose gel and the bands were visualized by ethidium bromide and photographed. Real-time quantitative PCR using the SYBR Green I dye fluoresces system (Applied Biosystems) was performed for the relative quantification of the mRNA expression according to a previously described method (Drost et al. 2009). The sequences for the primers were as follows: CD44 primer forward 5′-CAGACCTGCCCAATGCCTTTGATGGACC -3′, reverse 5′-CAAAGCCAAGGCCAAGAGGGATGCC -3′, Desmin primer forward 5′- CTTCAGGAACAGGTC -3′, reverse 5′-ATCTCGCAGGTGTAGGAC-3′ 245 bp, and β-actin primer as a positive control, forward 5′-GGACTTCGAGCAAGAGATGG-3′, reverse 5′-AGCACTGTGTTGGCGTACAG-3′, 233 bp.
Statistical analysis.
Statistical significant differences between two groups were determined using Student’s t test and analysis of variance for multiple group comparisons. All data are presented as ±SD is an index of the variability of the original data points and reported as ±SEM if the study were repeated of three independent experiments. Statistical significance was set at P < 0.05.
Results
Microscopic cell morphology.
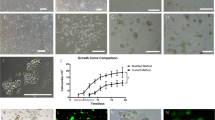
MSCs cultures derived from adipose connective tissue, bone marrow, and skeletal muscle tissue were observed with phase-contrast microscopy. After being cultured, the isolated cells were heterogeneous in shape, and many cells are seen floating on the surface. After 24 h, few numbers of cells are adherent to the culture plate and appeared in separated colonies. The adherent cells are reached 80% confluence after 6–7 d. The rate of growth of BM-MSCs is slower than that of Ad-MSCs and MC-MSCs as it reaches confluent at 12th day. Single cells had a fibroblast like shape. The cells were short and fusiform with clear, elliptical nuclei (Fig. 1A–C ). From p2, the cells appeared to be distributed evenly and had uniform morphology. After induction of differentiation, the cells were observed after first, second, and fourth wk. In the first week after induction, the rate of proliferation of the cells was slowed down, and a lot of cells are detached. After the second week, the orientation the treated groups of cells became nearly the same, and the cells became long spindle shaped with double nuclei and arranged parallel to each other (Fig. 1D–F ). The untreated control groups of cells attained different shape and orientation with haphazard arrangement in different directions (Fig. 1G–I ).
MSCs proliferation assay.
Figure 2 demonstrated the in vitro cell proliferation assay of the three rat isolated MSCs lines: AD-MSCs, BM-MScs, and MC-MSCs. AD-MSCs showed the highest proliferation rate, while BM-MSCs had the lowest one. At the eight day of cultivation, there were statistical significant differences between the triplicate average growth rate of the three MSCs lines (AD-MSCs and BM-MSCs, P = 0.0001; AD-MSCs and MC-MSCs, P = 0.008; BM-MSCs and MC-MSCs, P = 0.0006).
Immunocytofluorescence staining.
The isolated MScs were cultured and stained immunocytochemically with anti-CD90, anti-CD44, anti-CD45, anti-CD31, and anti-CD34 antibodies. MSCs from the three different tissue showed strong expression of stem cell surface marker CD90 and CD44 (Fig. 3A–D and D–F, respectively), while they showed obvious negative expression of CD45, CD31, and CD34 (Fig.3G–I , 3J–L , and 3M–O , respectively). After induction of differentiation, the untreated cell lines were negative for desmin antibody (Fig. 6A ).In comparison, the AZA-treated cell lines showed strong expression of desmin protein throughout the period of observation (Fig. 6B–D ).
Immunofluorescence staining for merged DAPI stained nuclei (blue); CD90, Alexa Flour 647 (red); CD44, Alexa Flour 647 (red); CD45, Alexa Flour 647 (red); CD31, Alexa Flour 647 (red); and CD34, Alexa Flour 647 (red) in isolated MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue were evaluated by confocal laser microscopy. In (A–C) and (D–F), MSCs relevant to CD90- and CD44-positive expression were detected respectively. However, in (G–I), (J–L), and (M–O) MSCs relevant to CD45-, CD31-, and CD34-negative expression were detected, respectively.
Flow cytometric expression of MSCs and myogenic markers.
FACS analysis of isolated stem cells demonstrated that the three populations of isolated MSCs were negative for CD45, CD31, and CD34 (Fig. 4a , G–I, M–O, and P–R, respectively). In contrast, MSC markers CD90 and CD44 were detected in all of them (Fig. 4a , D–F, and J–L, respectively). AD-MSCs had the highest percentage of CD90- and CD44-positive-expressing cells (99% and 96%) followed by BM-MSCs (97% and 94%), and lastly, MC-MSCs (92% and 91%). By statistical analysis of percentage of CD90-expressing cells, we found a statistical significant differences between AD-MSCs and MC-MSCs (P = 0.009) and also between BM-MSCs and MC-MSCs (P = 0.042), but no significant difference between AD-MSCs and BM-MSCs (Fig. 4b ).
Expression of MSCs surface markers were measured by FACS analysis in MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. (A) Representative plots of IgG-matched isotopic controls (plots; A–C), CD90 (plots; D–F), CD45 (plots; G–I), CD44 (plots; J–L), CD31 (plots; M–O), and CD34 (plots; P–R) expression of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue of p2 (passage 2), respectively. (B) Summarized data of percentage of CD90-expressing MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue of p2, respectively. Data are expressed as relative mean fluorescence intensity ± SEM. All significant differences were marked with *1–*2. *1, P = 0.009; *2, P = 0.042; NS non-significant.
Expression of MSCs surface marker CD44 in MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. (a) Representative PCR analysis of CD44 and β-actin mRNA expression in MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue (lanes 1–3, respectively). (b) Summarized data of relative mRNA levels of CD44 in MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. AD-MSCs expressed the highest CD44 mRNA among all cell lines. Each point represents triplicate averages with ±SEM bars. β-actin was used as the endogenous RNA control to normalize for differences in the amount of total RNA. All significant differences were marked with *1–*2. *1, P = 0.008; *2, P = 0.021; NS non-significant.
Immunofluorescence staining for merged DAPI-stained nuclei (blue) and desmin–Alexa Flour 647 (red) were evaluated by confocal laser microscopy. In (A), negative expression of desmin in the cytoplasm of control untreated MSCs, while in (B–D), significant positive expression of desmin in the cytoplasm of treated MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue, respectively, were detected 4 wk after induction of differentiation.
Intracellular skeletal muscle marker (myogenin) was observed throughout the culture period by FACS analysis. During the whole culture duration, the AZA-treated MSCs of all the three tissues had more strong myogenin expression than the untreated MSCs, and this is more remarkable after the first week when the peak of myogenin expression was detected. After the first week, we found that the expression of myogenin was gradually decreased in all cell lines (Fig. 7a ). The peak of myogenin expression reached 93% in differentiated MC-MSCs, 83.3% in BM-MSCs, and 77% in AD-MSCs. By statistical analysis of percentage of myogenin-expressing cells, significance differences were found between AZA-treated and untreated groups and between the three populations except between treated BM-MSCs and treated AD-MSCs that showing no significant difference (Fig. 7b ).
Expression of striated muscle marker myogenin was measured by FACS analysis in untreated and 5-azacytidine-treated MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue after induction of myogenic differentiation. (A) Representative plots of IgG-matched isotopic controls expression (plots; A–C), myogenin expression 1 wk after induction (in untreated control and 5-azacytidine treated MSCs; plots; D–F) and myogenin expression 2 wk after induction (in 5-azacytidine treated MSCs; plots; G–I). (B) Summarized data of percentage of myogenin expression 1 wk after induction in untreated and 5-azacytidine-treated MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue, respectively. Data are expressed as relative mean fluorescence intensity ± SEM. All significant differences were marked with *1–*5. *1, P = 0.012; *2 P = 0.049; *3, P = 0.0001; *4, P = 0.0001; *5, P = 0.0001; NS non-significant.
Reverse transcription polymerase chain reaction analyses.
For confirmation of our results, PCR analysis showed that CD44 is expressed in all isolated MSCs from the three different tissues (Fig. 5a ). In addition, by RT-PCR analysis, the relative expression of CD44 mRNA in all isolated MSCs from the three different tissues was evaluated with AD-MSCs having the highest expression level. Furthermore, relative expression of CD44 mRNA showed significant differences between AD-MSCs and MC-MSCs (P = 0.008) and also between BM-MSCs and MC-MSCs (P = 0.021), but not between AD-MSCs and BM-MSCs, which showed no significant difference (Fig. 5b ).
With regards myogenic-differentiated cells, we found that the striated muscle marker, desmin, is highly expressed throughout observation period by PCR analysis; however, its expression in AZA-treated cells was higher than that in untreated cells (Fig. 8a ). Furthermore, by RT-PCR analysis, the relative expression of desmin mRNA in untreated and AZA-treated MSCs after induction of myogenic differentiation was evaluated with its peak was discovered at the 28th day, whereas after this time, its expression decreased. AZA-treated MC-MSCs had the highest relative desmin mRNA expression. Moreover, relative expression of desmin mRNA characteristically showed significant differences between AZA-treated and untreated groups and between the AZA-treated MSCs of three populations except between AZA-treated AD-MSCs and AZA-treated BM-MSCs that showed no significant difference (Fig. 8b ).
Expression of striated muscle marker desmin in untreated and 5-azacytidine-treated MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue 4 wk after induction of myogenic differentiation. (a) Representative PCR analysis of desmin and β-actin mRNA expression in untreated (lanes 1–3) and 5-azacytidine-treated (lanes 4–6) MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue, respectively. (b) Summarized data of relative mRNA levels of desmin in untreated and 5-azacytidine-treated MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue 4 wk after induction of myogenic differentiation. Treated MSCs derived from skeletal muscle tissue expressed the highest desmin mRNA among all cell lines. Each point represents triplicate averages with ±SEM bars. β-Actin was used as the endogenous RNA control to normalize for differences in the amount of total RNA. All significant differences were marked with *1–*5. *1, P = 0.006; *2, P = 0.031; *3, P = 0.001; *4, P = 0.003; *5, P = 0.0006; NS non-significant.
Discussion
MSCs are being evaluated as therapeutic agents in animal models of paralysis, stroke, heart attack, and neurodegenerative disease as well as in human clinical trials for osteogenesis imperfecta (Phinney 2002). MSCs were first described in 1968 by Friedenstein et al. who discovered that MSCs adhered to tissue culture plates, resembled fibroblasts in morphology, and grew in the form of colonies, and these characteristics have been identified in MSCs from numerous species including human, rats, mice, rabbits, and monkeys (Friedenstein et al. 1968).
According to the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy, the criteria to define MSCs include the expression of CD90, CD105, CD44, and CD73 and lack of expression of CD45, CD34, CD31, CD14 or CD11b, CD79a, and CD19 in addition to the ability to proliferate as plastic adherent cells in standard culture conditions and to differentiate into multiple cell lineages in vitro (Dominici et al. 2006).
In this study, we were able to isolate AD-MSCs from the rat adipose tissue of the subcutaneous inguinal and abdominal region (Zuk et al. 2002) and MC-MSCs from gastrocnemius muscle using the enzyme digestion method, and we isolated BM-SCs from bone marrow of tibias and femurs by using flushing method (Yoshimura et al. 2007; Drost et al. 2009). The MSCs from these three sources are cultured and purified by their adherent ability. In addition, the cells grow in colonies, have uniform morphology, and being highly proliferative from the proliferation assay data; however, we noticed that bone marrow had slower rate of growth and reached the 80% confluence at day 12. However, other two populations reached it at day 7. Our finding is in agreement with Musina et al. 2005 who noticed that BM-MSCs had slower rate of growth than other two populations (Musina et al. 2005; Sakaguchi et al. 2005). On the same concept, it was reported that there is a difference between rat and human BM-MSCs. Although in rat cell number per colony is lower in bone marrow than in other mesenchymal tissues, resulting in a lower expansion potential of rat BM-MSCs, but in human, cell number per colony is much greater in bone marrow than in other mesenchymal tissues, and consequently, BM-MSCs expansion potential is much greater (Yoshimura et al. 2007).
As regards to FACS results, CD90 and CD44 were expressed in each population of the cells, but CD45, CD31, and CD34 were not expressed, and this confirms that these are stem cells according to the International criteria reported (Dominici et al. 2006). Furthermore, AD-MSCs showed the highest percentage of CD90- and CD44-expressing cells. Furthermore, there was statistical significant difference between AD-MSCs and MC-MSCs but no significant difference between AD-MSCs and BM-MSCs. From our documented results, we concluded that AD-MSCs and BM-MSCs have a similar expression pattern of surface markers with no statistical significance difference between them by FACS analysis for CD90. This similarity was previously reported (Lee et al. 2004; Sakaguchi et al. 2005). The immunostaining results also confirm the results of FACS. These results were further supported by gene expression of MSCs surface marker CD44 by PCR and RT-PCR analysis.
After we add the AZA for 24 h in the experimental group, we analyzed the expression of myoblast markers myogenin and desmin at days 7 and 14 and day 28 from induction of differentiation. According to the FACS analysis, PCR and RT-PCR and immunostaining results showed that the myoblast markers are expressed in the AZA-treated experimental group in higher rate than the untreated control group.
FACS results showed expression peak of skeletal muscle marker myogenin at the end of first week and reached 93% in differentiated MC-MSCs, 83.3% in BM-MSCs, and 77%in AD-MSCs. By statistical analysis, significance differences were found between treated and untreated groups and between the three populations except between treated AD-MSCs and treated BM-MSCs that showed no significant difference. This result is in accordance with the study of Lee et al., who demonstrated that AD-MSCs and BM-MSCs have the same in vitro differentiation ability (Lee et al. 2004).
In addition, we found that the expression of myogenin decreased after the first week in all cell lines. These data are agreed with the previous data, which referred that myogenin is expressed in early period of myogenic differentiation of BM-MSCs and its peak is by the end of the first wk (Gang et al. 2004). In our study, we could prove the same finding in the other two populations that was not reported before.
Our results were further supported by gene expression by PCR and RT-PCR analysis. We found that expression of striated muscle marker desmin was noticed all over the period of observation, but the peak was at the 28th d. Furthermore, quantitative relative expression of desmin by real-time-PCR characteristically showed significant difference between AZA-treated and untreated groups and between the three populations except between treated BM-MSCs and treated AD-MSCs that showed no significant difference, which confirms the FACS analysis results.
From our results, it is obvious that MC-MSCs showed the highest rate of myogenic differentiation potential and expression of myoblastic markers, while the other two populations had nearly similar rate. This can be explained by the fact that the adult skeletal muscle has the ability for generating new fibers depending on a reserve population of cells called satellite cells, which lie underneath the basal lamina. In response to environmental changes, such as injury, satellite cells become activated and proliferated, giving rises to myoblasts, which undergo terminal differentiation and ultimately fuse to each other to form new myotubes or become incorporated into pre-existing muscle fibers. Satellite cells represent one choice of cell-transplantation approaches for degenerative muscle diseases aimed at rescuing muscle damage by delivery of cells that can differentiate into skeletal muscle (Cossu and Mavilio 2000). In accordance to this, our results showed that MC-MSCs had higher rate of myogenic differentiation potential than the other two populations, which confirm the results of previous study(Yoshimura et al. 2007).
Although MC-MSCs give higher percentage in myogenic differentiation, BM-MSCs and AD-MSCs still can be valuable alternatives. This is augmented by the previous study, which stated that muscle satellite cells lack a sufficient differentiation capacity after several cycles of cell expansion, rendering it difficult to generate a sufficient number of well-differentiated cells to create large constructs of skeletal muscle tissue, as needed for a possible clinical application (Carlson and Conboy 2007). These two populations had nearly similar myogenic differentiation rate and showed good percentage of expression of myoblast markers. In addition, from our isolation results, we showed that AD-MSCs had high rate of growth on cultivation and had the highest percentage of CD90- and CD44-expressing cells. Furthermore, AD-MSCs can be easily isolated from subcutaneous tissue because it is abundant and readily accessible with liposuction, lipoplasty, and lipectomy surgeries (Mizuno 2009; Hass et al. 2011). AD-MSCs are multipotent and hold promise for a range of therapeutic applications (Rangwala and Lazar 2000). Although BM-MSCs were well-known type in regeneration medicine, its isolation procedure is invasive, painful, and associated with the risk of infection (Hass et al. 2011).
We would like to emphasize the limitations of our study. Only two adult rats were used in this study, but we could attain our results, and by this, we followed the ethical principles on animal experimentation according to laws and regulations in our institution (Kobe 2011). In addition, further in vitro and in vivo studies would be potentially important to evaluate physiological characteristics of differentiated myogenic cells and to experimentally examine their ability for autotransplantation and repair of striated muscle disorders. In addition, although this study focused comparing efficiency of isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue, which were previously studied (Lee et al. 2004; Musina et al. 2006; Yoshimura et al. 2007; Drost et al. 2009; Fu et al. 2010; Wu et al. 2010; Hass et al. 2011), but based on the data of this study, we could report our results and conclusions depending not only on descriptive data but also percentages and valuable statistical results.
In conclusion, the current study, investigated the efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. We could isolate MSCs from these three different tissues, and we succeed to differentiate these cells to myocytes. Our study demonstrated that MSCs isolated from these three tissues have the same morphology and phenotype, but AD-MSCs were the most easily accessible and had the highest rate of growth on cultivation and the highest percentage of surface marker expression. Moreover, although MC-MSCs showed the highest rate of myogenic differentiation potential and expression of myoblast markers, AD-MSCs and BM-MSCs still can be a valuable alternative. The differentiated myoblastic cells could be an available new choice for myoblastic auto-transplantation in regeneration medicine for repair of skeletal muscle and striated sphincteric disorders.
References
Adhim Z.; Matsuoka T.; Bito T.; Shigemura K.; Lee K. M.; Kawabata M.; Fujisawa M.; Nibu K.; Shirakawa T. In vitro and in vivo inhibitory effect of three Cox-2 inhibitors and epithelial-to-mesenchymal transition in human bladder cancer cell lines. Br. J. Cancer 105: 393–402; 2011.
Ahmed S. A.; Gogal Jr. R. M.; Walsh J. E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170: 211–224; 1994.
Asakura A.; Komaki M.; Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68: 245–253; 2001.
Beauchamp J. R.; Heslop L.; Yu D. S.; Tajbakhsh S.; Kelly R. G.; Wernig A.; Buckingham M. E.; Partridge T. A.; Zammit P. S. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. The Journal of cell biology 151: 1221–1234; 2000.
Beier J. P.; Bitto F. F.; Lange C.; Klumpp D.; Arkudas A.; Bleiziffer O.; Boos A. M.; Horch R. E.; Kneser U. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell biology international 35: 397–406; 2011.
Boonen K. J.; Post M. J. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue engineering 14: 419–431; 2008.
Caplan A. I. Mesenchymal stem cells. J. Orthop. Res. 9: 641–650; 1991.
Carlson M. E.; Conboy I. M. Loss of stem cell regenerative capacity within aged niches. Aging cell 6: 371–382; 2007.
Cossu G.; Mavilio F. Myogenic stem cells for the therapy of primary myopathies: wishful thinking or therapeutic perspective? J. Clin. Invest. 105: 1669–1674; 2000.
Curzer H. The ethics of embryonic stem cell research. The Journal of medicine and philosophy 29: 533–562; 2004.
De Bari C.; Dell’Accio F.; Luyten F. P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis and rheumatism 44: 85–95; 2001a.
De Bari C.; Dell’Accio F.; Tylzanowski P.; Luyten F. P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis and rheumatism 44: 1928–1942; 2001b.
Dominici M.; Le Blanc K.; Mueller I.; Slaper-Cortenbach I.; Marini F.; Krause D.; Deans R.; Keating A.; Prockop D.; Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317; 2006.
Dreyfus P. A.; Chretien F.; Chazaud B.; Kirova Y.; Caramelle P.; Garcia L.; Butler-Browne G.; Gherardi R. K. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am. J. Pathol. 164: 773–779; 2004.
Drost A. C.; Weng S.; Feil G.; Schafer J.; Baumann S.; Kanz L.; Sievert K. D.; Stenzl A.; Mohle R. In vitro myogenic differentiation of human bone marrow-derived mesenchymal stem cells as a potential treatment for urethral sphincter muscle repair. Ann. N. Y. Acad. Sci. 1176: 135–143; 2009.
Friedenstein A. J.; Petrakova K. V.; Kurolesova A. I.; Frolova G. P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6: 230–247; 1968.
Fu Q.; Song X. F.; Liao G. L.; Deng C. L.; Cui L. Myoblasts differentiated from adipose-derived stem cells to treat stress urinary incontinence. Urology 75: 718–723; 2010.
Gage FH (2000) Mammalian neural stem cells. Science 287: 1433–8
Gang E. J.; Jeong J. A.; Hong S. H.; Hwang S. H.; Kim S. W.; Yang I. H.; Ahn C.; Han H.; Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem cells (Dayton, Ohio) 22: 617–624; 2004.
Gimble J. M. Adipose tissue-derived therapeutics. Expert. Opin. Biol. Ther. 3: 705–713; 2003.
Gimble J. M.; Katz A. J.; Bunnell B. A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 100: 1249–1260; 2007.
Hass R.; Kasper C.; Bohm S.; Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9: 12; 2011.
Kobe U (2011) Laws and Regulations of Animal experimentation at Kobe University: http://www.kobe-u.ac.jp/research/animal-experiments/laws-regulations.htm.
Lee R. H.; Kim B.; Choi I.; Kim H.; Choi H. S.; Suh K.; Bae Y. C.; Jung J. S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 14: 311–324; 2004.
Liu Z. J.; Zhuge Y.; Velazquez O. C. Trafficking and differentiation of mesenchymal stem cells. J. Cell. Biochem. 106: 984–991; 2009.
Meirelles Lda S.; Nardi N. B. Methodology, biology and clinical applications of mesenchymal stem cells. Front. Biosci. 14: 4281–4298; 2009.
Mizuno H. Adipose-derived stem cells for tissue repair and regeneration: ten years of research and a literature review. Journal of Nihon Medical School = Nihon Ika Daigaku zasshi 76: 56–66; 2009.
Mollmann H.; Nef H. M.; Voss S.; Troidl C.; Willmer M.; Szardien S.; Rolf A.; Klement M.; Voswinckel R.; Kostin S.; Ghofrani H. A.; Hamm C. W.; Elsasser A. Stem cell-mediated natural tissue engineering. Journal of Cellular and Molecular Medicine 15: 52–62; 2011.
Musina R. A.; Bekchanova E. S.; Sukhikh G. T. Comparison of mesenchymal stem cells obtained from different human tissues. Bulletin of Experimental Biology and Medicine 139: 504–509; 2005.
Musina R. A.; Bekchanova E. S.; Belyavskii A. V.; Sukhikh G. T. Differentiation potential of mesenchymal stem cells of different origin. Bulletin of Experimental Biology and Medicine 141: 147–151; 2006.
Phinney D. G. Building a consensus regarding the nature and origin of mesenchymal stem cells. J Cell Biochem Suppl 38: 7–12; 2002.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–7
Rangwala S. M.; Lazar M. A. Transcriptional control of adipogenesis. Annu. Rev. Nutr. 20: 535–559; 2000.
Sakaguchi Y.; Sekiya I.; Yagishita K.; Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis and Rheumatism 52: 2521–2529; 2005.
Wakitani S.; Saito T.; Caplan A. I. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 18: 1417–1426; 1995.
Wu G.; Zheng X.; Jiang Z.; Wang J.; Song Y. Induced differentiation of adipose-derived stromal cells into myoblasts. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao 30: 285–290; 2010.
Yamamoto N.; Akamatsu H.; Hasegawa S.; Yamada T.; Nakata S.; Ohkuma M.; Miyachi E.; Marunouchi T.; Matsunaga K. Isolation of multipotent stem cells from mouse adipose tissue. J. Dermatol. Sci. 48: 43–52; 2007.
Yoshimura H.; Muneta T.; Nimura A.; Yokoyama A.; Koga H.; Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and tissue research 327: 449–462; 2007.
Zuk P. A.; Zhu M.; Ashjian P.; De Ugarte D. A.; Huang J. I.; Mizuno H.; Alfonso Z. C.; Fraser J. K.; Benhaim P.; Hedrick M. H. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell 13: 4279–4295; 2002.
Zuk P. A.; Zhu M.; Mizuno H.; Huang J.; Futrell J. W.; Katz A. J.; Benhaim P.; Lorenz H. P.; Hedrick M. H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering 7: 211–228; 2001.
Grant support
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Meligy, F.Y., Shigemura, K., Behnsawy, H.M. et al. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. In Vitro Cell.Dev.Biol.-Animal 48, 203–215 (2012). https://doi.org/10.1007/s11626-012-9488-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-012-9488-x