Abstract
Hepatocyte growth factor (HGF) can induce proliferation and migration of intestinal epithelial cells and has also been shown to be important in wound healing of inflamed mucosal tissues. HGF is known to be expressed along with interleukin-1 (IL-1) by inflamed mucosal tissues, yet the effect of HGF on IL-1-induced proinflammatory cytokine responses by colonic epithelial cells is unknown. In this report, we have examined the effect of HGF on IL-1-induced secretion of IL-8 by the Caco-2 colonic epithelial cell line. HGF stimulation alone had no effect on the secretion of IL-8 by the Caco-2 cells. However, culture of the cells with HGF and suboptimal levels of IL-1 resulted in a significant enhancement of IL-8 secretion compared to cells cultured with IL-1 alone. A similar effect was seen with HGF and IL-1 simulation of monocyte chemoattractant protein-1 secretion by the rat IEC-6 intestinal epithelial cell line. The enhancing effect of HGF was seen regardless of whether the culture medium contained serum or not. Simultaneous stimulation with HGF and IL-1 was required for the enhancing effect as cells pretreated with HGF for 24 h and then stimulated with IL-1 alone secreted IL-8 levels similar to that of cells stimulated with IL-1 alone. These results suggest that in addition to wound healing, HGF may play a role in the IL-1-induced chemokine response of epithelial cells in inflamed mucosal tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocyte growth factor (HGF; also known as Scatter Factor) is known to induce proliferation and migration of a variety of normal and cancer cell types (Jiang et al. 2005). HGF is produced by fibroblasts stromal cells and fat-storing cells and may be stored in the extracellular matrix (Jiang et al. 2005). Produced as an inactive pro-HGF form, HGF can be activated by HGF Activator (Jiang et al. 2005). In addition to a role in epithelial cell proliferation, migration, and wound healing, Crestani et al. 2002 have shown that neutrophils can produce significant levels of HGF providing a link between this cytokine and the inflammatory response.
In the intestine, HGF appears to be produced by fibroblasts, but not by the intestinal epithelial cells (Goke et al. 1998; Matsubara et al. 1998). However, epithelial cells can produce the HGF activator necessary to activate pro-HGF (Matsubara et al. 1998). HGF appears to have an important role in wound healing of epithelial monolayers (Dignass et al. 1994), and several reports have linked HGF to repair of the mucosa during inflammatory disease. Kitamura et al. (2000) have shown that the expression of HGF and its receptor, c-Met, are increased in ulcerative colitis tissue and the expression of HGF mRNA is increased in mucosal tissues from rats with dextran-sulfate-induced colitis (Ortega-Cava et al. 2002). Furthermore, recent studies in rats with two different forms of experimentally induced colitis have shown that administration of HGF can limit inflammation and induce a repair of the mucosa caused by the colitis (Tahara et al. 2003; Numata et al. 2005).
Although the role of HGF in wound healing is pivotal, little is known of the effect of HGF on the inflammatory response. HGF mRNA expression occurs at the same time as IL-1 mRNA expression during experimental colitis (Ortega-Cava et al. 2002) and IL-1 is known to be a major proinflammatory cytokine in intestinal inflammatory diseases (Ludwiczek et al. 2004). Furthermore, one report has suggested that HGF can induce rat hepatocytes to produce rat cytokine-induced neutrophil chemoattractant (CINC; Kaibori et al. 2004), a chemokine that is a member of the interleukin-8 (IL-8) family. These chemokines can recruit inflammatory cells, such as neutrophils for IL-8, into the area of infection, thereby initiating or enhancing inflammation. Therefore, we have investigated the effect of HGF on IL-1-induced IL-8 secretion by the colonic cell line Caco-2. The Caco-2 cell line was chosen for majority of the experiments in this study, as this cell line is known to produce an array of cytokines similar to that of normal colonic epithelial cells, and IL-1 can stimulate these cells to produce significant levels of IL-8 (Jung et al. 1995; Reinecker et al. 1995; Vitkus et al. 1998). Our studies indicate that HGF alone had no effect on IL-8 secretion by Caco-2 cells, but HGF could significantly enhance IL-1-induced IL-8 secretion by these cells. A similar effect was seen with HGF and IL-1 stimulated secretion of the chemokine monocyte chemoattractant protein-1 (MCP-1) by rat IEC-6 cells. Furthermore, the effect of HGF on the Caco-2 cells required the presence of HGF at the time of IL-1 stimulation. These results suggest that HGF may be an important regulator of proinflammatory cytokine responses by epithelial cells in mucosal inflammatory responses.
Materials and Methods
Cell culture. The Caco-2 human colonic adenocarcinoma cell line (ATCC HTB-37; Manassas, VA) was cultured in Rapid Prototyping and Manufacturing Institute (RPMI)-1640 (Cellgro, Washington, DC) containing l-glutamine, supplemented with 10% Fetal Bovine Serum (FBS; Hyclone, Logan, UT), 2 g/L sodium bicarbonate (J.T. Baker, Phillipsburg, NJ), 25 U/ml penicillin (Cellgro), 25 μg/ml streptomycin (Cellgro), 2 mM l-glutamine (Cellgro), and 10 mM nonessential amino acids (Sigma, St. Louis, MO). This medium was referred to as 10% FBS-RPMI. The rat IEC-6 intestinal epithelial cell line (ATCC CRL 1592) was cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone) with 5% FBS, penicillin, streptomycin, l-glutamine, and 0.1 μg/ml insulin (Sigma; 5% FBS-DMEM). The Caco-2 and IEC-6 cells were maintained in 75-cm2 tissue culture flasks at 37°C in a 90% air–10% CO2 humid environment.
The cells were removed by brief trypsin-ethylenediaminetetraacetic acid (EDTA; Sigma) treatment and were cultured for 24 h in 24-well tissue culture plates at 5 × 104 cells/well. The supernatant was then removed and replaced with 10% FBS-RPMI (or 5% FBS-DMEM for the IEC-6 cells) containing recombinant human (rh) HGF (50, 100, or 200 ng/ml) (Sigma or Invitrogen/Biosource, Carlsbad, CA) alone or in combination with rhIL-1β (R&D Systems, Minneapolis, MN) at 0.5 ng/ml. Culture supernatants were harvested 24 h posttreatment and stored at −80°C. In some experiments, the Caco-2 cells were cultured in serum-free RPMI containing insulin, transferrin, and selenium (ITS-RPMI; BD Biosciences, Bedford, MA).
Determination of secreted IL-8 or MCP-1 levels in the culture supernatants. The IL-8 content in the Caco-2 cell culture supernatants was determined using the DuoSet enzyme-linked immunosorbent assay (ELISA) development systems for human IL-8 (R&D Systems). The MCP-1 levels in the IEC-6 cell culture supernatants was determined using the Rat MCP-1 ELISA Kit from Invitrogen/Biosource. The absorbances of the samples were measured using a Bio-Tek ELX 808 microplate reader (Winooski, VT).
Statistics. The results from triplicate cultures in each experiment were analyzed for significant differences by ANOVA and Fisher’s protected least significant difference test.
Results
HGF has been shown to induce epithelial cells, including Caco-2 cells, to proliferate (Dignass et al. 1994; Goke et al. 1998). However, the time frame of for cell proliferation is much longer than that of cytokine secretion in response to IL-1 stimulation, which can often occur within 8 to 24 h. Therefore, we first determined the effect of HGF on the proliferation of Caco-2 cells after 24 h of stimulation with or without IL-1. As shown in Fig. 1, the total cell numbers per well were unchanged at 24 h regardless of the presence of HGF or IL-1. Although, the cell numbers may change after 24 h, the results in Fig. 1 indicated that we could continue our experiments to determine the effect of HGF and IL-1 on Caco-2 IL-8 secretion in 24-h cultures without having to consider the proliferative effect of HGF on these cells.
Culture of the Caco-2 cells with HGF for 24 h does not affect total cell numbers. Caco-2 cells were cultured at 5 × 104 cells/well in 10% FBS-RPMI for 24 h. The culture supernatant was then removed and replaced with ITS-RPMI alone or with 100 ng/ml HGF, 1 ng/ml IL-1β, or both. After 24 h, the cells were removed by trypsin and EDTA treatment and the cells were counted using a hemacytometer. Shown are the means ± SD from triplicate cultures of a representative experiment from four separate experiments.
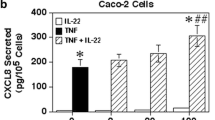
Next, Caco-2 cells were cultured with a concentration of IL-1β (0.5 ng/ml) previously shown to be suboptimal for IL-1 effects on cytokine secretion (Vitkus et al. 1998). Culture of the Caco-2 cells with levels of HGF capable of inducing the cells to proliferate (50 ng/ml HGF or greater; Goke et al. 1998) resulted in no significant effect of HGF on IL-8 secretion by the Caco-2 cells (Fig. 2). However, culture of the Caco-2 cells with both IL-1 and HGF at the same time yielded significantly enhanced levels of IL-8 secretion compared to the cells stimulated with IL-1 alone (Fig. 2). Of interest, when optimal levels of IL-1 were used (1 ng/ml), the addition of HGF still resulted in significant, but smaller increases in IL-8 secretion, yet only with 100 and 200 ng/ml of HGF (data not shown). These results suggest that HGF by itself cannot induce IL-8 secretion by epithelial cells, but it can significantly enhance IL-1-stimulated IL-8 secretion with suboptimal levels of IL-1.
HGF significantly enhances IL-1-stimulated IL-8 secretion by Caco-2 cells. Caco-2 cells (5 × 104 cells/well) were cultured for 24 h in 10% FBS-RPMI before adding fresh medium with or without HGF or 0.5 ng/ml IL-1β. After 24 h, the culture supernatants were collected and secreted IL-8 levels were determined by specific ELISA. Shown are the means ± SD from triplicate cultures of a representative experiment from three separate experiments. Asterisk indicates a significant difference from cultures stimulated with IL-1 alone (p < 0.001).
To confirm this enhancing effect of HGF with IL-1 stimulation, we next determined the effect of HGF on IL-1 stimulated monocyte chemoattractant protein-1 (MCP-1) secretion by the rat IEC-6 intestinal epithelial cell line. Because rats do not produce IL-8, MCP-1 was chosen as an alternative chemokine, as we have shown that the IEC-6 cells produce MCP-1 in response to IL-1 stimulation (Matagrano et al. 2003). Culture of the IEC-6 cells with HGF alone resulted in no significant effect on MCP-1 secretion; however, culture of the cells with both IL-1 and HGF resulted in a significant increase in MCP-1 secretion compared to that of cells stimulated with IL-1 alone (Fig. 3). This confirms the enhancing effect of HGF on IL-1-simulated chemokine secretion and that HGF alone had no effect on chemokine responses. In addition, culture of the cells with HGF, IL-1, or both had no significant effect on the number of cells per well at the end of the experiments, indicating that cell proliferation was not a factor in the 24 h of stimulation with the cytokines (data not shown).
HGF significantly enhances IL-1 stimulated MCP-1 secretion by rat IEC-6 cells. IEC-6 cells at 5 × 104 cells/well were cultured for 24 h before adding fresh medium with or without HGF at 100 ng/ml or IL-1β at 0.5 ng/ml. The culture supernatants were then collected at 24 h and secreted MCP-1 levels were determined by ELISA. Shown are the means ± SD from triplicate cultures of a representative experiment from three separate experiments. Asterisk indicates a significant difference from cultures stimulated with IL-1 alone (p < 0.001).
HGF is normally produced in an inactive pro-HGF form. However, the HGF used in these experiments was recombinant and the preparations included both pro-HGF and active HGF forms (Sigma Product No. H1404 information datasheet). Serum contains several factors such as kallikrein and coagulation factor XIa, which can activate pro-HGF (Jiang et al. 2005). Therefore, we next investigated whether culturing the cells in serum-free medium would alter the effect of HGF on IL-1-stimulated IL-8 secretion. As seen in Fig. 4, culturing the cells in serum-free medium did not significantly alter the enhancing effect of HGF on IL-1-stimulated IL-8 secretion. This suggests that the enhancing effect of HGF may not be linked to the activation of pro-HGF, as active HGF was present in the preparation and providing an environment that could result in activation of pro-HGF (serum-containing medium) had no further effect. Of note, the actual levels of secreted IL-8 seen in Fig. 4 were higher than those seen in Fig. 2 probably because of the experiments being performed at different times. However, the overall effect of HGF and IL-1 were similar in both experiments.
The enhancing effect of HGF on IL-1-stimulated IL-8 secretion is not affected by the presence of fetal bovine serum in the culture medium. Caco-2 cells were cultured at 5 × 104 cells/well in 10% FBS-RPMI for 24 h before adding fresh 10% FBS-RPMI or ITS-RPMI alone or with 100 ng/ml HGF, 0.5 ng/ml IL-1β, or both cytokines. The cells were then cultured for 24 h and the culture supernatants were collected to determine secreted IL-8 levels. Shown are the means ± SD from triplicate cultures of a representative experiment from three separate experiments. Asterisk indicates a significant difference from cultures stimulated with IL-1 alone (p < 0.01).
Finally, it is possible that the effect of HGF may be to activate the Caco-2 cells to a state in which they were more sensitive to IL-1 stimulation. This could be either by inducing the expression of IL-1 or other receptors or possibly by inducing the production of a secondary cytokine or factor that enhanced IL-1-induced IL-8 secretion. Therefore, Caco-2 cells were pretreated for 24 h with HGF (100 ng/ml) before washing the cells and then stimulating with IL-1 alone for 24 h. As shown in Fig. 5, pretreatment of the Caco-2 cells with HGF alone followed by stimulation of the cells with IL-1 alone resulted in secreted IL-8 levels, which were not significantly different from cells pretreated with culture medium and then stimulated with IL-1 alone. Furthermore, the cells pretreated with HGF followed by IL-1 stimulation alone yielded secreted IL-8 levels that were significantly lower than those of the cells pretreated with culture medium and then stimulated simultaneously with both HGF and IL-1. In addition, cells that were pretreated with HGF and then left unstimulated yielded IL-8 levels, which were not significantly different from cells pretreated with culture medium and then stimulated with HGF alone. These results suggest that HGF does not activate the Caco-2 cells to a state in which they are more sensitive to IL-1 stimulation. Indeed, it appears that HGF stimulation must occur simultaneously with IL-1 stimulation for the enhancing effect of HGF to be seen.
Pretreatment of the Caco-2 cells with HGF did not enhance IL-1-stimulated IL-8 secretion. Caco-2 cells at 5 × 104 cells/well were cultured for 24 h in 10% FBS-RPMI before adding fresh medium alone or medium containing 100 ng/ml HGF (for Pre-HGF and Pre-HGF + IL-1 cultures). After 24 h, the culture supernatants were removed and fresh medium alone (for unstimulated and Pre-HGF cultures), 100 ng/ml HGF (for HGF cultures), 0.5 ng/ml IL-1β (for IL-1 and Pre-HGF + IL-1 cultures), or both HGF and IL-1 (for HGF + IL-1 cultures) were added. The cells were cultured for 24 h and the culture supernatants were then collected for IL-8 determination. Shown are the means ± SD from triplicate cultures of a representative experiment from three separate experiments. The bars indicate cultures compared with the appropriate p values. Asterisks indicate a significant difference between cultures.
Discussion
The complex interplay of cytokines and cells forms a regulatory network during mucosal inflammatory responses (McGee 1999). Proinflammatory cytokines such as IL-1, IL-8, and MCP-1 induce or enhance the inflammation, whereas other cytokines induce wound healing. HGF has been shown to play a significant role in wound healing in mucosal inflammatory responses (Ortega-Cava et al. 2002; Tahara et al. 2003; Numata et al. 2005). Some cytokines may play a dual role in both inflammation and wound healing, such as transforming growth factor-β, which has antiinflammatory functions, but can also enhance IL-1 stimulated IL-6 (McGee et al. 1993) and monocyte chemoattractant protein-1 secretion (Matagrano et al. 2003) by intestinal epithelial cells. In this report, we have found that in addition to its role in wound healing, HGF can also enhance IL-1-stimulated IL-8 or MCP-1 production by epithelial cell lines, suggesting that HGF may play a role in inflammation as well.
A previous report has shown that HGF alone can induce hepatocytes to produce the chemokine CINC (Kaibori et al. 2004). HGF has also been shown to induce squamous cell carcinoma cell lines to produce significant levels of IL-8 and vascular endothelial cell growth factor (VEGF; Dong et al. 2001; Worden et al. 2005), yet HGF was unable to induce normal keratinocytes to produce either of these factors (Dong et al. 2001). Similar to their findings with normal keratinocytes, we found that HGF alone had no effect on IL-8 or MCP-1 secretion, suggesting that the inability of HGF to induce IL-8 production may be common to epithelial cell types. Others have also shown that IL-1 can induce malignant keratinocytes to produce IL-8 (Wolf et al. 2001). However, our study is the first to show that HGF can significantly enhance IL-1-stimulated IL-8 and MCP-1 secretion by epithelial cell lines. It will be interesting to determine if HGF can enhance IL-1-stimulated IL-8 secretion by other epithelial cell types, including keratinocytes.
Of interest was the finding that the enhancing effect of HGF on IL-1-stimulated IL-8 secretion required simultaneous treatment with both stimuli. This suggests that the HGF effect was not mediated by a second factor induced by HGF, which then enhanced IL-1 effects. It is quite possible that stimulation by HGF resulted in an intracellular signal, which acted in concert with the IL-1 signal to enhance IL-8 mRNA expression or secretion. Recent reports have shown that HGF stimulation results in the activation of the transcription factor NF-κB through the Akt signaling pathway (Muller et al. 2002; Fan et al. 2005). The expression of the IL-8 gene is strongly upregulated by NF-κB activation (Roebuck 1999) and IL-1 is a potent inducer of NF-κB activation (O’Neill and Greene 1998). Perhaps HGF activation of the Akt to NF-κB signaling pathway alone is insufficient to activate IL-8 gene expression but sufficient to enhance IL-1-stimulated activation of NF-κB activation and IL-8 gene expression. Alternatively, HGF may alter the secretion or protein translation of IL-8 independent of IL-1 activation of IL-8 gene transcription. These questions are now being examined in our laboratory.
A role for the HGF enhancement of IL-1-stimulated IL-8 or MCP-1 production remains a mystery at this time. Although HGF and IL-1 are present at the same time in inflammed mucosal tissues (Ortega-Cava et al. 2002), the levels of HGF mRNA expression were shown to increase, whereas IL-1 mRNA expression decreased. Perhaps the reduced levels of IL-1 with HGF may allow the epithelial cells to continue IL-8 production until IL-1 levels are diminished and HGF stimulation alone cannot sustain IL-8 secretion. Indeed, IL-8 may have a potential role in wound healing as an angiogenic factor (like VEGF) to stimulate the regrowth of the capillary bed in damaged intestinal tissues (Danese et al. 2006).
References
Crestani, B.; Dehoux, M.; Hayem, G., et al. Differential role of neutrophils and alveolar macrophages in hepatocyte growth factor production in pulmonary fibrosis. Lab. Invest. 82:1015–22; 2002.
Danese, S.; Sans, M.; de la Motte, C., et al. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 130:2060–2073; 2006.
Dignass, A. U.; Lynch-Devaney, K.; Podolsky, D. K. Hepatocyte growth factor/scatter factor modulates intestinal epithelial cell proliferation and migration. Biochem. Biophys. Res. Comm. 202:701–709; 1994.
Dong, G.; Xhen, Z.; Zhi-Yu, L., et al. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 61:5911–5918; 2001.
Fan, S.; Gao, M.; Meng, Q., et al. Role of NF-κB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 24:1749–1766; 2005.
Goke, M.; Kanai, M.; Podolsky D. K. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am. J. Physiol. 274:G809–G818; 1998.
Jiang, W. G.; Martin, T. A.; Parr, C., et al. Hepatocyte growth factor, its receptor, and their potential value in cancer therapies. Crit. Rev. Oncol. Hematol. 53:35–69; 2005.
Jung, H. C.; Eckmann, L.; Yang, S.-K., et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Invest. 95:55–65; 1995.
Kaibori, M.; Yanagida, H.; Nakanishi, N., et al. Effect of hepatocyte growth factor on induction of cytokine-induced neutrophil chemoattractang in rat hepatocytes. Transplant. Proc. 36:1977–1979; 2004.
Kitamura, S.; Kondo, S.; Shimomura, Y., et al. Expression of hepatocyte growth factor and c-met in ulcerative colitis. Inflamm. Res. 49:320–324; 2000.
Ludwiczek, O.; Vannier, E.; Borggraefe, I., et al. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin. Exp. Immunol. 138:323–329; 2004.
Matagrano, L. B.; Magida, J. A.; McGee, D. W. TGF-β1 enhances the secretion of monocyte chemoattractant protein-1 by the IEC-18 intestinal epithelial cell line. In Vitro Cell. Dev. Biol. 39:183–186; 2003.
Matsubara, Y.; Ichinose, M.; Yahagi, N., et al. Hepatocyte growth factor activator: a possible regulator of morphogenesis during fetal development of the rat gastrointestinal tract. Biochem. Biophys. Res. Comm. 253:477–484; 1998.
McGee, D. W. Inflammation and Mucosal Cytokine Production. In: Ogra, P. L.; Mestecky, J.; Lamm, M. E.; Strober, W.; Bienenstock, J.; McGhee, J. R. eds. Mucosal Immunology. San Diego, CA: Academic Press; 1999:559–573.
McGee, D. W.; Beagley, K. W.; Aicher, W. K.; McGhee, J. R. TGF-β and IL-1β act in synergy to enhance IL-6 secretion by the intestinal epithelial cell line, IEC-6. J. Immunol. 151:970–978; 1993.
Muller, M.; Morotti, A.; Ponzetto, C. Activation of NF-κB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol. Cell. Biol. 22:1060–1072; 2002.
Numata, M.; Ido, A.; Moriuchi, A., et al. Hepatocyte growth factor facilitates the repair of large colonic ulcers in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats. Inflamm. Bowel Dis. 11:551–558; 2005.
O’Neill, L. A. J.; Greene, C. Signal transduction pathways activated by the IL-1 receptor family: ancient signaling machinery in mammals, insects, and plants, J. Leukoc. Biol. 63:650–657; 1998.
Ortega-Cava, C. F.; Ishihara, S.; Kawashima, K., et al. Hepatocyte growth factor expression in dextran sodium sulfate-induced colitis in rats. Dig. Dis. Sci. 47:2275–2285; 2002.
Reinecker, H.-C.; Loh, E. Y.; Ringler, D. J. et al. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease. Gastroenterology. 108:40–50; 1995.
Roebuck, K. A. Regulation of interleukin 8 gene expression. J. Interferon Cytokine Res. 19:429–38; 1999.
Tahara, Y.; Ido, A.; Yamamoto, S., et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. J. Pharmacol. Exp Ther. 307:146–151; 2003.
Vitkus, S. J. D.; Hanifin, S. A.; McGee, D. W. Factors affecting Caco-2 intestinal epithelial cell interleukin-6 secretion. In Vitro Cell. Dev. Biol. 34:660–664; 1998.
Wolf, J. S.; Chen, Z.; Dong, G.; Sunwoo, J. B.; Bancroft, C. C.; Capo, D. E.; Yen, N. T.; Mukaida, N.; Van Waes, C. IL (interleukin)-1α promotes nuclear factor-κB and AP-1 induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin. Cancer Res. 7:1812–1820; 2001.
Worden, B.; Yang, X. P.; Lee, T. L.; Bagain, L.; Yeh, N. T.; Cohen, J. G.; Van Waes, C.; Chen, Z. Hepatocyte growth factor/scatter factor differentially regulates expression of proangiogenic factors through egr-1 in head and neck squamous cell carcinoma. Cancer Res. 65:7071–7080; 2005.
Acknowledgments
This study was supported by US PHS Grant 54049.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: J. Denry Sato
Jillian Grygas, Nicole Steiger, and Carrie L. LeSeur contributed equally to this manuscript.
Editor: J. Denry Sato
Rights and permissions
About this article
Cite this article
Grygas, J., Steiger, N., LeSeur, C.L. et al. Hepatocyte growth factor enhances IL-1β stimulated IL-8 secretion by Caco-2 epithelial cells. In Vitro Cell.Dev.Biol.-Animal 43, 147–152 (2007). https://doi.org/10.1007/s11626-007-9018-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-007-9018-4