Abstract
Background
Recent data illustrates improved outcomes when adhering to early drain removal following pancreatoduodenectomy (PD). This study aims to explore the potential benefits of expanding the timeframe for early drain removal.
Methods
Six hundred forty PDs were originally managed by selective drain placement and early removal. Outcomes were reappraised in the framework of a novel proposal; intraoperative drains were omitted based on a low-risk profile (Fistula Risk Score 0–2), followed by drain removal at PODs 1, 3, and 5 if drain fluid amylase (DFA) fell below specific cutoffs based on optimized negative predictive values (NPV) for clinically relevant postoperative pancreatic fistula (CR-POPF). Characteristics of the remaining cohort with drains in situ on POD5 were examined using multivariable analysis (MVA).
Results
Intraoperative FRS would preclude drains from 230 (35.9%) negligible/low-risk cases with a cohort CR-POPF rate of 1.7%. Of the remaining patients, 30.5% would have drains removed on POD1 based on a DFA threshold of 300 IU/L (NPV = 98.4%), demonstrating a 1.6% CR-POPF rate. On POD3, drains could be removed in the residual cohort from 21.1% of patients with DFA ≤ 150 IU/L (NPV = 96.6%), reflecting a 3.4% CR-POPF rate. On POD5, a DFA threshold of 50 IU/L (NPV = 84%) identified 16.3% more patients whose drains could be removed. The remaining cohort (POD5 DFA > 50 IU/L), “enriched” for fistula development and reflecting just 18.4% of the original patients, displays a 61% CR-POPF rate. Among these patients on POD5, a DFA threshold > 2000 IU/L best predicted subsequent CR-POPF (PPV = 89.5%), and MVA revealed a positive association between pancreatic cancer/pancreatitis (OR = 4.37, p = 0.022) and longer operations (OR = 3.74, p = 0.014) with CR-POPF development.
Conclusion
Early drain removal is a dynamic concept and can be employed throughout the postoperative time course using conditional thresholds to better identify patients at risk for CR-POPF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recent growth of fistula risk prediction methods following pancreatoduodenectomy (PD) has provided an opportunity for the development of fistula mitigation strategies on a risk-stratified basis. One such emerging approach is early removal of intraoperatively placed peritoneal drains, which was first explored by Kawai et al.1 In a non-randomized, sequential cohort series, they described that patients who had drains removed on postoperative day (POD) 4 had lower rates of pancreatic fistula and infectious complications when compared to those who had removal on POD8. Those authors not only provided evidence for the benefits of early removal but also made the first suggestion of what could be considered an “early” removal time point at POD4. Furthermore, after Molinari et al. reported that a POD1 drain fluid amylase (DFA) above 5000 IU/L was a significant predictor of pancreatic fistula,2 the same group from Verona incorporated this factor into a prospective, randomized trial.3 When focusing on the lower-risk cohort with a DFA-1 < 5000 IU/L, drain removal on POD3 demonstrated significant reductions in fistula occurrence, length of stay, and other important perioperative outcomes when compared to drain removal on POD5 or later.3
Several subsequent studies have suggested that the point of removal could be safely moved even earlier. Fong et al. suggested that a drain amylase level less than 600 IU/L on POD1 would identify patients for safe, early removal, given a high negative predictive value (NPV).4 Additionally, the work of McMillan et al. has extended the notion of “early” even further to the time of anastomotic creation in the operation, demonstrating that intraoperative drains could be safely omitted in negligible/low-risk cases5,6 as judged by the Fistula Risk Score (FRS).7 Furthermore, drains that were placed in moderate/high FRS cases (FRS 3–10) could be removed if POD1 amylase was less than 5000 IU/L.6 More recently, investigators at Baylor University have proposed additional value by removing drains using sequential DFA assessments over the early postoperative time frame.8
Despite these continual enhancements of early drain removal, with strong evidence of benefit, surgeons have been slow to adopt the practice. This is in spite of the fact that they profess to respect drain amylase a dominant driver of drain management decision making.9 In an international survey of management practices by surgeons who perform pancreatectomy, 45% reported removing drains based on an early DFA level, while this practice was affirmed by just 32% of North American surgeons.10 In an examination of actual implementation patterns through the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) dataset, Beane et al. found that DFA-1 was only recorded in 21.5% of cases, while drains were removed before POD3 in only 28.8% of patients who had a DFA-1 less than 5000 IU/L, equating to just 8% of all PDs performed in the dataset.11
According to these previous developments, the concept of early drain removal has shown progression and refinement over time, although surgeons appear hesitant to adopt the practice. Through data reappraisal, the aim of this study was to evaluate the potential effectiveness of early drain removal as a dynamic and fluid process, expanding the timeframe of removal from the time of the operation onward through the early postoperative recovery period.
Methods
All consecutive PDs performed from 2014 to 2017 by 13 pancreatic surgical specialists at the University of Pennsylvania and the University of Verona were included in this retrospective study. This study was approved by the IRB at both institutions. PD was generally performed as a pylorus-preserving variant with an end-to-side, double-layered, duct-to-mucosa pancreaticojejunostomy. Dunking/invagination was performed 25% of the time, and pancreaticogastrostomy was rare (4.8%). Prophylactic octreotide was infrequently applied (16.6%, early in the series by one of the institutions; never by the other), as were either internal or external transanastomotic stents (27.1%), given findings by prior studies from the Pancreas Fistula Study Group.12,13,14 Other fistula mitigation strategies such as the Roux limb construction, biologic sealants, and autologous patches were never employed.
During this time period, these patients were managed by a general policy of selective drain placement and early drain removal, extending the series previously described by McMillan et al.6 The FRS was calculated intraoperatively and drains were omitted if the FRS risk zone was negligible (0) or low (1–2), while drains were placed if the risk fell in the moderate to high categories (3–10)7 (Supplemental Table 1). At Penn, a single closed-suction drain is placed in proximity to both anastomoses. At Verona, two Penrose-like drains are placed, one near each anastomosis (PJ and HJ). After assessing DFA-1, drains were removed on POD3 if the day 1 value fell below 5000 IU/L. Additional DFA values were recorded on days 3 and 5 if a drain remained at that point. Approximately 20% of patients did not follow this protocol, at the surgeon’s discretion, most often with inappropriate placement of drains intraoperatively in low/negligible risk patients.6 Pathology, pancreatic duct diameter, pancreatic gland texture, and intraoperative blood loss were all categorized according to the previously established FRS criteria.7
Definition of Outcomes
The primary outcome of interest was clinically relevant postoperative pancreatic fistula (CR-POPF), which was defined as grades B or C according to the most recent ISGPS consensus revision.15 Fistulas were generally managed with prolonged drainage, antibiotics, interventional radiology procedures, and infrequently, reoperation as previously described in detail.16 Other outcomes included the occurrence of any complication or severe complications, which were graded according to the accordion severity grading system and corresponded to Accordion ≥ 1 and Accordion ≥ 3, respectively.17 Fistula severity was assessed by calculating the average complication burden (ACB) of fistula from a modified accordion scale which designated the severity weighting of fistulas based on the type of intervention used18 (Supplemental Table 2). These outcomes, along with initial hospital stay, readmission, reoperation, and death were all recorded within 90 days of the operation. Outcome data was incomplete in 21.7% of cases.
Derivation of Conceptual Framework
The actual outcomes of these patients were then reassessed within the novel, hypothetical framework of this study. The previously calculated FRS was utilized to strictly determine which patient would receive intraoperative drains (FRS 3–10) and those who would not (FRS 0–2). Previous data has suggested that drains could be safely omitted in the negligible/low-risk cohort.5 The negative predictive value (NPV) of various DFA cutoffs for CR-POPF was calculated using actual drain amylase values accrued on POD1, POD3, and POD5. The cutoff that provided the greatest NPV for each individual POD was then selected as the conditional threshold for that day. On POD3, a cutoff of 150 IU/L rather than 100 IU/L was chosen to include a greater proportion of patients under the cutoff in favor of a slightly higher NPV. Patients whose DFA fell below the POD1 cutoff were designated as having drains removed at that point in time, and this same selection was applied if DFA fell below the cutoffs on POD3 and POD5. “Early drain removal” refers to the group of patients who would have drains omitted in the operating room or those who would have drains removed on POD1, POD3, or POD5. The percentage of patients designated for early removal at each time point was based upon the cohort of patients that did not have drains removed immediately prior in the sequence. The group of initial moderate/high-risk FRS patients who progressed through the sequence without DFA values falling below any of the thresholds were designated the “enriched” cohort which would have drains maintained after POD5 given evidence of a significantly greater risk for CR-POPF. The outcomes of the groups that were designated as “early drain removal” were compared in aggregate to those of the “enriched” cohort. The discriminatory power of each cutoff in the sequence for ultimate CR-POPF formation was evaluated. A total of 13.1% of patients were ultimately excluded from the outcomes analysis due to a missing DFA value at any day along the pathway. Predictors of CR-POPF in the “enriched” group of patients were assessed. A DFA cutoff with the highest positive predictive value (PPV) for CR-POPF on POD5 was determined.
Statistical Analysis
Continuous variables are reported as means ± standard deviations or median and interquartile range (IQR) while categorical variables are reported as frequencies. The Mann-Whitney U test and Pearson χ2 test were used to analyze continuous nonparametric variables and categorical variables, respectively. Multivariable logistic regression modeling with backward stepwise elimination (p ≤ 0.05 for entry, p > 0.10 for elimination) was used to evaluate predictors of CR-POPF in the “enriched” cohort and was adjusted for 28 patients and operative factors. The area under the ROC curve was calculated for each cutoff in the sequence and used to estimate strength of prediction. All analyses were conducted using IBM SPSS 24.0 (IBM Corporation, Armonk, NY). A p value of < 0.05 was considered significant, and all tests were two-sided.
Results
Patient Characteristics
In this series of 640 PDs, 59.7% of the patients were male and the mean age was 63.3 ± 11.2 years. An American Society of Anesthesiologists score ≥ 3 was recorded in 31.1% of patients, and 46.7% had an overweight or obese BMI (> 25). The most common indication for PD in the series was pancreatic cancer (58.2%). The mean FRS was 3.6 (± 2.3), and 10.9% of cases fell into the high-risk FRS category (7–10). The overall CR-POPF rate was 15.2% (13.3% grade B, 1.9% grade C) (Table 1). Any complication (Accordion ≥ 1) occurred in 52.7% of patients while severe complications (Accordion ≥ 3) occurred in 15% (Table 1). The median initial hospital length of stay (LOS) was 9 days (IQR 7–15) (Table 1). Reoperations occurred 7.2% of the time, while 90-day readmissions were 7.6% (Table 1). The overall 90-day mortality rate was 2.8% (Table 1).
Proposed Early Removal Pathway
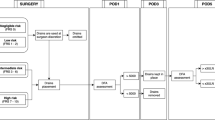
At the time of intraoperative FRS calculation, 35.9% (N = 230) of patients fell in the negligible and low-risk categories (FRS 0–2) and would have drains omitted. The CR-POPF rate in this group was 1.7% (N = 4), giving the FRS threshold an NPV of 98.3% (Fig. 1a). Next, of the remaining patients with drains in situ on POD1, a DFA ≤ 300 IU/L yielded the highest NPV (98.4%) (Table 2). 30.5% (N = 125) of these patients would therefore have drains removed at that time, yielding a 1.6% (N = 2) CR-POPF rate in that cohort (Fig. 1b). On POD3, 21.1% (N = 58) of the remaining patients had DFA ≤ 150 IU/L and would be subjected to drain removal then. The NPV and CR-POPF rate in this group were 96.6% (Table 3) and 3.4% (N = 2), respectively (Fig. 1c). Finally, on POD5, an additional 16.3% (N = 25) of the remaining patients had an optimal DFA ≤ 50 IU/L and would have drains removed then. The CR-POPF rate in this group was 16% (N = 4), and the NPV of the POD5 DFA cutoff was 84% (Table 4). The rate of CR-POPF in the cohort excluded from analysis due to missing DFA values (13.1%) was 15.5%, falling within general norms of CR-POPF rates expressed in the literature.
Proposed early drain removal pathway based on intraoperative determination of the fistula risk score and omission of drains in low-risk cases (a), drain removal on POD1 if DFA ≤ 300 IU/L (b), removal on POD3 in patients with DFA ≤ 150 IU/L (c), removal on POD5 if DFA ≤ 50 IU/L (d). DFA, drain fluid amylase; CR-POPF, clinically relevant fistula; FRS, fistula risk score; POD, postoperative day
The remaining patients whose POD5 DFA was > 50 IU/L, and would have drains left in place, represented the “enriched” cohort. This comprises 18.4% (N = 118) of all patients in the series while producing a 61% (N = 72) CR-POPF frequency (Fig. 1d). Furthermore, these CR-POPFs accounted for 85.7% of all CR-POPFs in the entire series of 640 PDs.
Comparative Outcomes
The outcomes of the “enriched” cohort on POD5 were significantly worse than those who were designated for no placement or early removal. In addition to significantly different CR-POPF rates (61% vs. 2.7%), the “enriched” cohort displayed higher rates of any complication, severe complication, readmission, reoperation, and longer LOS (Table 1). Additionally, grade C fistulas occurred with significantly greater frequency (6.8% vs. 0.5% overall, p < 0.001) in the “enriched” cohort when compared to the early removal group. However, when CR-POPFs did occur, their severity did not differ between the “enriched” cohort and the early removal group (ACB = 0.3411 ± 0.187 vs. 0.325 ± 0.244, respectively; p = 0.171).
Characteristics of the “Enriched” Cohort
When compared to the early removal group, the “enriched” cohort had higher rates of obesity, less neoadjuvant chemotherapy and obstructive jaundice, fewer vascular resections, greater PG reconstruction, softer gland texture, higher rates of pathology other than pancreatic cancer or pancreatitis, and greater use of transanastomotic stents (Table 5). Within this cohort, the median day of drain removal was POD9 (IQR 6–24). Furthermore, those patients who went on to develop CR-POPF had significantly greater median day of drain removal than those who did not have a CR-POPF (22 days [8–34] vs. 6 days [5–7], p < 0.001). A POD5 DFA threshold of 2000 IU/L provided the greatest PPV (89.5%) for CR-POPF at this point in time.
Predictors of Clinically Relevant Fistula
Within the “enriched” cohort, length of operation (> 405 min), pathology (pancreatic cancer/pancreatitis), decreasing pancreatic duct size, and POD5 DFA (> 2000 IU/L) were positively associated with CR-POPF upon univariable analysis (Supplemental Table 3). After multivariable analysis, length of operation > 405 min (OR = 3.74, 95% C.I. = 1.31–10.69, p = 0.014) and pancreatic cancer/pancreatitis pathology (OR = 4.37, 95% C.I. = 1.24–15.40, p = 0.014) were the only significant predictors of CR-POPF. Assessing the discriminatory capability of each cutoff in the sequence for CR-POPF revealed improvements in prediction as time progressed: intraoperative FRS (AUC = 0.688), POD1 DFA (AUC = 0.756), POD3 DFA (AUC = 0.816), and POD5 DFA (AUC = 0.880). The proposed sequence also displayed a greater capacity for segregating CR-POPF away from the early removal group than the current standard of the POD1 DFA 5000 IU/L threshold. Using POD1 DFA > 5000 IU/L alone yielded CR-POPF rates of 48.4% above 5000 and 10.8% below (4.5:1 ratio), whereas the “enriched” cohort displayed a 61% rate while the early removal group was only 2.7% CR-POPF (22.5:1 ratio).
Discussion
By integrating previously established methods of fistula prediction, this study was able to offer a novel and dynamic framework for early drain removal following PD. The proposed management sequence follows this course:
-
1.
Omission of drains intraoperatively for patients with negligible/low risk (FRS 0–2).
-
2.
In patients with intraoperative drain placement, removal on POD1 if DFA is ≤ 300 IU/L.
-
3.
In remaining patients with drains, removal on POD3 if DFA is ≤ 150 IU/L.
-
4.
In remaining patients with drains, removal on POD5 if DFA is ≤ 50 IU/L.
-
5.
In the remaining patients with a DFA-5 > 50 IU/L, drain maintenance and subsequent removal using clinical discretion in this “enriched risk” group.
Utilization of the FRS intraoperatively enabled identification of over one third of the patients in this series for safe drain omission with a low CR-POPF rate. Previous prospective work has already provided evidence that drains can be safely omitted in these cases.6 Furthermore, another study demonstrated that drains are in fact detrimental in negligible and low-risk FRS cases, resulting in higher rates of CR-POPF.5 Therefore, omission of drains in these cases is not only benign, rather it is the optimal action in these instances. The data in this series serves to reinforce both the utility of FRS in determining fistula risk at the outset of anastomotic construction, as well as the virtually insignificant inherent danger of low-risk cases in developing CR-POPF. Furthermore, applying an intraoperative trigger for drain omission predates the removal timeframe and refines the notion of “early” to include the intraoperative state.
The value of using POD1 DFA cutoffs as predictors of CR-POPF has been validated across multiple studies.19 When examining DFA on POD1, as well as POD3 and POD5, this study employs the NPV for CR-POPF when identifying the optimal threshold for early removal. This emphasis aligns with the intended purpose of using amylase thresholds as early removal criteria. Isolating the cutoff with the greatest NPV maximizes the probability that a CR-POPF will not occur below that cutoff, and therefore minimizes the chance of a fistula occurring in the absence of a drain. Fong et al. have also utilized this approach in their recent study, proposing that a POD1 DFA level below 600 IU/L minimizes fistula risk. The threshold in the current study differs from this number; however, the cohort utilized is smaller due to intraoperative exclusion of drains based on FRS, which may require a more rigorous threshold to obtain a high NPV. The threshold obtained by Fong et al. was also derived using all POPFs, including biochemical leaks (ISGPF grade A), whereas the thresholds derived in the current study focused specifically on the development of clinically relevant situations (ISGPF grade B/C).15 This was also the case for the original proposal by the Verona group calling for DFA-1 > 5000 IU/L as an optimal prediction threshold.2,3
Using the combination of both POD1 and POD3 amylase thresholds for early removal has previously been proposed by Villafane-Ferriol et al.8 Although the cutoffs used in this prior study were derived in a different manner, the current study drew upon the same principle they espoused, namely that multiple data points sequentially throughout the postoperative period increase the ability to predict a patient’s trajectory. Along these lines, the current study also includes POD5 data in addition to POD3 to determine if this additional point could enhance identification of patients for safe early drain removal. The progressive improvements in the AUCs over time serve to reinforce this reasoning. Notably, the NPV of the POD5 cutoff is less than the NPVs calculated prior in the sequence of this study. This reduction in certainty may be explained by the possible migration of drains from their originally placed site near the anastomosis. The utility of DFA for clinical judgment is predicated on the belief that these values reflect drains positioning in the immediate vicinity of a leak. Therefore, drain migration may be responsible for a failure to detect CR-POPF in some cases, driven by essentially a “false-negative” drain amylase value. Nevertheless, the rate of CR-POPF in the POD5 DFA < 50 IU/L cohort is roughly equivalent to the average overall CR-POPF rate in the series. Furthermore, when viewed in aggregate with the other groups that were designated for early removal, the CR-POPF rate is just 2.7%. Therefore, this slightly lower NPV on POD5 could be justified as maximizing the degree of designation for early removal while still maintaining an overall modest risk for CR-POPF development.
After progressing through the proposed sequence using the FRS and amylase cutoffs, this study identifies an “enriched” cohort of patients with substantially elevated risk of developing CR-POPF. Based on this sizeable risk, this group encompasses patients who will most likely benefit from continuation of prophylactic drains. In the event of a CR-POPF, intraperitoneal drains have previously been associated with reduced mortality when compared to CR-POPFs in the absence of drains.5 Additionally, drains have also displayed a propensity for reducing the severity of fistula when compared to fistulas lacking drainage following distal pancreatectomy (DP).20 By maintaining drains in only this cohort with enhanced risk, the benefits of prophylactic drains will likely be maximized while the potential harms to cases without an apparent need for drainage will be largely avoided. Furthermore, the CR-POPFs in the early removal group do not substantially differ in severity when compared to the “enriched” cohort, supporting the notion that early removal is not necessarily more dangerous.
The “enriched” cohort is comprised of patients with higher rates of obesity and a greater proportion of high-risk pathology (other than pancreatic cancer/pancreatitis) when compared to the patients with early removal. The association of longer operations with CR-POPF may result from more challenging dissections in obese patients, which has previously been demonstrated.21 Furthermore, longer operations were also independently associated with increasing overall morbidity and other complications, and this relationship undoubtedly exists within the current study.21 Interestingly, the pathology association with CR-POPF in the “enriched” cohort is the inverse of the relationship proposed by the FRS. Pancreatic cancer and pancreatitis were both designated as “low-risk” pathologies when the FRS was conceived, and it is difficult to discern why the relationship is inverted in this case.
Further exploration of the “enriched” cohort reveals that a POD5 DFA threshold of 2000 IU/L has the greatest PPV for CR-POPF. At this point in time, PPV is deemed to have value for a variety of reasons. Clinicians realize that clinical evidence of a fistula generally emerges around this time, or soon thereafter,22 and high DFA on POD5 may be one of the earliest signs that a leak is manifest. Furthermore, patients finding themselves in the “enriched” cohort may provide clinicians an appropriate target for increased surveillance and intervention. Concern for increased fistula risk at this point in time may lead clinicians to obtain laboratory values (WBC, CRP, etc.), CT scans, administer therapeutic octreotide, or alter intake status.
The current study is not without limitations and must be interpreted with consideration. The outcomes reported within the proposed framework were reappraised and did not actually occur as a result of the proposed actions. Variables describing the character of drain effluent or drain volume were not available and likely guided surgeon’s decisions to maintain or remove drains during the original care of these patients. This study by no means seeks to replace the value of clinical judgment of effluent or other signs of clinical distress but rather attempts to propose a supplement to these decisions. Complete outcome data was also missing from a small portion of cases (21.7%).
Conclusion
This study proposes a dynamic framework for early drain removal that is effective in identifying a large proportion of patients for safe removal across multiple time points. By employing conditional thresholds with strong negative predictive values for CR-POPF intraoperatively to POD5, this study isolates a portion of patients with sizable fistula risk that may benefit from continuation of prophylactic drains, if not other fistula mitigation approaches. This framework may serve as the basis for future prospective studies exploring the refinement of early drain removal.
References
Kawai M, Tani M, Terasawa H, et al. Early removal of Prophylactic Drains Reduces the Risk of Intra-abdominal Infections in Patients With Pancreatic Head Resection: Prospective Study for 104 Consecutive Patients. Ann Surg. 2006;244(1):1. https://doi.org/10.1097/01.sla.0000218077.14035.a6
Molinari E, Bassi C, Salvia R, et al. Amylase Value in Drains After Pancreatic Resection as Predictive Factor of Postoperative Pancreatic Fistula: Results of a Prospective Study in 137 Patients. Ann Surg. 2007;246(2):281. https://doi.org/10.1097/sla.0b013e3180caa42f
Bassi C, Molinari E, Malleo G, et al. Early Versus Late Drain Removal After Standard Pancreatic Resections: Results of a Prospective Randomized Trial. Ann Surg. 2010;252(2):207. https://doi.org/10.1097/sla.0b013e3181e61e88
Fong ZV, Correa-Gallego C, Ferrone CR, et al. Early Drain Removal—The Middle Ground Between the Drain Versus No Drain Debate in Patients Undergoing Pancreaticoduodenectomy: A Prospective Validation Study. Ann Surg. 2015;262(2):378. https://doi.org/10.1097/sla.0000000000001038
McMillan MT, Fisher WE, Van Buren G, et al. The Value of Drains as a Fistula Mitigation Strategy for Pancreatoduodenectomy: Something for Everyone? Results of a Randomized Prospective Multi-institutional Study. J Gastrointest Surg. 2015;19(1):21–31. https://doi.org/10.1007/s11605-014-2640-z
McMillan MT, Malleo G, Bassi C, et al. Multicenter, Prospective Trial of Selective Drain Management for Pancreatoduodenectomy Using Risk Stratification. Ann Surg. 2017;265(6):1209. https://doi.org/10.1097/sla.0000000000001832
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A Prospectively Validated Clinical Risk Score Accurately Predicts Pancreatic Fistula after Pancreatoduodenectomy. J Am Coll Surg. 2013;216(1):1–14. https://doi.org/10.1016/j.jamcollsurg.2012.09.002
Villafane-Ferriol N, Buren G Van, Mendez-Reyes JE, et al. Sequential drain amylase to guide drain removal following pancreatectomy. HPB (Oxford). 2018. https://doi.org/10.1016/j.hpb.2017.11.008
McMillan MT, Malleo G, Bassi C, et al. Pancreatic fistula risk for pancreatoduodenectomy: an international survey of surgeon perception. HPB. 2017;19(6):515–524. https://doi.org/10.1016/j.hpb.2017.01.022
McMillan MT, Malleo G, Bassi C, Sprys MH, Vollmer CM. Defining the practice of pancreatoduodenectomy around the world. HPB. 2015;17(12):1145–1154. https://doi.org/10.1111/hpb.12475
Beane JD, House MG, Ceppa EP, Dolejs SC, Pitt HA. Variation in Drain Management After Pancreatoduodenectomy: Early Versus Delayed Removal. Ann Surg. 2017:1. https://doi.org/10.1097/sla.0000000000002570
McMillan MT, Christein JD, Callery MP, et al. Prophylactic octreotide for pancreatoduodenectomy: more harm than good? HPB. 2014;16(10):954–962. https://doi.org/10.1111/hpb.12314
Sachs TE, Pratt WB, Kent TS, Callery MP, Vollmer CM. The pancreaticojejunal anastomotic stent: Friend or foe? Surgery. 2013;153(5):651–662. https://doi.org/10.1016/j.surg.2012.11.007
McMillan MT, Ecker BL, Behrman SW, et al. Externalized Stents for Pancreatoduodenectomy Provide Value Only in High-Risk Scenarios. J Gastrointest Surg. 2016;20(12):2052–2062. https://doi.org/10.1007/s11605-016-3289-6
Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161(3):584–591. https://doi.org/10.1016/j.surg.2016.11.014
Maggino L, Malleo G, Bassi C, et al. Decoding Grade B Pancreatic Fistula: A Clinical and Economical Analysis and Subclassification Proposal. Ann Surg. 2018:1. https://doi.org/10.1097/sla.0000000000002673
Strasberg SM, Linehan DC, Hawkins WG. The Accordion Severity Grading System of Surgical Complications. Ann Surg. 2009;250(2):177. https://doi.org/10.1097/sla.0b013e3181afde41
McMillan MT, Christein JD, Callery MP, et al. Comparing the burden of pancreatic fistulas after pancreatoduodenectomy and distal pancreatectomy. Surgery. 2016;159(4):1013–1022. https://doi.org/10.1016/j.surg.2015.10.028
Giglio MC, Spalding DRC, Giakoustidis A, et al. Meta-analysis of drain amylase content on postoperative day 1 as a predictor of pancreatic fistula following pancreatic resection. Br J Surg. 2016;103(4):328–336. https://doi.org/10.1002/bjs.10090
Ecker BL, McMillan MT, Allegrini V, et al. Risk Factors and Mitigation Strategies for Pancreatic Fistula After Distal Pancreatectomy: Analysis of 2026 Resections From the International, Multi-institutional Distal Pancreatectomy Study Group. Ann Surg. 2017:1. https://doi.org/10.1097/sla.0000000000002491
Maggino L, Liu JB, Ecker BL, Pitt HA, Vollmer CM. Impact of Operative Time on Outcomes after Pancreatic Resection: A Risk-Adjusted Analysis Using the American College of Surgeons NSQIP Database. J Am Coll Surg. 2018. https://doi.org/10.1016/j.jamcollsurg.2018.01.004
McMillan MT, Vollmer CM, Asbun HJ, et al. The Characterization and Prediction of ISGPF Grade C Fistulas Following Pancreatoduodenectomy. J Gastrointest Surg. 2016;20(2):262–276. https://doi.org/10.1007/s11605-015-2884-2
Author information
Authors and Affiliations
Corresponding author
Additional information
All authors listed in this manuscript have fulfilled the four ICMJE criteria for authorship.
Rights and permissions
About this article
Cite this article
Seykora, T.F., Maggino, L., Malleo, G. et al. Evolving the Paradigm of Early Drain Removal Following Pancreatoduodenectomy. J Gastrointest Surg 23, 135–144 (2019). https://doi.org/10.1007/s11605-018-3959-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-3959-7