Abstract
Objective
We summarized the diagnosis, surgical strategies, and long-term follow-up outcomes in our large series of solid pseudopapillary tumors (SPTs) of pancreas.
Methods
In this retrospective analysis, we collected data pertaining to pancreatic SPTs diagnosed in 115 patients between July 2003 and February 2013.We analyzed the demographic characteristics, clinical presentations, operative strategies, perioperative details, and follow-up outcomes.
Results
Abdominal pain was the most frequent symptom (40.0 %). The most frequent location of SPT was pancreatic tail (36.5 %). We performed 33 cases of pancreaticoduodenectomy, 15 cases of middle pancreatectomy, 19 cases of distal pancreatectomy with spleen preservation, 28 cases of distal pancreatectomy with splenectomy, and 18 cases of enucleation. Two patients suffered tumor recurrence and required a second resection of the recurrent tumor.
Conclusions
Complete resection of the tumor is associated with good survival, even in patients with vessel involvement or metastases. In patients with tumor recurrence, a second resection resulted in long-term survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid pseudopapillary tumor (SPT) is a rare primary neoplasm of the pancreas, first described by Frantz1 in 1959. Accounting for 1–3 % of all pancreatic neoplasms, SPT occurs primarily in young women.2 The clinicopathologic features of SPT are unique: slow-growing, low-grade malignancy.3 The prognosis after complete surgical resection is quite favorable. A 5-year survival rate higher than 95 % has been reported in several studies.4
To our knowledge, our series represents the largest number of SPT cases from a single institution. We summarized the demographic characteristics, clinical presentations, radiologic features, pathology, surgical strategies, and long-term follow-up outcomes of SPT for a better understanding of its natural history and prognosis.
Materials and Methods
A total of 115 patients were diagnosed as pancreatic SPT (PSPT) at our institution from July 2003 to February 2013. Data were collected retrospectively by chart review. A surgical complication was defined as perioperative if it occurred within 30 days of surgery. We conducted telephone interviews and/or outpatient interview to follow up these patients. This study was approved by the Ethics Committee of Sichuan University.
Results
Patient Characteristics
The demographic characteristics of patients included in our study are shown in Table 1. Of the 115 patients diagnosed as PSPT, 100 patients (87.0 %) were female, with a female-to-male ratio of 6.7:1. The mean age at diagnosis was 35 years (range 13 to 63 years). Compared with other studies, most of patients (38 cases, 33.1 %) in our series were diagnosed as SPT in the fourth decade of life. The signs and symptoms included upper abdominal pain in 46 patients (40.0 %), abdominal discomfort in 19 patients (16.5 %), and palpable abdominal mass in 17 patients (14.8 %). Only one patient (0.9 %) was presented with jaundice. There were 32 (27.8 %) asymptomatic patients with the diagnosis made by occasional ultrasonography (US) or computed tomography (CT).
Preoperative Examination
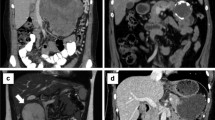
Preoperative examination included tumor marker studies, US, CT, or magnetic resonance imaging (MRI). Positron emission tomography was carried out in one patient. Typical ultrasonographic manifestations of PSPT include well-circumscribed hypoechoic solid lesions, without main pancreatic duct dilatation. The typical computed tomographic appearance of SPT was well-circumscribed cystic and solid masses with heterogeneous enhancement (Fig. 1a). In patients with tumors smaller than 3-cm diameter, the cystic portion was not apparent. SPT usually appeared as pancreatic tumors without apparent pancreatic duct dilatation or parenchymal atrophy. Only three cases of SPT presented dilatation of the bile duct and main pancreatic duct. Calcification of mass appeared in 18 patients and large cystic masses appeared in 7 patients. The SPT in MRI showed low signal intensity in unenhanced T1-weighted images, high signal intensity in T2-weighted images, and early heterogeneity and progressive enhancement. Positron emission tomography of one patient in our series showed increased fluorodeoxyglucose uptake in SPT. The serum levels of carbohydrate antigen 19-9 and carcinoembryonic antigen were in the normal range in 113 patients. Only one patient presented elevated total bilirubin and presented jaundice.
The clinical presentations of SPT are shown in Table 2. The most frequent locations of SPT were pancreatic tail (42 cases, 36.5 %) and pancreatic head (37 cases, 32.1 %), followed by pancreatic neck and body (23 cases, 20.0 %), pancreatic body (8 cases, 7.0 %) and the uncinate process of the pancreas (4 cases, 3.5 %). Only one patient (0.9 %) presented multiple lesions of pancreatic head and tail. The mean size of the tumors was 6.3 cm (range 1 to 25 cm).
Surgery and Postoperative Complications
The surgical details and postoperative complications of our patients are shown in Table 3. All the 115 patients except for one underwent complete surgical resection including 33 cases of pancreaticoduodenectomy, 15 cases of middle pancreatectomy, 19 cases of distal pancreatectomy with spleen preservation, 28 cases of distal pancreatectomy with splenectomy, and 18 cases of enucleation. Among the patients undergoing distal pancreatectomy, six cases were performed laparoscopically. Enucleation of pancreatic head lesion and distal pancreatectomy with splenectomy of pancreatic tail lesion was carried out in a patient with simultaneous pancreatic head and tail SPT. We performed tumor fine needle biopsy and cholangiojejunostomy in a patient with a 9-cm SPT in the pancreatic head, and extensive portal vein and superior mesenteric vein involvement and multiple liver metastases. Interestingly, we successfully performed distal pancreatectomy with splenectomy and pancreaticoduodenectomy with portal vein resection in two patients, respectively, diagnosed as unresectable SPT by laparotomy elsewhere. Distal pancreatectomy with splenectomy and wedge resection of the liver metastases were conducted in another patient with pancreatic body SPT and two isolated liver metastases in the left lobe and right posterior lobe. Distal pancreatectomy with splenectomy, subtotal gastrectomy and wedge resection of the liver metastases were carried out in a case involving SPT of pancreatic body, with gastric body involvement and liver metastases. Simultaneous resections of SPT and liver metastases were done with another three patients. Two patents suffering from SPT with transverse colon involvement underwent resection of SPT and colectomy.
The operative time and estimated blood loss varied with the tumor size and surgical strategies. The mean operative time was 235 min (range 155 to 420 min), and the mean estimated blood loss was 317 ml (range 50 to 1,500 ml). The overall complication rate was 19.1 % (n = 22), with no perioperative deaths. Complications included postoperative pancreatic fistula (13 cases, 11.3 %), followed by pulmonary infection and fluid collection (3 cases, 2.6 %), incision infection (2 cases, 1.7 %), and alimentary tract hemorrhage (1 case, 0.9 %). Pancreatic fistula was treated with percutaneous drainage. Alimentary tract hemorrhage required emergency laparotomy and discharge on the eighth postoperative day after the second surgery. A month after discharge, the patient suffered from abdominal pain and was diagnosed as abdominal infection. He was cured by percutaneous drainage and antibiotics.
Pathological Features
Gross Appearance
The gross appearance of SPT varies with the size of the tumor. Typically, SPT is well capsulated and demarcated from the pancreas, with a mixture of solid, cystic, and pseudopapillary patterns in various proportions (Fig. 1b). However, the smaller tumors may be less likely to show cystic changes and often appear as uncapsulated solid masses with varying degrees of fibrosis.
Histology
Microscopically, SPT is remarkably uniform, with a combination of solid pseudopapillary or hemorrhagic pseudocystic structures in various proportions.5 The histologic appearance varies across different regions of the tumor.6 Typically, the solid area of SPT appears microscopically as uniform, poorly cohesive polygonal cells surrounding delicate blood vessels (Fig. 2a).7 The SPT cells usually manifest decreased mitotic activity, and true necrosis is uncommon. However, cystic degeneration is common in “papillary-cystic” or “solid and cystic” SPT, especially in tumors larger than 3 cm.
Immunohistochemistry
Generally, the neoplastic cells are stained positive for vimentin in all tumors, and most cases express α1-antitrypsin, α1-antichymotrypsin, and neuron-specific enolase.5 Immunohistochemical staining was performed in selected cases. CD56 was positive in all 74 cases, beta-catenin was positive in all 63 cases (Fig. 2b), neuron-specific enolase was positive in 26 out of 27 cases, vimentin was positive in all 34 cases, α1-antichymotrypsin was positive in six out of seven cases, and progesterone receptor was positive in all eight cases.
Follow-Up and Survival
All patients underwent US or CT every 6 months to 1 year after surgery. Follow-up data were collected by telephone or outpatient interview. The mean follow-up period was 58 months (range 6 to 121 months). The overall survival was 98.3 % (113 out of 115 cases), and the survival of patients with combined pancreatic and liver/gastric resection was 75 % (6 out of 8 patients). The survival curve is shown in Fig. 3. The patient who underwent distal pancreatectomy with splenectomy, subtotal gastrectomy, and wedge resection of the liver metastases died of arrhythmia 3 months after surgery. The patient who underwent cholangiojejunostomy died of liver failure 18 months after surgery. The tumor-free survival was 96.6 %. Two patients (1.7 %) suffered tumor recurrence. One patient who underwent distal pancreatectomy and splenectomy developed local recurrence at the pancreatic stump 3 months after surgery and underwent a second laparotomy for recurrent tumor. This patient who was followed up for 21 months never presented with tumor recurrence again. Another patient who underwent pancreaticoduodenectomy developed isolated liver metastases 11 months after the first surgery. Resection of the liver metastases resulted in a 38-month tumor-free period. Seven patients presented with postsurgical dyspepsia and two patients suffered from insulin-dependent diabetes.
Discussion
The SPT diagnostic and therapeutic guidelines remain unclear.8 Our study represents the largest number of patients with SPT from a single institution and summarizes its diagnosis, surgical strategies, and long-term outcomes.
The molecular events associated with the development of SPT have recently been reported.9 Genetic profile of SPT differs from that of pancreatic adenocarcinoma. SPT is characterized by activating β-catenin gene mutations, which interfere with protein phosphorylation.10 Translocation of β-catenin into the nucleus regulates the transcription of the growth regulatory genes cyclin D1 and c-myc.9 Furthermore, β-catenin interacts with E-cadherin, preventing normal cell-to-cell interactions.11
Compared with other pancreatic tumors, SPT is most commonly present in females in their third or fourth decade. The mean age of patients at diagnosis in our series was 35 years, which was higher compared with several other series.5,12,13 Abdominal pain was the most frequent symptom, probably due to tumor compression. Jaundice was rare, occurring only in one patient in our series, even in the presence of a huge SPT associated with pancreatic head. The proportion of asymptomatic patients (27.8 %) was higher in our series compared with other studies. Pancreatic tail and head were the most common locations of SPT, consistent with other studies. The tumor markers, such as carbohydrate antigen 19-9 and carcinoembryonic antigen, were always normal. Only two patients in our series presented a slight elevation of carbohydrate antigen 19-9.
Advances in imaging strategies have improved the accuracy of the preoperative diagnosis of SPT.14 The radiological presentations of SPT are unique.15 The typical CT presentation of SPT is a heterogeneous pancreatic mass with cystic and solid components. The solid portion of SPT was typically enhanced similar to pancreatic parenchyma in arterial and venous phases.16 Calcification may be present in some cases, whereas dilation of pancreatic duct is rare. MR imaging, with superior contrast resolution, displays intratumoral hemorrhage, and the capsule of the SPT is better than CT.17 Contrast-enhanced US is another useful modality to establish an accurate diagnosis, with a typical peripheral rim enhancement in the early dynamic phases.18 A percutaneous or endoscopic fine needle aspiration may establish an accurate preoperative diagnosis.19 However, the procedure may cause tumor cell dissemination.20 Furthermore, Kim et al.21 reported in their study that only 11 of the 24 (46 %) patients who underwent fine needle aspiration were correctly diagnosed. Radiologic diagnosis is adequate, especially for surgery. Overall, the diagnosis of SPT should always be suspected in young women with a solid or cystic pancreatic mass. The final diagnosis is based on pathology and immunohistochemistry.
Surgery is the first choice of treatment once the diagnosis of PSPT is established.22 Recurrence rate is estimated in 10–15 % of patients after resection,23 whereas only two patients (1.7 %) suffered from tumor recurrence in our series. Pancreatoduodenectomy, distal pancreatectomy (with or without splenectomy), middle pancreatectomy, or enucleation depends on tumor location, size, and invasive potential. Distal pancreatectomy with or without splenectomy is performed for tumors located in the pancreatic body or tail. Tumors located in the neck or body of pancreas, without vessel involvement, require middle pancreatectomy with distal pancreatojejunostomy to preserve the rim of the head, the uncinate process, and the tail portion. For tumors located in the pancreatic head or uncinate process, a classic or pylorus-preserving pancreatoduodenectomy is indicated. Due to low-grade malignancy and the surrounding dense fibrous capsule, enucleation is indicated for smaller tumors distant from the main pancreatic duct, without affecting long-term survival. Extensive lymphadenectomy is not necessary due to infrequent nodal metastases.24 No patient in our series suffered from lymph node metastases. Tumors with vessel involvement or metastases are not always unresectable.8 Our series showed 32 cases of vessel involvement including 21 cases of splenic vessels and 11 cases of superior mesenteric vein or/and portal vein. Only one case of SPT was unresectable due to extensive involvement of superior mesenteric vein and portal vein and multiple liver metastases. Surgical resection is indicated for metastases.25 Our series included one case of gastric involvement, two cases of transverse colon involvement, and five cases of liver metastases. Except for one (described above), we performed complete tumor resection plus wedge resection of the liver metastases, gastrectomy, or colectomy resulting in good survival. Chemotherapy25 or radiotherapy26 may be of value in patients with unresectable SPT.
The first laparoscopic distal pancreatectomy was reported in 1996.27 Compared with open surgeries, laparoscopic distal pancreatectomy offers advantages in terms of earlier oral intake, limited blood loss, shorter posthospital stays, and higher spleen preservation.28 Six patients successfully underwent laparoscopic spleen preservation, and distal pancreatectomy in our series, without perioperative complications. During the follow-up, no tumor recurrence or metastases were detected. Laparoscopic surgery is safe and feasible in the selected cases.
Patients with SPT undergoing surgical resection achieved good long-term survival. Only two patients (1.7 %) suffering from local recurrence or liver metastases underwent second resection, with a 21 and 38 months disease-free period during the follow-up, respectively. Only two patients (1.7 %) died during the follow-up: one patient with tumor resection died of arrhythmia, and one patient without tumor resection died of liver failure. No local recurrence or metastases occurred in the other patients.
In conclusion, SPT is a rare pancreatic tumor, with a low-grade malignancy and strong female preponderance. Complete resection is associated with good survival, even in patients with vessel involvement or metastases. Patients with tumor recurrence also showed long-term survival, after a second resection. As a minimal invasive procedure, laparoscopic surgery for SPT is a promising approach in selected cases.
References
Frantz V: Tumors of the Pancreas. Atlas of Tumor Pathology, Section vii, Fascicles 27 and 28. Washington, DC: Armed Forces Institute of Pathology 1959:32–3.
Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE: Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery 2008, 143:29–34.
Adams AL, Siegal GP, Jhala NC: Solid pseudopapillary tumor of the pancreas: a review of salient clinical and pathologic features. Adv Anat Pathol 2008, 15:39–45.
Sperti C, Berselli M, Pasquali C, Pastorelli D, Pedrazzoli S: Aggressive behaviour of solid-pseudopapillary tumor of the pancreas in adults: a case report and review of the literature. World J Gastroenterol 2008, 14:960–5.
Papavramidis T, Papavramidis S: Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005, 200:965–72.
Francis WP, Goldenberg E, Adsay NV, Steffes CP, Webber JD: Solid-pseudopapillary tumors of the pancreas: case report and literature review. Curr Surg 2006, 63:469–72.
Butte JM, Brennan MF, Gonen M, Tang LH, D'Angelica MI, Fong Y, Dematteo RP, Jarnagin WR, Allen PJ: Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg 2011, 15:350–7.
Wang XG, Ni QF, Fei JG, Zhong ZX, Yu PF: Clinicopathologic features and surgical outcome of solid pseudopapillary tumor of the pancreas: analysis of 17 cases. World J Surg Oncol 2013, 11:38.
Reddy S, Cameron JL, Scudiere J, Hruban RH, Fishman EK, Ahuja N, Pawlik TM, Edil BH, Schulick RD, Wolfgang CL: Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg 2009, 208:950–7; discussion 7–9.
Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y, Kobayashi Y: Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res 2001, 61:8401–4.
Chetty R, Serra S: Membrane loss and aberrant nuclear localization of E-cadherin are consistent features of solid pseudopapillary tumour of the pancreas. An immunohistochemical study using two antibodies recognizing different domains of the E-cadherin molecule. Histopathology 2008, 52:325–30.
Goh BK, Tan YM, Cheow PC, Chung AY, Chow PK, Wong WK, Ooi LL: Solid pseudopapillary neoplasms of the pancreas: an updated experience. J Surg Oncol 2007, 95:640–4.
Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, Xu Q: Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature. World J Gastroenterol 2010, 16:1209–14.
Nadler EP, Novikov A, Landzberg BR, Pochapin MB, Centeno B, Fahey TJ, Spigland N: The use of endoscopic ultrasound in the diagnosis of solid pseudopapillary tumors of the pancreas in children. J Pediatr Surg 2002, 37:1370–3.
Matos JM, Grutzmann R, Agaram NP, Saeger HD, Kumar HR, Lillemoe KD, Schmidt CM: Solid pseudopapillary neoplasms of the pancreas: a multi-institutional study of 21 patients. J Surg Res 2009 Nov;157(1):e137–42.
Reddy S, Wolfgang CL: Solid pseudopapillary neoplasms of the pancreas. Adv Surg 2009, 43:269–82.
Yu MH, Lee JY, Kim MA, Kim SH, Lee JM, Han JK, Choi BI: MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR Am J Roentgenol 2010, 195:1324–32.
D'Onofrio M, Malago R, Vecchiato F, Zamboni G, Testoni M, Falconi M, Capelli P, Mucelli RP: Contrast-enhanced ultrasonography of small solid pseudopapillary tumors of the pancreas: enhancement pattern and pathologic correlation of 2 cases. J Ultrasound Med 2005, 24:849–54.
Song JS, Yoo CW, Kwon Y, Hong EK: Endoscopic ultrasound-guided fine needle aspiration cytology diagnosis of solid pseudopapillary neoplasm: three case reports with review of literature. Korean J Pathol 2012, 46:399–406.
Fais PO, Carricaburu E, Sarnacki S, Berrebi D, Orbach D, Baudoin V, de Lagausie P: Is laparoscopic management suitable for solid pseudo-papillary tumors of the pancreas? Pediatr Surg Int 2009, 25:617–21.
Kim CW, Han DJ, Kim J, Kim YH, Park JB, Kim SC: Solid pseudopapillary tumor of the pancreas: can malignancy be predicted? Surgery 2011, 149:625–34.
Ji S, Xu J, Zhang B, Xu Y, Liu C, Long J, Ni Q, Yu X: Management of a malignant case of solid pseudopapillary tumor of pancreas: a case report and literature review. Pancreas 2012, 41:1336–40.
Geers C, Moulin P, Gigot JF, Weynand B, Deprez P, Rahier J, Sempoux C: Solid and pseudopapillary tumor of the pancreas—review and new insights into pathogenesis. Am J Surg Pathol 2006, 30:1243–9.
Machado MCC, Machado MAC, Bacchella T, et al.: Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery 2008, 143:29–34.
Rebhandl W, Felberbauer FX, Puig S, Paya K, Hochschorner S, Barlan M, Horcher E: Solid-pseudopapillary tumor of the pancreas (Frantz tumor) in children: report of four cases and review of the literature. J Surg Oncol 2001, 76:289–96.
Matsunou H, Konishi F: Papillary-cystic neoplasm of the pancreas. A clinicopathologic study concerning the tumor aging and malignancy of nine cases. Cancer 1990, 65:283–91.
Becmeur F, Hofmann-Zango I, Moog R, Sauvage P: [Small bowel obstruction and laparoscopic treatment in children]. J Chirurg 1996, 133:418–21.
Kang CM, Choi SH, Hwang HK, Lee WJ, Chi HS: Minimally invasive (laparoscopic and robot-assisted) approach for solid pseudopapillary tumor of the distal pancreas: a single-center experience. J Hepatobiliary Pancreat Sci 2011, 18:87–93.
Acknowledgments
This work is supported by The Research Special Fund for Public Welfare Industry of Health of China. No. 201202007
Author information
Authors and Affiliations
Corresponding author
Additional information
The abstract of this study was present at 39th PSGBI, 27th–29th November 2013, Liverpool, UK.
Rights and permissions
About this article
Cite this article
Cai, Y., Ran, X., Xie, S. et al. Surgical Management and Long-Term Follow-Up of Solid Pseudopapillary Tumor of Pancreas: A Large Series from a Single Institution. J Gastrointest Surg 18, 935–940 (2014). https://doi.org/10.1007/s11605-014-2476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2476-6