Abstract
Introduction
Management of gastroesophageal reflux disease (GERD) is based on the concept that gastric contents, principally acid and pepsin, are responsible for symptoms of reflux and esophageal injury. Pharmacologic treatment is based on the principle that controlling intragastric pH will affect esophageal healing and subsequently symptom relief.
Results and Discussion
Control of pH can be accomplished with antisecretory agents, principally proton pump inhibitors (PPIs). The majority of patients respond to a single daily dose of a PPI; however, some will require higher doses, and a small percentage are “refractory” to twice daily dosing of these drugs. The success of these agents, and in fact the reasons for “failure,” is elucidated by understanding the mechanism of action of PPIs and the effect of dose timing and meals on their efficacy.
Conclusion
Awareness of new concerns regarding potential side effects of PPIs when used long-term require careful thought as GERD is a chronic disease with most needing some form of medical treatment over time. This article reviews the pharmacologic properties of PPIs and the impact on the treatment of GERD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that 30–40% of the US population have some symptoms of gastroesophageal reflux disease (GERD).1 The most common symptoms are heartburn and/or regurgitation with an unknown number of patients with extraesophageal symptoms, such as cough, laryngitis, or wheezing.2,3 Chronic, frequent heartburn is the major risk factor for the development of esophageal adenocarcinoma, the fastest rising cancer in white men in the USA.
GERD is caused by the retrograde movement of gastric contents, mainly acid and pepsin, into the esophagus thereby causing symptoms of reflux and injury to the esophageal mucosa. Antisecretory agents, such as proton pump inhibitors (PPIs), increase intragastric pH thereby promoting esophageal mucosal healing and subsequent symptom relief. Understanding the mechanism of action of PPIs and the importance of dose timing in relation to meals is critical to optimize treatment for GERD. A majority of patients respond to a single daily dose of PPI. However, there are those who will require higher doses of medication and a small percentage that are considered refractory to PPIs. PPIs are safe medications with a low side effect profile; however, there is a new concern regarding the potential for long-term effects of these medications in the treatment of a chronic GERD sufferers. This article will explain the pharmacology of PPIs and their impact on the treatment of GERD.
Acid Production
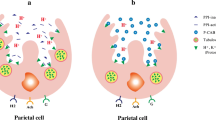
The parietal cell is the key player of acid secretion in the stomach responsible for an average of 2 L of gastric acid daily.4 It expresses receptors for stimulators of acid secretion, including gastrin released from G cells, acetylcholine released from the vagus nerve, and histamine. Gastrin and acetylcholine cause the release of histamine from enterochromaffin-like cells (ECL). Activation of gastrin and acetylcholine receptors results in the activation of the protein kinase C phosphoinositide signaling pathway. Histamine leads to activation of adenylate cyclase and increase in cyclic AMP.5,6 Both of these signaling pathways regulate a series of kinase cascades that control the acid secreting, H+/K+ATPase (proton pump), the target of the PPI.
PPIs inhibit only active pumps. A single dose of a PPI does not inhibit all pumps and does not result in profound inhibition of acid secretion. Acid production is inhibited with subsequent PPI doses, taking 5–7 days to achieve a steady state. Acid inhibition is never complete because of continued synthesis of new proton pumps. When PPIs are given twice daily, more active proton pumps are exposed to drug, and steady-state inhibition of gastric acid secretion is achieved more rapidly and more complete.7
PPIs are weak bases, incompletely absorbed, and have short half lives (0.6–1.9 h).
PPIs accumulate and activate in the acid environment at the secretory canalicular surface of the parietal cell. The inactive benzimidazole is converted to a cationic tetracyclic sulfonamide, which bind to the alpha subunit of the H+ K+ ATPase enzyme, irreversibly inhibiting acid production in about 70% of active pumps.5,8 Acid secretion returns when new H+ K+ ATPase molecules are converted from inactive status in the tubulovesicle to active form at the canalicular surface.9 This averages 36–72 h. PPIs decrease daytime, nocturnal, and meal-stimulated acid secretion.10 The slower a PPI is cleared from the plasma; the more of it is available to be delivered to the proton pump.11
All available PPIs are indicated for once daily dosing, usually in the morning. Food affects the bioavailability of each molecule, so it is our practice to recommend that all PPIs be given prior to meals for optimal efficacy. This is based on the concepts outlined above and results of intragastric pH studies in which superior daytime pH control (time intragastric pH > 4) was seen when the PPI was taken before breakfast compared to an empty stomach (Fig. 1).11
Percentage time for which gastric pH <4 when taking PPI (either omeprazole 20 mg or lansoprazole 30 mg) each morning, either 15 min before a breakfast meal or without food and drink (except for water), until 12 noon. In this box-and-whiskers plot, the median values are indicated as the transverse line within the box, the interquartile range as the vertical extent of the box, and total range as the whiskers. Acid suppression was significantly more effective when medication was taken with breakfast than without (p < 0.01).
PPIs are responsible for inhibiting gastric acid secretion, thereby decreasing potential damage to the esophageal mucosa. In addition, by raising gastric pH, the conversion of pepsinogen to pepsin, another player of mucosal damage, is inhibited. As one would expect, greater duration of gastric acid suppression affords greater healing rates of erosive esophagitis (Table 1).
Most patients respond to once-a-day PPI. However, some patients, particularly those with extraesophageal symptoms or complicated disease need higher doses. Splitting the dose and giving a PPI twice daily, before breakfast and dinner, results in superior nocturnal intragastric pH control, when compared to a double dose given once daily (Fig. 2).
Pharmacodynamic Effects
Omeprazole (also available over the counter at a 20 mg dose), lansoprazole, pantoprazole, rabeprazole, esomeprazole, and the newest PPI omeprazole sodium bicarbonate immediate release (IR-OME, Zegerid®) are all available for the treatment of GERD. PPIs inhibit daytime, nocturnal, and meal-stimulated acid secretion to a significantly greater degree than H2RAs and have largely replaced these agents in antireflux therapy.
Usually, delayed release PPIs are administered before the first meal of the day. If a second dose is needed, it should be given before the evening meal. With the exception of IR-OME, bedtime dosing is discouraged because proton pumps are not stimulated to a great degree during the sleeping period. When PPIs are administered twice daily, more active proton pumps are exposed to drug, and steady-state inhibition of gastric acid secretion is quicker and more complete. Daily dosing of PPIs are thus more effective than on demand or intermittent dosing in maintenance of symptom relief and healing of erosive esophagitis.
Patients with Helicobacter pylori gastritis involving the gastric corpus have enhanced overnight intragastric pH control with PPIs compared to those who are H. pylori negative.12 However, H. pylori infection has little effect on pH control and thus has no role in the management of GERD. We do not routinely test for H. pylori in our GERD patients and find that it has no effect on outcome.
Immediate-Release Omeprazole
The newest PPI, immediate-release omeprazole sodium bicarbonate (IR-OME), offers another option for nighttime heartburn sufferers. IR-OME suspension, when administered at bedtime, achieved enhanced control of nocturnal gastric pH when compared to pantoprazole 40 mg.13 Additionally, IR-OME given prior to breakfast and dinner, was found to be more effective in controlling overnight pH as compared to twice daily pantoprazole.13
An open-label, randomized, crossover study (N = 54) compared intragastric pH with IR-OME suspension 40 mg, lansoprazole 30 mg, and esomeprazole 40 mg given at bedtime (10:00 p.m.) on an empty stomach for 7 days.14 In the first half of the nighttime, intragastric pH >4 were higher after IR-OME compared to esomeprazole or lansoprazole (p < 0.001, both comparisons).15 Acid control with IR-OME was significantly better than lansoprazole (p < 0.001) and comparable to esomeprazole for the entire nighttime period. The percentage of time with gastric pH >4 for the entire 24-h period was 43.6% after treatment with IR-OME vs. 59% with esomeprazole (p < 0.001) and 27.8% with lansoprazole (p < 0.001), when compared with both IR-OME and esomeprazole (Table 2).14
This study further supports the longstanding suggestion that delayed release PPIs are not optimally effective when given at bedtime. Lansoprazole offered a slow onset of overnight control with little increase the next day. Esomeprazole had a slower onset, with little time pH >4 in the first part of the sleeping period, however, had excellent 24-h control. As such, delayed release PPIs are best given before the evening meal if control is needed overnight.16
Side Effects of PPIs
PPIs have a low side effect profile and are considered to be safe medications. Frequent side effects seen in trials include headache, abdominal pain, and diarrhea. However, they are no greater in frequency than placebo.15
The increased levels of gastrin from gastric acid suppression and its trophic effects on stomach mucosa have also been a concern. Fundic gland polyps, while seen in a small number of patients, have not been shown to lead to a negative outcome. In the absence of observational and case–control studies, we do not change or stop PPIs due to fundic gland polyps unless the patient requests an alternative approach. We do not perform surveillance endoscopies unless a patient has a change in symptoms.
Increased incidence of gastric carcinoids, once thought to be of concern, have not been proven to be true. Vitamin B12 deficiency was thought to be a potential problem but has not been shown in well-done studies except in patients with multiple endocrine neoplasias.17
Several newer issues have been raised, especially in regards to infectious diseases. Pneumonia has been reported to be higher in patients on PPI (and H2RAs) as compared to the patients not on treatment. The odds ratio for pneumonia was 1.8 compared to those not on PPI.18 The patients in the study had multiple comorbidities, making these results difficult to interpret. Additionally, case–control studies have reported an increase in the prevalence of Clostridium difficile infection while on PPIs. Odds ratios from 1.3 to 5.1 for infection have been reported.19–21 An increase in infection has not been substantiated in direct observational studies nor has the mechanism for this increase been elucidated. More importantly, the overall incidence of C. difficile infection in the general population is still quite low, so any small increase is unlikely to be a major clinical problem. Nonetheless, we must increase our awareness of the potential for C. difficile infection in patients on PPI, especially if they are on antibiotics, hospitalized, or in chronic care facilities.
A recent study reports an increase in the odds ratio for hip fractures for patients taking a PPI.22 There was increased risk if a patient was taking a PPI long term and on more than once a day dosing.23 The study states the multiple confounders were accounted for, including the severity and number of comorbid conditions in the comparator groups. Two other studies support this association. While the association is plausible, it has not been proven to be causal. Although the number of hip fractures is low, if confirmed, these data may affect the way we use PPIs long term particularly in patients at risk for fracture. At present, we remind patients at risk to discuss preventative measures with their primary care providers and use the lowest effective dose needed.
It is important to note that, in an observational study (N = 230) in which patients were followed while on continuous PPI, in doses of 20–160 mg/day for up to 11 years, none of the side effects above were seen.24
Multiple dose–response, meal, and dose timing pharmacodynamic studies reinforce the following key principles for clinical practice:
-
Daytime control of intragastric pH is superior to nighttime control when PPIs are given in the morning.25 A single daily dose given in the morning before breakfast will help most patients. A PPI given before dinner improves nighttime pH control without effect on daytime pH control.16 Bedtime dosing of IR-OME will shift this curve to better overnight pH control compared to daytime.14 Symptom studies are not available to compare these dose timings.
-
Intragastric pH studies support superiority of acid control with twice daily dosing compared to double dose once daily in patients needing higher doses than approved by the FDA.16
-
We recommend that all PPIs be dosed prior to meals for optimal efficacy. Our own data found superior daytime pH control (time intragastric pH >4) when PPI was taken before breakfast compared to an empty stomach.11 Despite knowing these data, the appropriate timing of a meal is still under emphasized by clinicians. Patients often use PPIs in the morning and do not eat breakfast, taking their drug after a meal or at bedtime. A small adjustment in timing to before dinner, or eating breakfast after the morning dose, may improve clinical outcome. Consider IR-OME in patients needing nighttime control, especially the early hours after going to sleep.
-
Optimal intragastric pH control is the key to effective treatment. Better control results in improved outcomes.26
Summary
PPIs are safe and effective agents for GERD. A single daily dose works for most. All available PPIs offer excellent symptom relief and healing of erosive esophagitis both short and long term. Esomeprazole 40 mg once daily offers small improvements in erosive esophagitis healing and symptom relief at 4 and 8 weeks compared to omeprazole 20 mg,27 lansoprazole 30 mg,28 and pantoprazole 40 mg.29 The clinical importance of these statistical differences continues to be debated. In practice, cost usually guides therapy. Omeprazole OTC is equal in strength to standard FDA approved dose by prescription. IR-OME offers options for patients with nighttime heartburn and off label as an on-demand PPI. Optimal efficacy of PPIs should ideally be given before a meal. Higher doses, if needed, should be given in split dose taking advantage of improved pH control when given twice daily. Careful vigilance and more studies are needed to evaluate the potential for side effects in those on long-term therapy. Until more studies are available, PPIs remain the treatment of choice for clinical management for the vast majority of patients with GERD.
References
Locke GR, Talley NJ, Fett SL et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population based study in Olmstead county, Minnesota. Gastroenterology 1997;112:1448.
Stanghellini V. Relationship between upper gastrointestinal symptoms and lifestyle, psychosocial factors and comorbidity in general population; results from the Domestic/International Gastroenterology Surveillance Study (DIGEST). Scand J Gastroenterol Suppl 1999;231:29–37.
Fennerty MB. Extraesophageal gastroesophageal reflux disease. Presentations and approach to treatment. Gastroenterol Clin North Am 1999;28:861–873.
Robinson M. Review article: Current perspectives on hypergastrinaemia and enterochromaffin-like-cell hyperdysplasia. Aliment Pharmacol Ther 1999;13(suppl 5):5–10.
Massoomi F, Savage J, Destache CJ. Omeprazole: a comprehensive review. Pharmacotherapy 1993;13(1):46–59.
Maton PN. Omeprazole N. Engl J Med 1991;324(14):965–975.
Bell NJV, Burget D, Howden CW et al. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion 1992;51(suppl 1):59–67.
Lew EA. Review article: Pharmacokinetic concerns in the selection of anti-ulcer therapy. Aliment Pharmacol Ther 1999;13(suppl 5):11–16.
Bensancon M, Simon A, Sachs G et al. Sites of action of the gastric H, K-ATPase with extracytoplasmic thiol reagents. J Biol Chem 1997;272:22438–22446.
Klinkenberg-Knol EC, Festen HPM, Meuwissen SGM. Pharmacological management of gastro-oesophageal reflux disease. Drugs 1995;49:695–710.
Hatlebakk JG, Katz PO, Castell DO. Proton pump inhibitors: better acid suppression when taken before a meal than without a meal. APT 2000;14(10):1267–1272.
Katsube T, Adachi K, Kawamura A et al. Helicobacter pylori infection influences nocturnal gastric acid breakthrough. Aliment Pharmacol Ther 2000;14(8):1049–1056.
Castell D, Bagin R, Goldlust B, Major J, Hepburn B. Comparison of the effects of immediate-release omeprazole powder for oral suspension and pantoprazole delayed-release tablets on nocturnal acid breakthrough in patients with symptomatic gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2005;21(12):1467–1474.
Katz PO, Koch FK, Ballard ED, Bagin RG, Gautille TC, Checani GC, Hogan DL, Pratha VSV. Comparison of the effects of immediate-release omeprazole oral suspension, delayed-release lansoprazole capsules and delayed-release esomeprazole capsules on nocturnal gastric acidity after bedtime dosing in patients with night-time GERD symptoms. Aliment Pharmacol Ther 2007;25:197–205.
Physicans’ Desk Reference. Product information for all proton pump inhibitors, 60th edn. Thomson PDR: Montvale, NJ, 2006
Hatlebakk JG, Katz PO, Kuo B et al. Nocturnal gastric acidity and acid breakthrough on different regimens of omeprazole 40 mg daily. Aliment Pharmacol Ther 1998;12:1235–1240.
Howden CW. Vitamin B12 levels during prolonged treatment with proton pump inhibitors. J Clin Gastroenterol 2000;30(1):29–33.
Laheij RJF, Sturkenboom MCHM, Hassing RJ et al. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA 2004;292(16):1955–1960.
Dial S, Alrasadi K, Manoukian C et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ: Can Med Assoc J 2004;171(1):33–38.
Dial S, Delaney JAC, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile- associated disease. JAMA 2005;294(23):2989–2995.
Williams C, McColl KEL. Review article: Proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther 2006;23(1):3–10.
Targownik LE, Lix LM, Metge CJ et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008;179(4):319–326.
Yang Y-X, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006;296(24):2947–2953.
Klinkenberg-Knol E, Nelis F, Dent J et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease. Gastroenterology 2000;118:661–669.
Tutuian R, Katz PO, Castell DO. A PPI is a PPI is a PPI: Lessons from prolonged intragastric pH monitoring. Gastroenterol 2000;118:A17.
Katz PO, Ginsberg GG, Hoyle PE, Sostek MB, Monyak JT, Silberg DG. Relationship between intragastric acid control and healing status in the treatment of moderate to severe erosive oesophagitis. Aliment Pharmacol Ther 2007;25(5):5617–5628.
Richter JE, Kahrilas PJ, Johanson J et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol 2001;96:656–665.
Castell DO, Kahrilas PJ, Richter JE et al. Esomeprazole (40 gm) compared with lansoprazole (30 mg0 in the treatment of erosive esophagitis. Am J Gastroenterol 2002;97(3):575–583.
Labenz J, Armstrong D, Lauritsen K et al. A randomized comparative study of esomeprazole 40 mg versus pantoprazole 40 mg for healing erosive oesophagitis: the EXPO study. Aliment Pharmacol Ther 2005;21(6):739–746.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katz, P.O., Zavala, S. Proton Pump Inhibitors in the Management of GERD. J Gastrointest Surg 14 (Suppl 1), 62–66 (2010). https://doi.org/10.1007/s11605-009-1015-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-009-1015-3