Abstract
Purpose
This study aimed to create an animal model of type Ia endoleak that creates persistent problems after thoracic endovascular aortic repair.
Materials and methods
In six swine, thoracic aortic aneurysms were created using the harvested jugular vein. We created a type Ia endoleak using a composite stent-graft comprising the first stent-graft (reverse-tapered: thicker part, 16 mm; thinner part, 10 mm) and the second stent-graft (tapered: thicker part, 18–20 mm; thinner part, 16 mm). This double-component stent-graft was deployed in the abdominal aorta and then moved upward to the proximal entry site of the thoracic aneurysm using the inflated balloon for precise positioning. After the surgical procedure and on postoperative day 8, aortography was performed to detect residual endoleak, and then the swine were euthanized.
Results
A stable aneurysm (mean size of all aneurysms, 16.8 ± 1.72 mm × 11.8 ± 2.32 mm) and type Ia endoleak were successfully observed in all swine. A single stent-graft was sufficient in one of the six swine.
Conclusion
A novel technique to create a type Ia endoleak model can be successfully developed in swine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endovascular aortic repair (EVAR) has become a common therapy for the treatment of aortic aneurysms over the last decade; however, several problems associated with EVAR remain in clinical settings. The major problem associated with EVAR is residual endoleak in the long-term period after the procedure. It has been reported that endoleaks occurred in 19.5% of cases after EVAR for a thoracic aortic aneurysm (TAA), and secondary procedures were needed in 79, 24, and 53% of cases for type I, type II, and type III endoleaks, respectively [1]. Type Ia endoleaks are highly associated with aneurysm dilation and rupture compared to other types of endoleaks; thus, it is essential to consider a secondary procedure for type Ia endoleaks. There are two types of secondary treatment for type Ia endoleaks, i.e., endovascular treatment and conventional open surgery; however, if additional treatment is needed, the less invasive endovascular treatment is preferred.
Aortic extension is a form of endovascular procedure that is the first choice of treatment for endoleaks; however, type Ia endoleak is sometimes uncontrollable due to the lack of a sufficient proximal neck. Other procedures, such as direct puncture have been reported but are not common [2,3,4]. Thus, an experimental model of type Ia endoleak is required to evaluate these methods and to develop new treatments.
Materials and methods
Animal preparation
Procedures were performed on the swine (n = 6, Duroc, all female), with a mean weight of 53.7 ± 1.86 kg. After fasting for 24 h, the swine were tranquilized with an intramuscular injection containing a mixture of hydrochloric acid medetomidine (80 μg/kg), butorphanol tartrate (1 mg), and ketamine (5 mg/kg) and then anesthetized using inhalational 2% isoflurane. After endotracheal intubation with an endotracheal tube (7.0 mm, Univent Endotracheal Tube OLB type; Fuji Systems Corporation, Tokyo, Japan), general anesthesia was maintained with 1.5–2% isoflurane. The heart rate, blood pressure, end-tidal carbon dioxide, and blood oxygen saturation were constantly monitored using an anesthetic apparatus. Venous access was established for periprocedural hydration and drug administration. All procedures were performed in a hybrid operating room using an X-ray system (AlluraXper FD20; Philips Healthcare, Best, Netherlands). This study was approved by the Institutional Laboratory Animal Care and Use Committee of Boston Scientific (S16-079, S16-125) and conducted according to the Guidelines for the Proper Conduct of Animal Experiments by the Science Council of Japan.
Vein graft harvesting
Under sterile conditions, the maximum possible length of the left jugular vein was harvested. When the size of the harvested vein was not sufficiently long to create an aneurysm graft, the right jugular vein was used as a substitute. Moreover, when the vein size was insufficient after opening the vein, the two short sides were sewn together to create a wider column (Figs. 1, 2). As the length of the vein graft needs to cover the circumference of the neck width of the aneurysm, the graft needed to be at least 30 mm long, including the suture margins.
a Photograph exhibiting a graft harvested from the left jugular vein (white arrow: jugular vein, black circle: long axis of the vein graft, white circle: short axis of the vein graft). b Photograph exhibiting the columnar graft stitched together on a short axis after folding longitudinally once the graft was cut open (white arrow: jugular vein, black circle: long axis of the vein graft, white circle: short axis of the vein graft). c Photograph exhibiting the exposed aorta (thick black arrow: exposed thoracic aorta, white asterisk: cranial side, black asterisk: caudal side). d Photograph exhibiting the saccular aneurysm created by the vein graft (thick white arrow: saccular aneurysm, white asterisk: cranial side, black asterisk: caudal side)
Creation of a thoracic aneurysm
In the right decubitus position, left thoracotomy was performed in the fourth or fifth intercostal space (Fig. 1). Before opening the pleura, differential lung ventilation was commenced. The thoracic aorta was exposed, and the surrounding tissues were detached from the aorta to secure sufficient space to clamp. After heparin (100 IU/kg) was administered, the aorta was side-clamped and incised longitudinally for approximately three quarters of its diameter. The vein graft was sutured to form a saccular aneurysm using a 5-0 Prolene (ETHICON).
Creation of type Ia endoleak using stent-grafts
After changing the position from the right lateral to the supine position, laparotomy was performed. The iliac artery was taped, and an 8-Fr 11-cm sheath (Medikit Co. Ltd., Tokyo, Japan) was inserted. Aortography was performed with a 5-Fr pigtail catheter (Glidecath, Terumo, Europe NV, Belgium) to confirm the creation of a TAA. We measured the diameter of the aorta and the size of the aneurysm using an aortographic image (Fig. 3). The sheath was then changed to a 12-Fr DrySeal Sheath (W. L. Gore and Associates, Flagstaff, AZ).
a Aortography image before deployment of double-component stent-graft after creation of a thoracic aortic aneurysm (black arrowhead: the thoracic aorta, black arrow: a saccular aneurysm, white arrow: a pigtail catheter). b Fluoroscopic image during precise positioning using the inflated balloon after deployment of double-component stent-graft (white arrowhead: double-component stent-graft, white arrow: inflated balloon catheter). c, d Aortography images of the early (c) and late (d) phases after creating the type Ia endoleak in a thoracic aortic aneurysm (white arrowhead: stent-grafts, black arrow: contrast finding within the aneurysm)
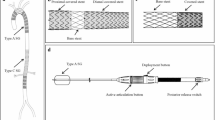
We selected a stent-graft size that was oversized but did not exceed 120% of each aortic diameter. The tapered stent-graft (GORE®EXCLUDER®, W.L. Gore & Associates, Inc., Flagstaff, AZ) that was clinically used as the leg device, was removed from the delivery sheath and remounted after changing the direction to create a reverse-tapered-shaped configuration when released from the delivery sheath in the aorta. In all but one case, we faced the problem that the first stent-graft unintentionally slipped down to the abdominal aorta from the thoracic aorta as soon as it was deployed. Thus, we had to make a contrivance. At first, the second stent-graft (tapered), whose proximal edge was larger than the distal end of the first stent-graft (reverse-tapered), was overlapped with the first stent-graft at the abdominal aorta. This double-component stent-graft was then moved upward with an inflated occlusion balloon catheter (Equalizer, Boston Scientific Corp, Marlborough, MA, USA) and positioned so that the entry site of the aneurysm was covered by a narrow portion of the stent-graft. The stent-graft was elaborately positioned under the guidance of intermittent aortography to confirm the entry site of the aneurysm. Finally, we performed ballooning to secure the stent-graft from slipping at the aneurysm site (Fig. 4). To confirm a type Ia endoleak of the TAA, aortography was performed to measure the aortic diameter, neck width, and sac depth of each aneurysm (Fig. 2). After closing the laparotomy, the swine were awakened with an intramuscular injection of hydrochloric acid atipamezole (200 μg/kg).
Scheme illustrating the method to create a type Ia endoleak model by combining two kinds of stent-grafts. First, a first stent-graft (reverse-tapered), which was a little larger than the native aorta, was placed in the lower level of the thoracic aorta (a). Next, a second stent-graft (tapered) was placed to overlap with the first stent-graft (b). Finally, this double-component stent-graft was moved upward with an inflated occlusion balloon catheter and laid over the anastomotic site of the aneurysm to incompletely cover the site to allow for a type Ia endoleak (c)
On postoperative day 8, the swine were anesthetized using the same method as on the first day of the experiment. In the supine position, a 7-Fr vascular sheath was inserted into the femoral artery. Aortography was performed to confirm residual type Ia endoleak. The swine were euthanized with an intravenous injection of pentobarbital sodium (75 mg/kg). No aortic dissection was observed macroscopically in the extracted aorta.
Results
The surgical procedure described above was accomplished in all the swine. The time taken for the entire process, including the administration of anesthesia, was 438 ± 195 min (240–720), and the time taken to create the TAA was 202 ± 67 min (140–330). The amount of bleeding was extremely minimal in all cases.
A stable saccular aneurysm was successfully created in all the swine. The mean aortic diameter and size of the aneurysm in the six swine were 14.4 ± 0.72 mm and 16.8 ± 1.72 mm (sac depth) × 11.8 ± 2.32 mm (neck width), respectively (Table 1). At autopsy, there was no change in the size of the sac or the shape of the aneurysm compared to its original size.
Details of the stent-graft size are illustrated in Table 2. In four cases, the first stent-graft was selected with a proximal end of 10 mm in one case and 12 mm in the other two cases, whereas the distal end was 16 mm in all cases. A second stent-graft was selected in five cases (proximal end measuring 18 mm in three cases and 20 mm in two cases, and distal end measuring 16 mm in all cases). A second stent-graft was not needed in one case only because the type Ia endoleak could be created using a first stent-graft.
Discussion
The main focus of this study was the creation of a durable type Ia endoleak model in swine, most notably the creation of a thoracic aneurysm via a thoracotomy and precise stent-graft positioning using a double stent-graft component.
There have been several attempts to create animal models of aneurysms over the last 2 decades. Hallisey et al. first reported the creation of an abdominal aortic aneurysm (AAA) by over dilation of the abdominal aorta with a balloon catheter in mongrel dogs [5]. All dogs, except for one in which operational death occurred, were alive for 30 days postoperatively, and aortography revealed that the aneurysms enlarged as time progressed. In a preliminary study, we used the same aortic dilatation technique and attempted to create aortic aneurysms by directly ballooning the thoracic aorta in one swine; however, the aorta ruptured immediately. Uson-Gargallo et al., reported that they created an AAA with gastric serosa; however, two of their five subjects (40%) died postoperatively due to aneurysm rupture. Furthermore, Maynar et al. prepared AAAs using peritoneal patches and reported that 15 of their 27 subjects (56%) died from a rupture within 2 weeks postoperatively [6, 7]. To advance the experiment to the next step, we used a jugular vein graft to create the aneurysm to make it tougher and less susceptible to rupture. In addition, we used a fresh vein graft and not a glutaraldehyde-treated vein graft, because a recent study reported that the intensities of a glutaraldehyde-treated homologous vein graft as a vein substitute in rabbits and an autologous vein graft were equivalent [8]. In our preliminary study, a small aneurysm created in a narrow part of the aorta, such as the abdominal aorta, had become fully thrombosed within the sac at the time of contrast. Therefore, we created an aneurysm in the thoracic aorta instead of the abdominal aorta for two reasons: to make the aneurysm larger and to facilitate stent-grafting. However, regarding the size of the aneurysm, neither a significantly small nor a significantly large aneurysm is good. Based on the experience of a preliminary study in which the sac depth was significantly large and the aneurysm ruptured, we set a guideline of not exceeding 1.5 times the aortic diameter. Depending on the body size of the individual, it is important to refer to the criteria for high-risk saccular aneurysm (sac depth/neck width > 0.8 or vertical diameter/horizontal diameter < 1.0) for appropriate size [9, 10].
Several endoleak models have been reported recently [11,12,13]. Fromageau et al., created aneurysms with a vein patch on the iliac arteries in dogs, which were thereafter subjected to endovascular therapy (EVT). Elastography of the aneurysms was performed, and residual type II endoleaks in the aneurysm sacs were observed by ultrasonography [11]. On one hand, Shi et al. [12] created aneurysms with a vein patch on the cervical arteries of dogs that were thereafter subjected to endovascular repair with a covered stent and were followed-up for 6 months after EVT. Nakai et al. [13] also used the inferior vena cava to create a type II endoleak model, and they performed embolization of the aneurysm using an n-butyl-2-cyanoacrylate-lipiodol-ethanol mixture in swine. The present study differs from these previous studies in two ways. First, we aimed to establish a method of creating a type Ia endoleak model. Second, we created an aneurysm in the thoracic aorta through a thoracotomy in an animal, and this has never been reported before. When attempting to create a type Ia endoleak model using EVAR, it is necessary to leave a small gap between the native aorta and the stent-graft for blood flow to enter the aneurysm. Therefore, a tapered stent-graft with thin and thick parts, such as the leg device was convenient. To make the ideal “reverse taper shape,” the leg device was removed from the delivery sheath once and remounted in reverse. The only commercially available device that can be used considering this clinically uncommon principle is the EXCLUDER leg device (GORE®EXCLUDER®, W.L. Gore & Associates, Inc., Flagstaff, AZ). The first reverse-tapered stent-graft was inserted from the iliac artery into the descending aorta. Next, the second slightly larger stent-graft was inserted into the aorta and positioned such that it overlapped with the first stent-graft. Finally, the complex of the two overlapping stent-grafts was moved to the site of the aneurysm using an inflated balloon catheter. Although the step for creating type Ia endoleak in this process appears complicated at first glance, the reason for using a double stent-graft component was that the first stent-graft would be swept away by the rapid aortic blood flow from the thoracic aorta to the abdominal aorta upon deployment. We believe that moving the stent-graft within the thoracic aorta while the balloon was inflated could damage the aortic intima; therefore, we devised a method to reduce damage to the aortic intima by inflating the balloon on the site of stent-graft overlap. Since we expected to move the stent-graft beforehand, we carefully selected the size of the stent-graft. The stent-graft size was selected so that the thicker portion of the first stent-graft would not exceed 120% of the native aorta, and the thicker portion of the second stent-graft would be larger than 120%, because it would be the landing zone, i.e., the fixed part of the stent-graft. Moreover, the double stent-graft component technique was useful in fine-tuning the stent-graft positioning.
This study has some limitations. First, we used only a small number of swine in view of animal protection. Second, the aneurysms developed using the vein graft are histologically different from real aneurysms, and the shape of all aneurysms was saccular, not fusiform. Third, the damage to the aortic intima caused by movement of the stent-graft with the inflated balloon was not histologically verified. Finally, the stent-grafts that we used were of the quick-release type, and selection of appropriate sizes was limited due to commercial availability. Thus, considering the limitations of our study, the development of a new, balloon-expandable, and reverse-tapered shape stent-graft might be further improved in the future. When not relying on the use of another device, rapid pacing might prevent the stent-graft from slipping down.
In conclusion, a durable type Ia endoleak model after EVAR for TAA was successfully created in swine using a novel technique. This model is expected to be clinically applicable in the future as it can be used to develop novel techniques for additional treatment of type Ia endoleaks after EVAR.
References
Morales JP, Greenberg RK, Lu Q, Cury M, Hernandez AV, Mohabbat W, et al. Endoleaks following endovascular repair of thoracic aortic aneurysm: etiology and outcomes. J Endovasc Ther. 2008;15:631–8.
Kreusch AS, Samuels S, Benenati JF, Schernthaner M, Uthoff H. Direct percutaneous sac injection for treatment of a thoracic type II endoleak. J Vasc Interv Radiol. 2013;24:1071–3.
Bangard C, Franke M, Pfister R, Deppe AC, Matoussevitch V, Maintz D, et al. Thoracic type Ia endoleak: direct percutaneous coil embolization of the aortic arch at the blood entry site after TEVAR and double-chimney stent-grafts. Eur Radiol. 2014;24:1430–4.
Katada Y, Kondo S, Tsuboi E, Nakamura K, Rokkaku K, Irie Y. Type IA endoleak embolization after TEVAR via direct transthoracic puncture. Jpn J Radiol. 2015;33:169–72.
Hallisey MJ. A transluminally created abdominal aortic aneurysm model. J Vasc Interv Radiol. 1997;8:305–12.
Usón-Gargallo J, Crisóstomo V, Loscertales B, Sun F, Sánchez-Margallo FM, Martín-Cancho MF, et al. A new model of abdominal aortic aneurysm with gastric serosa patch: surgical technique and short-term evaluation. J Invest Surg. 2006;19:97–104.
Maynar M, Qian Z, Hernandez J, Sun F, DeMiguel C, Crisostomo V, et al. An animal model of abdominal aortic aneurysm created with peritoneal patch: technique and initial results. Cardiovasc Intervent Radiol. 2003;26:168–76.
Moura R, Maffei FH, Mattar L, Fabris VE, Cury P, Lastória S, et al. Glutaraldehyde-treated homologous vein graft as a vein substitute: experimental study in rabbits. Int Angiol. 2009;28:113–9.
Natsume K, Shiiya N, Takehara Y, Sugiyama M, Satoh H, Yamashita K, et al. Characterizing saccular aortic arch aneurysms from the geometry-flow dynamics relationship. J Thorac Cardiovasc Surg. 2017;153:1413–20.
Akai T, Hoshina K, Yamamoto S, Takeuchi H, Nemoto Y, Ohshima M, et al. Biomechanical analysis of an aortic aneurysm model and its clinical application to thoracic aortic aneurysms for defining “saccular” aneurysms. J Am Heart Assoc. 2015;19:4.
Fromageau J, Lerouge S, Maurice RL, Soulez G, Cloutier G. Noninvasive vascular ultrasound elastography applied to the characterization of experimental aneurysms and follow-up after endovascular repair. Phys Med Biol. 2008;53:6475–90.
Shi WY, Gu JP, Li MH, Yan L, He X. The predictors of endoleaks after endovascular repair of experimentally produced fusiform carotid aneurysm in canine. Minim Invasive Ther Allied Technol. 2016;25:99–106.
Nakai M, Ikoma A, Loffroy R, Midulla M, Kamisako A, Higashino N, et al. Type II endoleak model creation and intraoperative aneurysmal sac embolization with n-butyl cyanoacrylate-lipiodol-ethanol mixture (NLE) in swine. Quant Imaging Med Surg. 2018;8:894–901.
Acknowledgements
We thank K. Sugimoto from the Fukushima Medical University for assisting us with the histopathological examinations. This study was supported by the staff of the Boston Scientific Institute for advancing science: Takahiro Uryu, Koichi Yamaguchi, Naoki Sako, Kota Yoshida, and Keisuke Takahashi. We would like to thank Editage (www.editage.jp) for the English language editing.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI (grant number JP15K19777).
Author information
Authors and Affiliations
Contributions
TT, YK, NK and SO designed and performed the research study; TT and HY wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics guidelines
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Takano, T., Katada, Y., Komaki, N. et al. A technique for creating an experimental type Ia endoleak model in the thoracic aorta of swine. Jpn J Radiol 39, 1127–1132 (2021). https://doi.org/10.1007/s11604-021-01144-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-021-01144-2