Abstract
Purpose
To assess the use of virtual monochromatic images (VMI) for discrimination of affected and non-affected bowel walls in patients with Crohn’s disease (CD) as well as to compare mural enhancement between patients with and without CD.
Materials and methods
This retrospective study included 61 patients (47 with CD, 14 without CD). Attenuation value (AV), signal-to noise ratio (SNR), and contrast-to-noise ratio (CNR) were obtained at VMI energy levels from 40 to 110 keV in 10 keV increment. Analyses were performed among affected and non-affected bowel walls in CD patients, as well as from bowel walls in patients without CD. Image quality and mural enhancement were evaluated at VMI energy levels at 40, 70, and 110 keV.
Results
At all energy levels of VMI, each quantitative data for AV, SNR, and CNR showed statistically significant difference between diseased and non-diseased bowel walls in CD patients. In the quantitative assessment of patients with and without CD, the optimal AV and SNR were obtained at 40 keV, and the optimal CNR was obtained at 70 keV. For the qualitative assessment, the best image quality and mural enhancement were obtained at 70 keV and 40 keV, respectively.
Conclusion
VMI are helpful for the differentiation of affected bowel walls in CD patients, providing high diagnostic accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease characterized by skip lesions and transmural inflammation [1]. With the improvement of multidetector computed tomography (CT), CT enterography (CTE) has become an important imaging modality of choice for evaluating CD [2, 3]. The main diagnostic purpose of CTE in the setting of CD is to differentiate active inflammation from fibrotic bowel strictures, thus enabling the most appropriate treatment. Bowel wall enhancement is the most sensitive indicator of active CD [4, 5]. Therefore, differences in mural enhancement can be used to differentiate diseased bowel from non-diseased bowel [6].
In conventional CT scanning, polychromatic X-ray beam, which consists of photons with a wide range of energies, is used. The tube peak voltage (kVp) that adjusts the upper limit of the X-ray energy spectrum affects tissue contrast [7]. However, in dual-energy CT, data are simultaneously obtained from high- and low-energy X-ray spectra [8, 9]. Dual-energy CT data can be demonstrated using non-material-specific, material-specific, and energy-specific display methods [10, 11]. In energy-specific methods, virtual monochromatic images (VMI) can be reconstructed. Virtual monochromatic images simulate Hounsfield unit (HU) values that correspond to imaging obtained using an X-ray beam composed of a single-energy value [12]. Virtual monochromatic images show alterations in HU values depending on the different energy levels of reconstruction [13]. Kiloelectron volt (keV) is used to report the X-ray energy instead of kVp [14]. Kiloelectron volt values can be used to maximize contrast between structures as lower keV images can make the iodine content within structures more prominent, while higher keV images can trivialize the iodine content [12, 15, 16].
Virtual monochromatic images have many applications in the clinical practice, including beam-hardening correction, metal artifact reduction, and optimization of image quality [17]. It is suggested that the use of optimal VMI can provide improved image quality than that obtained with a conventional single-energy 120-kVp technique [18, 19]. On the other hand, noise is an important factor to be considered when selecting the optimal reconstructed monochromatic energy level [17].
It has been demonstrated that VMI may supply more reliable attenuation values (AV) than conventional polychromatic imaging [19, 20]. Thus compared to conventional CT, dual-energy CT may improve conspicuity and enable additional information for assessment by accentuating distinctions in bowel wall enhancement [10]. However, reports of VMI of dual-energy CTE for the evaluation of patients with CD are limited. Therefore, the first objective of our study was to quantitatively assess AV, signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) of the affected and non-affected bowel segments in patients with CD using VMI at different energy levels to assign the optimal keV for the assessment of bowel wall enhancement. The second aim was to compare the mural enhancement between patients with and without CD using VMI.
Materials and methods
Study population
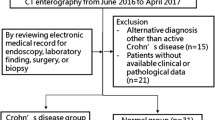
This retrospective study was approved by the instutional ethics committee and the requirement for informed consent was waived. CTE scans that were obtained between June 2016 and September 2017 in patients with known or suspected CD were reviewed.
Inclusion criteria were as follows: (a) adult patients (> 18 years old), (b) patients who underwent dual-energy CTE due to suspected CD, (c) patients who had clinical, laboratory, and histopathological data at the time of CTE including time interval 1 month after the scan.
All patients with imaging findings of CD that were identified on CTE underwent ileocolonoscopy. The electronic medical records of patients regarding findings of ileocolonoscopy, operation, histopathology, and laboratory were reviewed.
Dual-energy CTE acquisition and image reconstruction
All CTE scans were acquired using a single-source 64-slice rapid kV-switching dual-energy CT scanner (Discovery CT750 HD; GE Healthcare, WI, USA). To achieve better bowel distention in patients who underwent CTE, a lactulose solution (Duphalac; Abbott Biologicals, The Netherlands) was used. All patients were asked to drink 1500 mL of this solution diluted with water during 1 h prior to CTE examination. A bolus of intravenous iodinated contrast agent was administered at a rate of 3 mL/s followed by saline solution. The datasets were obtained in the portal venous phase by applying 65-s delay following intravenous administration of contrast agent with the bolus tracking method. CTE scans were acquired with spectral imaging mode, with fast tube voltage switching between 80 and 140 kVp on adjacent views during each single rotation.
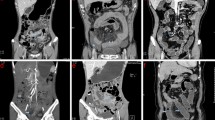
A tube current of 400 mA was used if the patient weight was less than 70 kg. Tube current was changed to 600 mA if the patient weight was more than 70 kg. Images were acquired using a 40 × 0.625 mm collimation, a 0.5-s rotation time, and pitch/speed of 1.375:1/55. Images were reconstructed on axial and coronal planes. CTE studies were transferred through the picture archiving computer system (PACS) to a workstation (AW Server, GE Healthcare, WI, USA). Using the Gemstone Spectral Imaging (GSI) viewer application, scans were reconstructed at VMI energy levels including 40 keV, 50 keV, 60 keV, 70 keV, 80 keV, 90 keV, 100 keV, and 110 keV (Fig. 1).
Quantitative image analysis
For patients with CD, abnormal or diseased bowel wall was radiologically identified when hyperenhancement of the bowel mucosa or bowel wall thickening were demonstrated [4, 12]. Qualitatively, mural hyperenhancement was determined by visual assessment of segmental hyperenhancement of the bowel wall comparing with the adjacent bowel loop with similar degree of distension. Quantitatively, hyperenhancement was evaluated using a diseased to non-diseased bowel loop enhancement ratio of more than 1.3 [4]. Two separate circular regions of interest (ROI) (area, 0.5 cm2) were placed within the diseased intestinal wall. A third ROI (area, 0.5 cm2) was placed within the gluteal muscle groups (Fig. 2). The ROI was placed away from tissue planes, intramuscular fat or calcifications as much as possible. A mean bowel attenuation was calculated from the AV that were obtained using two separate ROIs within the bowel wall. The standard deviation of the mean AV in the ROI represented the noise. The signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated for each dataset at each VMI from 40 to 110 keV. SNR was measured by dividing the mean AV of the diseased intestinal wall by the mean background noise. CNR was defined as the HU difference between the mean AV of the diseased bowel wall and gluteal muscle divided by the mean noise of the muscle.
Measurement of attenuation from bowel wall and gluteal muscle. Two circular regions of interest (ROI) (area 0.5 cm2) were placed within the diseased intestinal walls and the mean was obtained (one ROI placed within the diseased bowel wall is not shown in the figure). Another ROI (area, 0.5 cm2) was placed within the gluteal muscle
Similarly, ROIs were placed within the non-diseased bowel walls of patients with CD to assess bowel wall attenuation. SNR and CNR were calculated accordingly at each keV. In addition, for patients without CD, AV, SNR and CNR were obtained from bowel walls at VMI energy levels from 40 to 110 keV using the same approach.
Qualitative image analysis
Two radiologists, who were blinded to clinical data, performed qualitative image analysis of VMI at 40 keV, 70 keV, and 110 keV. Subjective analysis parameters included image quality, mural enhancement and subjective noise. Image quality was scored out of 5 where 1 = nondiagnostic, 2 = reduced image quality but interpretable, 3 = moderate image quality, 4 = good image quality, and 5 = excellent image quality. Mural enhancement was graded out of 5 where 1 = nondiagnostic, 2 = poor contrast, 3 = good contrast, 4 = very good contrast, and 5 = excellent contrast. Subjective noise was classified out of 5 where 1 = nondiagnostic, 2 = significant noise affecting diagnosis, 3 = noisy but diagnostic, 4 = minimal noise, and 5 = absence of noise.
Statistical analysis
AV, SNR, and CNR at different energy levels for diseased and non-diseased bowel walls in CD patients were compared using paired samples test. Receiver-operating characteristic (ROC) analysis was used to compare patients with and without CD in terms of AV, SNR, and CNR across different energy levels from 40 to 110 keV. Friedman test and Dunn’s test were used in the evaluation of qualitative image analysis. A p value of < 0.05 was considered to be statistically significant. All statistical analyses were assessed using IBM SPSS Statistics 23.0 (IBM Corp., Armonk, NY, USA).
Results
Of the 68 adult patients who were identified, 5 who did not have available clinical or pathological data and 2 who had an alternative diagnosis other than CD were excluded. The study included a total of 61 patients with and without CD. 47 patients (24 men and 23 women; median age 46 years, range 22–75 years) were diagnosed as having active CD on CTE and the affected bowel walls seen on CT were confirmed by ileocolonoscopy. Of 47 patients with CD, 11 underwent surgery. The remaining 14 patients (7 men and 7 women; median age 35.5 years, range 24–79 years) who underwent CTE due to suspected CD, had a negative diagnosis of CD with no specific finding of bowel disease.
Quantitative image analysis
The difference between affected and non-affected bowel walls of patients with CD at each reconstructed VMI for AV, SNR, and CNR are presented in Table 1. AV, SNR, and CNR were detected to be higher in diseased bowel walls than non-diseased bowel walls (Fig. 3). The difference between the abnormal and normal intestinal walls of CD patients in terms of attenuation, SNR, and CNR were statistically significant at each energy level of VMI (p < 0.001).
AV, SNR, and CNR of the diseased bowel walls of CD patients were compared with those that were obtained from the bowel walls of patients without CD. The area under ROC curve (AUC) across VMI from 40 to 110 keV for the evaluation of AV, SNR, and CNR are detailed in Table 2. In the assessment of patients with and without CD, 40 keV [(AUC): 0.913; 95% confidence interval (CI) 0.843–0.984)] provided the best AV. Optimal SNR and CNR values were observed at 40 keV (AUC: 0.807; 95% CI 0.679–0.935), and 70 keV (AUC: 0.906; 95% CI 0.82–0.987), respectively.
Qualitative image analysis
The subjective evaluation of image quality, mural enhancement, and noise is detailed in Table 3. Highest subjective scores for the assessment of image quality were observed at 70 keV. There were significant differences of image quality between the comparison of 40–110 keV and 70–110 keV (p = 0.006 and p < 0.001, respectively). Comparisons between 40 keV and both 70 keV and 110 keV in terms of mural enhancement were statistically significantly different (p < 0.001). Subjective noise significantly differed when both 40–70 keV and 40–110 keV were compared (p < 0.001).
Discussion
The rapid kV-switching dual-energy CT scanner that was used in our study has a single X-ray source that switches the voltage settings during a single gantry rotation. This configuration provides full field-of-view imaging. The dual-source dual-energy CT scanner uses two X-ray sources that operate at different tube potentials. This CT system enables to reduce the risk of spatial and temporal misregistration. One of the tubes that is located closer to the patient has a slightly smaller field-of-view, which might be a limitation of this system. The dual-layer or spectral detector dual-energy CT scanner grants spectral separation of the X-ray beam at the detector level. This arrangement allows for full field-of-view imaging and co-registration of the low- and high-energy data. The sequential acquisition dual-energy CT scanners changes kVp after each revolution of the CT gantry. The major limitation of this system is the overall long acquisition times. The split filter dual-energy CT scanner uses a single X-ray source and filters that block high and low keV photons, creating two different photon energy spectra in the z-axis. This CT system enables full field-of-view imaging; however, it usually requires a slow table speed and provides less robust spectral separation than other configurations [17, 21].
Our study demonstrated that there were substantial differences between the affected bowel walls and non-affected bowel walls in patients with CD at each energy level of VMI with regard to AV, SNR, and CNR. Dual-energy CT would be assumed to improve the conspicuity of distinctions in bowel wall attenuation with iodine quantification postprocessing techniques [22]. De Kock et al. reported that iodine quantification on dual-energy CTE can be used in distinguishing normal bowel from active inflammatory CD [23]. In another study, iodine concentration measured on spectral-detector-based dual-energy CTE found to be a convenient biomarker to monitor disease activity in CD [24]. However, there is relatively limited literature on the use of VMI for the evaluation of diseased bowel segments in patients with CD. Our study shows that VMI at different energy levels provide the advantage of differentiating abnormal bowel from normal bowel in CD patients.
In our study, the ideal CNR for detecting the enhancement of intestinal walls of patients with and without CD were obtained at 70 keV VMI. Previous studies suggested that the optimal iodine CNR is achieved at 60–70 keV, using either single-source or dual-source CT systems [17, 18, 20]. In a study that evaluated bowel wall enhancement in acute small bowel obstruction, dual-source dual-energy CT with 70 keV maximized the CNR of mural enhancement [25]. However, Lee et al. reported that 40 keV VMI on dual-layer dual-energy CT system yielded the best CNR for both affected intestinal walls of patients with CD and normal bowel walls of patients without CD [26]. They explained that the enhanced contrast at 40 keV might have replaced the increased noise that occurs in low keV images. It was suggested that the projection-based algorithm which was used by the spectral detector CT might have effectively reduced the noise at low keV VMI [26]. Future studies that will be comparing the different types of dual-energy CT systems will provide information to validate the results in the evaluation of mural enhancement.
VMI at 40 keV provided the best AV for bowel wall despite the increased noise in our study. Additionally, for the subjective analysis for image quality and mural enhancement, VMI at 40 keV and 70 keV were helpful to radiologists in the evaluation of patients with CD. In our study, VMI at a high-energy level like 110 keV did not make a significant difference for the assessment of bowel walls of CD patients. It might be attributed to the progressively increased noise at a rate that is inversely proportional to the energy separation between the low- and high-energy spectra [18, 20, 27]. This is also observed at VMI at energy levels lower than 60 keV; however, VMI at lower energy levels might contribute more effectively to the diagnosis of mural enhancement compared to VMI at higher energy levels.
There are several drawbacks in our study. First, the number of patients was comparatively small. Thus, studies with larger sample size using different dual-energy CT scanners are required to confirm the results. Second, placing ROIs within the thin bowel walls was challenging but efforts were made to accurately place uniform ROI for calculation. Two radiologists performed qualitative image analysis unanimously and the lack of interreader correlation was another limitation. Creation of post-processed images requires additional work and time, and this may seem as a limitation in busy clinical practice. However, VMI at 40 keV and 70 keV may be helpful in the evaluation of mural enhancement on CTE in CD patients without requiring additional radiation dose or contrast media.
In conclusion, VMI of dual-energy CTE are helpful in distinguishing diseased bowel walls from non-diseased bowel walls of patients with CD by assessing mural enhancement quantitatively. For the evaluation of patients with and without CD, the optimal AV and CNR of bowel walls are obtained using VMI at 40 keV and 70 keV, respectively.
References
Tochetto S, Yaghmai V. CT enterography: concept, technique, and interpretation. Radiol Clin North Am. 2009;47:117–32.
Elsayes KM, Al-Hawary MM, Jagdish J, Ganesh HS, Platt JF. CT enterography: principles, trends, and interpretation of findings. Radiographics. 2010;30:1955–70.
Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, et al. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128–34.
Bodily KD, Fletcher JG, Solem CA, Johnson CD, Fidler JL, Barlow JM, et al. Crohn disease: mural attenuation and thickness at contrast-enhanced CT enterography–correlation with endoscopic and histologic findings of inflammation. Radiology. 2006;238:505–16.
Booya F, Fletcher JG, Huprich JE, Barlow JM, Johnson CD, Fidler JL, et al. Active Crohn disease: CT findings and interobserver agreement for enteric phase CT enterography. Radiology. 2006;241:787–95.
Macari M, Balthazar EJ. CT of bowel wall thickening: significance and pitfalls of interpretation. AJR Am J Roentgenol. 2001;176:1105–16.
Yeh BM, Shepherd JA, Wang ZJ, Teh HS, Hartman RP, Prevrhal S. Dual-energy and low-kVp CT in the abdomen. AJR Am J Roentgenol. 2009;193:47–54.
Johnson TR. Dual-energy CT: general principles. AJR Am J Roentgenol. 2012;199:S3-8.
Karçaaltıncaba M, Aktaş A. Dual-energy CT revisited with multidetector CT: review of principles and clinical applications. Diagn Interv Radiol. 2011;17:181–94.
Silva AC, Morse BG, Hara AK, Paden RG, Hongo N, Pavlicek W. Dual-energy (spectral) CT: applications in abdominal imaging. Radiographics. 2011;31:1031–50.
Fornaro J, Leschka S, Hibbeln D, Butler A, Anderson N, Pache G, et al. Dual- and multi-energy CT: approach to functional imaging. Insights Imaging. 2011;2:149–59.
Fulwadhva UP, Wortman JR, Sodickson AD. Use of dual-energy CT and iodine maps in evaluation of bowel disease. Radiographics. 2016;36:393–406.
Mileto A, Barina A, Marin D, Stinnett SS, Roy Choudhury K, Wilson JM, et al. Virtual monochromatic images from dual-energy multidetector CT: variance in CT numbers from the same lesion between single-source projection-based and dual-source image-based implementations. Radiology. 2016;279:269–77.
Kalender WA, Perman WH, Vetter JR, Klotz E. Evaluation of a prototype dual-energy computed tomographic apparatus. I Phantom studies Med Phys. 1986;13:334–9.
Zhao LQ, He W, Li JY, Chen JH, Wang KY, Tan L. Improving image quality in portal venography with spectral CT imaging. Eur J Radiol. 2012;81:1677–81.
Albrecht MH, Vogl TJ, Martin SS, Nance JW, Duguay TM, Wichmann JL, et al. Review of clinical applications for virtual monoenergetic dual-energy CT. Radiology. 2019;293:260–71.
Marin D, Boll DT, Mileto A, Nelson RC. State of the art: dual-energy CT of the abdomen. Radiology. 2014;271:327–42.
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S. Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology. 2011;259:257–62.
Yu L, Leng S, McCollough CH. Dual-energy CT-based monochromatic imaging. AJR Am J Roentgenol. 2012;199:S9-15.
Yu L, Christner JA, Leng S, Wang J, Fletcher JG, McCollough CH. Virtual monochromatic imaging in dual-source dual-energy CT: radiation dose and image quality. Med Phys. 2011;38:6371–9.
Yeh BM, Obmann MM, Westphalen AC, Ohliger MA, Yee J, Sun Y, et al. Dual energy computed tomography scans of the bowel: benefits, pitfalls, and future directions. Radiol Clin North Am. 2018;56:805–19.
Murray N, Darras KE, Walstra FE, Mohammed MF, McLaughlin PD, Nicolaou S. Dual-energy CT in evaluation of the acute abdomen. Radiographics. 2019;39:264–86.
De Kock I, Delrue L, Lecluyse C, Hindryckx P, De Vos M, Villeirs G. Feasibility study using iodine quantification on dual-energy CT enterography to distinguish normal small bowel from active inflammatory Crohn’s disease. Acta Radiol. 2019;60:679–86.
Kim YS, Kim SH, Ryu HS, Han JK. Iodine quantification on spectral detector-based dual-energy CT enterography: correlation with Crohn’s disease activity index and external validation. Korean J Radiol. 2018;19:1077–88.
Darras KE, McLaughlin PD, Kang H, Black B, Walshe T, Chang SD, et al. Virtual monoenergetic reconstruction of contrast-enhanced dual energy CT at 70 keV maximizes mural enhancement in acute small bowel obstruction. Eur J Radiol. 2016;85:950–6.
Lee SM, Kim SH, Ahn SJ, Kang HJ, Kang JH, Han JK. Virtual monoenergetic dual-layer, dual-energy CT enterography: optimization of keV settings and its added value for Crohn’s disease. EurRadiol. 2018;28:2525–34.
Zhang D, Li XH, Liu B. Objective characterization of GE discovery CT750 HD scanner: gemstone spectral imaging mode. Med Phys. 2011;38:1178–88.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Guler, E., Unal, N.G., Hekimsoy, I. et al. Dual-energy CT enterography in evaluation of Crohn’s disease: the role of virtual monochromatic images. Jpn J Radiol 39, 341–348 (2021). https://doi.org/10.1007/s11604-020-01065-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-01065-6