Abstract
Double-layer (DL) electrodialysis cation exchange membranes (CEMs) were fabricated for copper ion removal from water. New membranes were prepared by deposition (chitosan-co-TiO2 nanoparticles) layer on polyvinyl chloride–based heterogeneous CEMs. The FTIR, FESEM, energy-dispersive X-ray analysis, and 3D-surface images were applied for membrane characterization. Membrane hydrophilicity, flux, conductivity, ion exchange capacity, permselectivity, current efficiency, energy consumption, and the reusability of membranes were studied. FTIR spectra confirmed formation of DL membrane decisively. FESEM and 3D-surface images also showed compact structure and smoother surface for the new DL membrane. The sodium flux exhibited a reducing trend for the DL membranes, whereas dialytic rate of copper ions showed an increasing behaviour. The modified membrane showed higher permselectivity in copper ion removal. Moreover, double-layer membranes showed high reusability potential after the regeneration. A negligible change was considered on dialytic rate for the cleaned membranes after regeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The treatment of seawater is an attractive way to increase the amount of fresh water and reduce water shortage tension. Many reported researches highlighted various water treatment methods to provide required water for utilizing in developed industries [1,2,3,4,5]. Membrane filtration processes are widely used as active separators for the aim [6,7,8,9]. Among these, ultrafiltration and nanofiltration as pressure-driven kinds separate multivalent ions, whereas they do not have enough ability to monovalent ions removal which leads to a lower degree of purification [10]. However, the only water molecules pass through the reverse osmosis membrane process, but the higher concentration of dissolved salt in the concentrate is usually discharged into the sea and causes environmental problems, and requires appropriate treatment [11]. Electrodialysis (ED) is an active process in not only brackish water desalination but also has utilized for industrial applications as well as manufacturing chemical products such as waste acids treatment or chlor-alkali processes. The base of this membrane separation technology is the selective migration of ions through the ion exchange membrane (IEM) [12]. To obtain cost-effective and highly efficient separation systems, ED undergoes some changes in operating conditions, geometrical correction of membrane, performance of electrodes, and IEM properties. The IEMs are subject to various modification techniques that can be classified as follows: (a) introducing organic/inorganic additives nanomaterials with special properties such as hydrophilic, electrical charge, and absorptivity in the polymeric membrane matrixes; (b) study on the use of a variety of ionic functional groups such as phosphonic acid, sulfonic acid, and carboxylic acid in the matrix of IEMs; (c) blend of different types of polymers in the membrane fabrication is employed to control the pores and the porosity of the membrane and to change the mechanical strength and hydrophobicity of the matrix; and (d) surface modification is another strategy by applying a thin modifier layer on the surface of the IEM [13,14,15,16,17,18,19]. Among these methods, surface modification by different techniques such as interfacial polymerization, surface coating, plasma treatment, grafting, ion implantation, and electrodeposition is one of the effective methods to increase the efficiency of IEMs. In these methods, a thin and uniform layer is bonded to the membrane surface to enhance the chemical properties. These changes include improvements in ion flux, hydrophilicity or hydrophobicity, surface charge density, conductivity and permselectivity, chemical and thermal stabilities, antibacterial and anti-organic-fouling property, surface adsorption, lower energy consumption, electrical resistance, and ultimately lower the cost of water treatment [20, 21]. Many studies reported the modification of membrane surfaces to enhance membrane properties and performance. The hydrogel, PANI, PAA-co-MWCNT, and acrylic acid/methyl methacrylate as some modifier layers were applied on the IEM surface to improve the ionic interactions with the membrane surface and control ionic percolation [14, 15]. Zhu et al. [22] studied the effect of different cross-linkers, including on the surface of CEMs. The modified membranes showed smoother surfaces and denser structures. The selectivity of membranes depends on the conjunction of many interlinked and important factors such as the created narrow ion transport channels and the changes in the exchanges sites concentration. NaCl rejection was reached (91.7%). Afsar et al. [23] prepared the novel polyvinyl alcohol (PVA)-based membranes that were prepared with cationic and anionic layers. They used from quaternized poly (2, 6-dimethyl-1, 4-phenylene oxide) and sulfonated poly (2, 6-dimethyl-1, 4-phenylene oxide) as a cationic and anionic layer, respectively. The deposited layers led to enhance permselectivity of the modified membranes. Hosseini et al. [15] use from poly(acrylic acid)-co-poly(methyl methacrylate) as surface modifier of poly(vinyl chloride)-based membranes by emulsion/graft polymerization technique. These membranes showed significant improvement in electrochemical properties such as selectivity, surface charge density, permeability, and ion exchange capacity.
Chitosan, as a multifunctional and biopolymer because of its high hydrophilicity, the existence of carboxyl and amino functional groups inside the backbone can be helpful in the preferential sorption and modification of IEMs [24,25,26]. Amino and hydroxyl functionalization can grow the reactivity of the CEMs surface, leading to an increase of Donnan potential and water affinity. Furthermore, the adsorption of heavy metal ions enhances. Low-cost chitosan as an abundant biopolymer was considered in the IEMs [27]. Hosseini et al. [28] fabricated the LBL CEMs to separate heavy metals by ED. Chitosan-co-activated carbon nanoparticles were applied as a coating layer on the PVC-based heterogeneous CEM. The LbL membranes indicated a more hydrophilic and smoother surface than the pristine membrane. These membranes showed a high capacity to remove heavy metals such as Cu2+, Pb2+, and Ni2+. Ebrahimi et al. [29] synthesized activated carbon-co-chitosan composite nanoparticles. The various concentrations of synthesized nanoparticles were employed in CEMs modification. The prepared membranes exhibited suitable capacity in Cu2+ removal. Salehi et al. [30] used the combination of chitosan and Fe3O4 nanoparticles for the surface modification of sulfonated polyvinyl chloride (SPVC) CEMs. The electrochemical properties of SPVC membranes were enhanced. The membrane permeability and permselectivity showed increasing trend. Tufa et al. [31] synthesized polypyrrole (PPy)/chitosan (CS) composites for the surface modification of cation exchange membranes by chemical polymerization. The modified membrane’s monovalent selectivity (Na +) enhanced threefold compared to the neat membranes.
Besides, nanoparticles have also been widely utilized to improve the properties of IEMs, such as thermal resistance, antifouling, chemical reactivity, mechanical properties, and chemical stability [30, 32]. Metal oxide nanoparticles, magnetic nanomaterials, graphene-based materials, multiwalled nanotubes, metal–organic framework-based materials (MOF), etc., have been of interest to many over the years. Among the most employed nanometal oxides (NMOs), titanium dioxide (TiO2) is particularly interested in its exemplary performance, such as good chemical stability, and high hydrophilicity and adsorption capacity. Studies have shown that adding nanoparticles improves the membrane surface and structure properties [33]. The use of TiO2 and modified TiO2 nanoparticles indicated the TiO2 capacity in desalination and wastewater treatment [34,35,36,37].
The synthesis of TiO2-based nanoparticles for the application in ion-exchange membranes showed higher pure water uptake, higher ion exchange capacity, and ionic permeability than the pristine membrane. The modified membranes showed superior permselectivity up to ∼99% [38]. Nemati et al. [39] investigated the different concentrations of TiO2 nanoparticles into the PVC as an anion exchange membrane matrix. The ion exchange capacity, membrane permselectivity, and transport number were enhanced by use of nanoparticles.
In the current study, double-layer electrodialysis cation exchange membranes were fabricated for copper ions removal from water. New membranes were prepared by deposition (chitosan-co-TiO2 nanoparticles) layer on polyvinyl chloride-based heterogeneous CEMs. To our knowledge, no research was found to fabrication of double-layer (Cs-TiO2 nanoparticles)/PVC-based CEM for the application in ED process in copper removal from water. The prepared membranes were characterized by exploiting FTIR, FESEM, and energy-dispersive X-ray (EDX) analysis. Furthermore, electrochemical properties, antifouling, and energy consumption of membranes were studied by a continuous electrodialysis pilot.
Materials and method
Materials
Polyvinylchloride (PVC) (S-7054, 490 g/l) was provided by Bandar Imam Petrochemical Company (BIPC), Iran. Chitosan (Cs, ACROS Inc., USA) and titanium oxide nanoparticles (TiO2, nanopowders, mean size∼60 nm, SSA > 55 m2/g) was also used in membranes fabrication. The glutaraldehyde aqueous solution (GA, Grade II, 25%, Singapore) also used as cross linker. Tetrahydrofuran (THF, 72.11 g/mol) provided by Merck Inc. was applied as solvent. Strongly acidic cation exchange resin supplied from Merck Inc., Germany was also used. A heterogeneous anion-exchange membrane (RALEX® AMH-PES), made by MEGA, Czech Republic, was applied during the ED process. Distilled water was used throughout the experiments. All other chemicals were purchased from Merck Inc, Germany. The electrochemical properties of used anionic membrane are given in Table 1.
Membrane preparation
The cation exchange membranes were fabricated by casting solution method. Firstly, PVC was dissolved in THF by a mechanical stirrer for 4 h. Then, pulverized fine resin particles added to the prior solution. The concentration of polymeric solution was (20:1) (v/w), and the ratio of used resin was (1:1) (w/w). Then, the polymeric solution was cast on a clean and dry glass plate at ambient temperature. After membranes drying at ambient temperature, dipped in deionized water. Finally, produced membranes were placed into 0.5 M NaCl solution.
Fabrication of DL membranes
For the first step, PVC-based CEMs were immersed into a polymeric solution of 1 wt% chitosan at ambient temperature for 20 min, and then sonicated for 20 min using an ultrasonic apparatus (Parsonic, IRAN). At the next step, treated membranes were placed into various solutions containing different percentages of TiO2 (Table 2), glutaraldehyde (2 wt%), and hydrochloric acid (1.4 ml). Then, modified membranes were placed in the oven for 2 h at 60 °C to form intra-crosslinking reactions and better compatibility between the membrane surface and Cs chains. Finally, all membranes were washed and immersed in distilled water for 4 h. The membrane thickness was measured around 80–90 μm by a digital caliper device.
Experimental continuous electrodialysis test cell
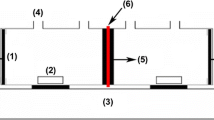
A simplified flow diagram of a continuous electrodialysis unit is shown in Fig. 1. The used equipment consists of two tanks, two pumps, and two flow meters, in which the electrolyte solution and the dilution feed are contained. The cell is packed with a pair of IEMs and a pair of electrodes (cathode and anode). Both electrodes are made of pure platinum and are connected to the DC power supply with 6 V of cell voltage. Each compartment of experimental cells was made of Plexiglas Spacer in the length of 3 mm and sealed by a rubber gasket with a 10 cm2 effective area. In all the experiments, sodium sulfate was used for the electrode chambers solution, and the outlet flow rate of the dilute and concentrated solutions was 30 cc/min.
Membrane characterization
Fourier transform infrared spectroscopy (FTIR) spectra were taken to obtain information about the chemical structure of prepared membranes. The morphology of membranes was also investigated by using Field Emission scanning electron microscopy (FESEM) analysis. Energy-dispersive X-ray analysis (EDX) is an analytical technique to apply in the chemical characterization or elemental analysis of membrane samples.
Surface hydrophilicity and water uptake studies
The interaction between membrane-solutes and membrane-solvents depends mainly on their hydrophobic or hydrophilic nature. For this purpose, a contact angle and water content measurements are done. In this case, the contact angle between water and membrane surface was measured using a contact angle measuring instrument to evaluate the membrane surface hydrophilicity. Deionized water was used as the probe liquid in all measurements. The contact angle was measured in five random locations for each sample to minimize the experimental error, and then, their average was reported. All experiments were carried out in ambient conditions. The water content was measured as the weight difference between the dried and swollen membranes. The wet membrane was weighed (OHAUS, Pioneer ™, Readability: 10−4 g, OHAUS Corp.) and then dried in an oven until a constant weight was obtained. The following equation (Eq. 1) can be used in water content calculation [40]:
Membrane electrochemical properties
The flux of ions (J) through the membrane could be evaluated by Eq. 2 as follows [41]:
where J is the ionic flux (mol m−2 s−1), Δn is the number of migrated ions (mol), t is the electrodialysis time (s), A is the membrane’s surface area (m2), tm is the membrane \(\mathrm{EC}=\frac{{\int }_{t=0}^{t=t}I\times V\times dt}{\Delta m}\) transport number, I is the current density (A), and F is the Faraday constant (96,485 As/mol), respectively.
Energy consumption (EC) of the electrodialysis process is determined by Eq. 3: [13]:
where V is the cell voltage and Δm is the mass of removal salt.
Current efficiency is also calculated as follows [42]:
where Co and Ci are dilute and feed concentrations (mol/m3), respectively; Qf is the dilute flow rate (m3/s); F is the Faraday constant (96,485 A s/mol); z is the charge of the ion; I is the current (A); and N is the number of cell pairs. The pH value of dilute and concentration chambers were controlled to avoid water dissociation phenomena in over-limiting current density.
The permselectivity of membranes is also quantitatively expressed based on the migration of counter-ions through the IEMs between Na+ and Cu2+ using Eq. 5: [40]:
where tCu2+ and tNa+ are the transport numbers in the membrane phase; CCu2+ and CNa+ are the concentration (M) of these ions in the compartment, and JCu2+ and JNa+ are the fluxes of Cu2+ and Na+ (mol m−2 s−1), respectively.
The ion exchange capacity (IEC) determination was also performed using titration method. For the IEC measurement, the membranes in acid form (H+) were converted to Na+ form by immersing in 1 M NaCl solution to liberate the H+ ions. The H+ ions in the solution were then titrated with 0.01 M NaOH and phenolphthalein indicator. The IEC can be calculated by Eq. 6 [38]:
where a is the milli-equivalent ion-exchange group in membrane and Wdry is the weight of dried membrane (g).
The electrical resistance of the equilibrated membrane was measured in NaCl solution with a concentration of 0.5 M by using an alternating current bridge with a 1500-Hz frequency. The membrane resistance is calculated using the different resistance between the cell and electrolyte solution [43]. The areal resistance membrane (r) was expressed as follows:
where Rm is membrane resistance and A is the surface area of the used membrane.
Results and discussions
Characterization of membrane
The membrane morphology and structure play a fundamental role in electron transport properties and electrochemical characteristics of them [44]. FESEM images from the surface of membranes with 12KX magnification are shown in Fig. 2. As shown, the surface of the virgin membrane is uniform. The membrane surface shifts to some porous and with typical ridges structure after chitosan deposition. Also, the use of chitosan-co-TiO2 nanoparticle layer on the membrane surface led to a denser and homogeneously surface because filling the hollow surfaces with the nanoparticles. Amino and hydroxyl groups of chitosan chain and polar functional groups on TiO2 molecules cause improved interactions due to creating a hydrogen bond network. These interactions enhanced compatibility between TiO2 and CS, which is greatly useful for avoiding interfacial deficiency between them. Therefore, the densification of the CS-co-TiO2 surface against the modification with CS is predictable. Moreover, it enhances the density of amino, hydroxyl, and other polar functional groups on the membrane surface, leading to improved membrane potential in the adsorption of metal ions. The fabrication of membrane structure with a smaller interfacial gap is necessary to ensure selective permeation of ED membranes [28].
Also, the EDX mapping analysis of nitrogen (N) depended on amino groups of chitosan chain for CS coating, and CS-co-TiO2 coating is shown in Fig. 3. According to the mapping analysis, it is clear that adding TiO2 makes a denser and uniform surface. It would improve the number of functional groups on the membrane surface.
Figure 4a–d shows the FTIR analysis of TiO2, chitosan, (PVC-co-resin) substrate CEM, and the modified (PVC-substrate)/(Cs-co-TiO2) DL membrane, respectively.
Strong absorption band in the range of 3600 to 3000 cm−1 attributed to the overlapping of N–H in Cs and O–H stretching vibrations in the surface of TiO2 nanoparticles; two peaks at 2970.44 cm−1 and 2912.28 cm−1 related to methylene C-H symmetrical stretching and asymmetric; the peak of moderate-intensity at 1641.82 cm−1 related to C = O stretching vibrations from amide groups or carboxyl due to interactions between GA and Cs [45]. GA-Cs grafting can enhance chitosan compatibility with PVC [28]. The peak at 1196.06 cm−1 is associated with the possible partial inter-chain interactions between C–Cl bonds in PVC and C–N bonds in the chitosan chain. These changes show the possible interaction between the PVC with –OH, –NH2 of Cs. Some peaks at the lower wavenumber region below 850 cm−1 attributed to the absorption band of O-Ti–O [45, 46]. Figure 5 shows the suggested reaction mechanics between Cs, TiO2, and GA on the surface of (PVC-co-resin) membrane. Studies showed that the stretching vibrations of amino, C–O, and hydroxyl groups are firmly attached to TiO2 nanoparticles, which promote the formation of CS –TiO2 through the electrostatic interaction of N–H–O–Ti bonds. Moreover, O–Ti–O bonds form between TiO2 and Cs. The CS and TiO2 chemically adsorbed and bonded into the Cs matrix due to a cross-linking process and the complex composite structure [47, 48]. Nonetheless, during the preparation of Cs–TiO2, the agglomeration of TiO2 nanoparticles is unavoidable due to creating Van der Waals influences between the chitosan and TiO2 nanoparticles [49].
Membrane surface hydrophilicity and water content
Obtained results presented in Table 3 revealed that deposition of chitosan along with increase of TiO2 concentration led to an improvement in membrane surface hydrophilicity and water content in prepared membranes. The water contact angle was measured 68.1° for the (PVC-co-resin)-(CS) membrane that was much lower than the measured one (109.2°) for virgin membrane. The contact angle was reduced up to 48.3° for (PVC-substrate)/(Cs-co-TiO2) membrane. At the same time, the water contact result illustrates a steady growth from 33 to 59% for samples S1 to S5, respectively.
This result introduces a more hydrophilic surface and nature of prepared membranes. Decrease of contact angle besides increased water content in these samples can be explained by the presence of polar functional groups of CS chain and their ability to create hydrogen bonds with water molecules (OH…O and OH…N). Also, the polar functional groups in the Cs chains are changed the interfacial free energy between the membrane and electrolyte environment. The decrease of water contact angle for the LbL membranes containing TiO2 could be substantiated by the fact that TiO2 spontaneously filled the valleys on the membrane surface, which produces a smoother surface for them. Furthermore, the contact angle results for the membranes agree with the results of the surface roughness analysis (Fig. 6), which means samples with lower surface roughness have lower contact angle values. This is a fact that contact angle is not only affected by the surface chemistry but also affects by the surface geometry. The suitable amount of membrane water content can better control the pathways of ion traffic and improve membrane selectivity. Additionally, the high water content can provide more and wider transfer channels for co and counter ion transportation that decrease the selectivity, and lead to a loose structure for the membranes. However, this is not always true and depends on the membrane structure and properties. The measurements were carried out three times for each sample, and then, their average value was reported to minimize experimental errors.
Membrane surface roughness
The average roughness (Ra) values and 3D images for the fabricated membranes are shown in Fig. 6. As evident in Fig. 6, the surface roughness of the modified membrane reduced compared with [PVC-co-resin] membrane. This reduction comes back to the filling of valleys on the membrane surface by the deposition of Cs-co-TiO2 layer. Also, the incorporation of TiO2 nanoparticles on the membrane surface makes a smoother surface than (PVC-co-resin)/CS membrane. Because the formation of hydrogen bonds among functional groups in TiO2 and Cs leads to improve compatibility valleys on the membrane surface and the decline of cracks or interfacial defects on the membrane surface, decline of membrane roughness could enhance the membrane hydrophilicity and decline the fouling phenomenon. Because valleys as steric hindrance prevent passing water molecules via cavities, therefore, hydrogen bonding between the surface of membrane and water molecules cannot form easily.
Ion exchange capacity and electrical resistance
IEC results (Table 4) indicated that utilizing CS and TiO2 on PVC-substrate led to change in ion exchange capacity in prepared membranes. Initially, deposition of Cs on PVC-based CEM led to some decrease in membrane IEC which may be assigned to resin particles surrounding or covering by the Cs chains that would restrict their accessibility. Slight increase of membrane IEC by utilizing of TiO2 NPs in surface layer is attributed to improvement of membrane wettability and possibly formation of voids in this layer that could promote the possibility of ion exchange by the ionic functional groups. Decrease of membrane IEC at higher TiO2 ratio also may be assigned to NPs agglomerations.
The membrane areal electrical resistance (MAER) (Table 4) was also enhanced by formation of Cs layer on the PVC-based substrate which may be due to increase of membrane thickness after coating process. Decrease of MAER by incorporating of TiO2 NPs in the surface layer may be due to increase of membrane water uptake that would enhance the ions percolation through the membrane.
Desalination study
The sodium flux is shown in Fig. 7a. Results showed that sodium flux was reduced by introducing (Cs-co-TiO2) layer on the PVC-based membrane surface. Moreover, current efficiency reduced, and energy consumption increased by the deposition of new layer on the PVC-based substrate (Fig. 7b). These trends attributed to the increase of membrane thickness by introducing (Cs-co-TiO2) layer along with formation of a compact structure on the surface of virgin membrane (see FESEM images) that would limit the transportation of sodium ions across the membrane and reduced the flux.
Heavy metal ion removal
The presence of heavy metals such as copper ions as toxic metal ions is a threat to public health. Copper ions should be removed from the water. Nanomaterials in nanocomposite membranes are an efficient method in heavy metal removal [50]. The prepared membranes were employed to investigate Cu2+ ion removal from wastewater. An aqueous solution of Cu(NO3)2 with a concentration of 20 ppm Cu2+ and 0.05 M Na2SO4 was applied as feed and electrolyte chamber, respectively. The removal of Cu2+ ions was determined by atomic emission spectroscopy (Shimadzu, Japan). Figure 8 shows the flux of Cu2+ ions by prepared membranes. As evident in this figure, the flux of Cu2+ ions was reduced initially by introducing CS layer on the PVC-based substrate which may be due to positive electric charge of the second layer [51]. After that, introducing the TiO2 NPs into surface layer caused to increase of Cu2+ ions flux due to unique adsorptive characteristic of used nanoparticles in heavy metals adsorption. The increase of negative electric charge on the DL membrane due to abundant hydroxyl groups provided by TiO2 nanoparticles could enhance the adsorption potential. EDX analysis (Fig. 9) shows the adsorption of Cu2+ ions by the DL membranes. Some decline of dialytic rate at higher loading TiO2 nanoparticles may be attributed to the tight and rigid surface of the membrane that restricts the transportation of metal ions. Moreover, the possible agglomeration of nanoparticles at high concentration (Fig. 10) would decline the adsorptive capacity of them.
At the same time, the given results illustrate an increase in permselectivity \(({P}_{{\mathrm{Na}}^{+}}^{{\mathrm{Cu}}^{2+}})\) for the prepared membrane that may be assigned to the adsorptive characteristic of Cs and TiO2 in heavy metal ions compared to monovalent ions. The heavy metal ions tend to create complexes with polar functional groups of modified membranes.
A typical comparison between the prepared membranes in this study with commercial HCEM (RALEX® CMH-PES) in Cu removal from water in ED process showed good ability for the modified membranes. The Cu flux measured 0.99 (mol/m2 S × 105) for S3, whereas that was 0.89 (mol/m2 S × 105) for commercial membrane along with higher permselectivity for S3 compared to commercial membrane (\(\frac{{{P}_{s}}^{\mathrm{S}3,{\mathrm{Cu}}^{2+}/{\mathrm{Na}}^{+}}}{{{P}_{\mathrm{s}}}^{\mathrm{commercial},{\mathrm{Cu}}^{2+}/{\mathrm{Na}}^{+}}}>1.8\)).
Investigation the reusability and antifouling properties of modified membrane
Decrease of Cu2+ dialytic rate was measured -44.7% after 1 h for the pristine membrane whereas that was − 38.7% and − 50.2%. or S3 and S4, respectively (Fig. 11).
The reusability study of prepared membrane was done for the fouled membranes with Cu2+ ions. Firstly, fouled membranes were washed by ultrasonic cleaner bath (Parsonic, 100 W, 28 kHz, PARS NAHAND Co., Iran) for 1 h, and then kept in distilled water for 2 h. The washed membrane was then employed in the ED process again to investigate the ability of used membranes in the removal of Cu2+ ions.
Also, as shown in Fig. 12, after the cleaning process, dialytic rate was improved over + 3.1% and + 0.99% for S3 and S4, unexpectedly. The use of ultrasound waves during the cleaning process can lead to relaxation of CS chains that could increase their latitude by rearranging polymer chains [28]. This can enhance membrane surface area, which may increase the removal efficiency. Moreover, the presence of highly hydrophilic groups on the membrane surface and the reduction of membrane roughness leads to promote membrane flux. The experiments were repeated twice for each sample.
Conclusion
In this study, new LBL membranes were prepared by deposition of CS-co-TiO2 layer on PVC-based heterogeneous CEMs. FTIR spectra confirmed the formation of new layer on the PVC-based substrate. The FESEM images showed a compact structure for the prepared membranes. Also, some agglomerations considered on the membrane surface at high TiO2 nanoparticles. All double-layer membranes showed smoother surfaces than the virgin membrane. It was found that the sodium flux was reduced by deposition of the CS-co-TiO2 layer on the PVC-based CEMs. However, double-layer membranes showed higher dialytic rate in Cu2+ removal than pristine membrane. Among the double-layer membranes, S3 with (0.25: 1) (w/w) (TiO2/Cs) ratio exhibited highest dialytic rate in copper removal compared to others. Moreover, results showed an increasing trend in permselectivity \(({P}_{{\mathrm{Na}}^{+}}^{{\mathrm{Cu}}^{2+}})\) for the DL membranes obviously. The double-layer membranes showed high reusability potential after the regeneration. A negligible change was considered on dialytic rate for the cleaned membranes (S3 and S4) after regeneration.
References
Joseph L, Jun B-M, Flora JR, Park CM, Yoon Y (2019) Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere
Bandehali S, Moghadassi A, Parvizian F, Hosseini SM (2019) A new type of [PEI-glycidyl POSS] nanofiltration membrane with enhanced separation and antifouling performance. Korean J Chem Eng 36:1657–1668
Bandehali S, Moghadassi A, Parvizian F, Shen J, Hosseini SM (2020) Glycidyl POSS-functionalized ZnO nanoparticles incorporated polyether-imide based nanofiltration membranes for heavy metal ions removal from water. Korean J Chem Eng 37:263–273
Bandehali S, Moghadassi A, Parvizian F, Zhang Y, Hosseini SM, Shen J (2020) New mixed matrix PEI nanofiltration membrane decorated by glycidyl-POSS functionalized graphene oxide nanoplates with enhanced separation and antifouling behaviour: Heavy metal ions removal. Sep Purif Technol 242:116745
Bandehali S, Parvizian F, Moghadassi A, Hosseini SM (2020) High water permeable PEI nanofiltration membrane modified by L-cysteine functionalized POSS nanoparticles with promoted antifouling/separation performance. Sep Purif Technol 237:116361
Vaselbehagh M, Karkhanechi H, Mulyati S, Takagi R, Matsuyama H (2014) Improved antifouling of anion-exchange membrane by polydopamine coating in electrodialysis process. Desalination 332:126–133
Hosseini SM, Karami F, Farahani SK, Bandehali S, Shen J, Bagheripour E, Seidypoor AJ (2020) Tailoring the separation performance and antifouling property of polyethersulfone based NF membrane by incorporating hydrophilic CuO nanoparticles. Korean J Chem Eng 37:866–874
Khosravi MJ, Hosseini SM, Vatanpour V (2022) Performance improvement of PES membrane decorated by Mil-125(Ti)/Chitosan nanocomposite for removal of organic pollutants and heavy metal. Chemosphere 290:1–14
Mirzamohammadi M, Koudzari Farahani S, Parvizian F, Hosseini SM (2021) Surface modification of nanofiltration membrane using polyvinyl alcohol and chitosan-functionalized activated carbon nanoparticles. Iran J Polym Sci Technol 34:349–358
Baker RW (2012) Membrane technology and applications, John Wiley & Sons
Irfan M, Wang Y, Xu T (2019) Novel electrodialysis membranes with hydrophobic alkyl spacers and zwitterion structure enable high monovalent/divalent cation selectivity. Chem Eng J 123171
Lai KC, Chen JW, Chen WT, Wan TJ, Wen JC, Shu CM (2014) Applications of the Taguchi method for key parameter screening in electrodialysis reversal used for high salinity wastewater, CLEAN–Soil, Air. Water 42:1751–1758
Nemati M, Hosseini SM, Parvizian F, Rafiei N, Van der Bruggen B (2019) Desalination and heavy metal ion removal from water by new ion exchange membrane modified by synthesized NiFe 2 O 4/HAMPS nanocomposite. Ionics1–11
Farrokhzad H, Darvishmanesh S, Genduso G, Van Gerven T, Van der Bruggen B (2015) Development of bivalent cation selective ion exchange membranes by varying molecular weight of polyaniline. Electrochim Acta 158:64–72
Hosseini SM, Rahzani B, Asiani H, Khodabakhshi A, Hamidi A, Madaeni S, Moghadassi A, Seidypoor A (2014) Surface modification of heterogeneous cation exchange membranes by simultaneous using polymerization of (acrylic acid-co-methyl methacrylate): Membrane characterization in desalination process. Desalination 345:13–20
Bandehali S, Parvizian F, Moghadassi A, Hosseini SM (2020) Chapter 5 - Nanomaterials for the efficient abatement of wastewater contaminants by means of reverse osmosis and nanofiltration. In: Bonelli B, Freyria FS, Rossetti I, Sethi R (eds) Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier, pp 111–144
Bandehali S, Parvizian F, Moghadassi AR, Hosseini SM, Shen JN (2020) Fabrication of thin film-PEI nanofiltration membrane with promoted separation performances: Cr, Pb and Cu ions removal from water. J Polym Res 27:94
Bandehali S, Parvizian F, Moghadassi A, Shen J, Hosseini SM (2020) Improvement in separation performance of PEI-based nanofiltration membranes by using L-cysteine functionalized POSS-TiO2 composite nanoparticles for removal of heavy metal ion. Kor J Chem Eng 37:1552–1564
Hosseini SM, Afshari M, Fazlali AR, KoudzariFarahani S, Bandehali S, Van der Bruggen B, Bagheripour E (2019) Mixed matrix PES-based nanofiltration membrane decorated by (Fe3O4–polyvinylpyrrolidone) composite nanoparticles with intensified antifouling and separation characteristics. Chem Eng Res Des 147:390–398
Ariono D, Wenten IG (2017) Surface modification of ion exchange membranes: Methods, characteristics, and performance. J Appl Polym Sci 134:45540
Bandehali S, Parvizian F, Moghadassi AR, Hosseini SM (2019) Copper and lead ions removal from water by new PEI based NF membrane modified by functionalized POSS nanoparticles. J Polym Res 26:211–219
Zhu J, Liao J, Jin W, Luo B, Shen P, Sotto A, Shen J, Gao CJ (2019) Effect of functionality of cross-linker on sulphonated polysulfone cation exchange membranes for electrodialysis. React Funct Polym 138:104–113
Afsar NU, Shehzad MA, Irfan M, Emmanuel K, Sheng F, Xu T, Ren X, Ge L, Xu T (2019) Cation exchange membrane integrated with cationic and anionic layers for selective ion separation via electrodialysis. Desalination 458:25–33
Qian X, Li N, Wang Q, Ji S (2018) Chitosan/graphene oxide mixed matrix membrane with enhanced water permeability for high-salinity water desalination by pervaporation. Desalination 438:83–96
Salehi E, Daraei P, Shamsabadi AA (2016) A review on chitosan-based adsorptive membranes. Carbohyd Polym 152:419–432
Lizardi-Mendoza J, Monal WMA, Valencia FMG (2016) Chemical characteristics and functional properties of chitosan. In: Chitosan in the preservation of agricultural commodities. Elsevier, pp. 3–31
Jang S-C, Tsen W-C, Chuang F-S, Gong C (2019) Simultaneously enhanced hydroxide conductivity and mechanical properties of quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. Int J Hydrogen Energy 44:18134–18144
Hosseini SM, Alibakhshi H, Jashni E, Parvizian F, Shen JN, Taheri M, Ebrahimi M, Rafiei N (2020) A novel layer-by-layer heterogeneous cation exchange membrane for heavy metal ions removal from water. J Hazard Mater 381:120884
Ebrahimi M, Van der Bruggen B, Hosseini SM, Askari M, Nemati M (2019) Improving electrochemical properties of cation exchange membranes by using activated carbon-co-chitosan composite nanoparticles in water deionization. Ionics 25:1199–1214
Salehi E, Hosseini SM, Ansari S, Hamidi A (2016) Surface modification of sulfonated polyvinylchloride cation-exchange membranes by using chitosan polymer containing Fe 3 O 4 nanoparticles. J Solid State Electrochem 20:371–377
Tufa RA, Piallat T, Hnát J, Fontananova E, Paidar M, Chanda D, Curcio E, di Profio G, Bouzek K (2020) Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem Eng J 380:122461
Hosseini SM, Madaeni S, Heidari A, Amirimehr A (2012) Preparation and characterization of ion-selective polyvinyl chloride based heterogeneous cation exchange membrane modified by magnetic iron–nickel oxide nanoparticles. Desalination 284:191–199
Yang Y, Zhang H, Wang P, Zheng Q, Li J (2007) The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J Membr Sci 288:231–238
Zhang Q, Cui Z, Li W (2020) High permeability poly(vinylidene fluoride) ultrafiltration membrane doped with polydopamine modified TiO2 nanoparticles. Chin J Chem Eng
Nayak V, Soontarapa K, Balakrishna RG, Padaki M, Zadorozhnyy VY, Kaloshkin SD (2020) Influence of TiO2 charge and BSA-metal ion complexation on retention of Cr (VI) in ultrafiltration process. J Alloys Compd 832:153986
PakdelMojdehi A, PourafshariChenar M, Namvar-Mahboub M, Eftekhari M (2019) Development of PES/polyaniline-modified TiO2 adsorptive membrane for copper removal. Colloids Surf A: Physicochem Eng Asp 583:123931
Parvizian F, Ansari F, Bandehali S (2020) Oleic acid-functionalized TiO2 nanoparticles for fabrication of PES-based nanofiltration membranes. Chem Eng Res Des 156:433–441
Hosseini SM, Jashni E, Amani S, Van der Bruggen B (2017) Tailoring the electrochemical properties of ED ion exchange membranes based on the synergism of TiO2 nanoparticles-co-GO nanoplates. J Colloid Interf Sci 505:763–775
Hosseini SM, Nemati M, Jeddi F, Salehi E, Khodabakhshi AR, Madaeni SS (2015) Fabrication of mixed matrix heterogeneous cation exchange membrane modified by titanium dioxide nanoparticles: mono/bivalent ionic transport property in desalination. Desalination 359:167–175
Hosseini SM, Sohrabnejad S, Nabiyouni G, Jashni E, Van der Bruggen B, Ahmadi A (2019) Magnetic cation exchange membrane incorporated with cobalt ferrite nanoparticles for chromium ions removal via electrodialysis. J Membr Sci 583:292–300
Hosseini SM, Moradi F, KoudzariFarahani S, Bandehali S, Parvizian F, Ebrahimi M, Shen JN (2021) Carbon nanofibers/chitosan nanocomposite thin film for surface modification of poly(ether sulphone) nanofiltration membrane. Mater Chem Phys 269:124720.
Sadrzadeh M, Mohammadi T (2009) Treatment of sea water using electrodialysis: Current efficiency evaluation. Desalination 249:279–285
Sata T (2007) Ion exchange membranes: preparation, characterization, modification and application. Royal Soc Chem
Kononenko N, Loza N, Shkirskaya S, Falina I, Khanukaeva DY (2015) Influence of conditions of polyaniline synthesis in perfluorinated membrane on electrotransport properties and surface morphology of composites. J Solid State Electrochem 19:2623–2631
Xiao G, Su H, Tan T (2015) Synthesis of core–shell bioaffinity chitosan–TiO2 composite and its environmental applications. J Hazard Mater 283:888–896
Yang D, Li J, Jiang Z, Lu L, Chen X (2009) Chitosan/TiO2 nanocomposite pervaporation membranes for ethanol dehydration. Chem Eng Sci 64:3130–3137
Saravanan R, Aviles J, Gracia F, Mosquera E, Gupta VK (2018) Crystallinity and lowering band gap induced visible light photocatalytic activity of TiO2/CS (Chitosan) nanocomposites. Int J Biol Macromol 109:1239–1245
Huang KS, Grumezescu AM, Chang C-Y, Yang C-H, Wang C-Y (2014) Immobilization and stabilization of TiO2 nanoparticles in alkaline-solidificated chitosan spheres without cross-linking agent. Int J Latest Res Sci Technol 3
Anaya-Esparza LM, Ruvalcaba-Gómez JM, Maytorena-Verdugo CI, González-Silva N, Romero-Toledo R, Aguilera-Aguirre S, Pérez-Larios A, Montalvo-González EJ (2020) Chitosan-TiO2: A versatile hybrid composite. Mater Chem Front 13:811
Ghaemi N (2016) A new approach to copper ion removal from water by polymeric nanocomposite membrane embedded with γ-alumina nanoparticles. Appl Surf Sci 364:221–228
Jashni E, Hosseini SM, Shabanian M, Sadrzadeh M (2022) Silane functionalized graphene oxide-bound polyelectrolyte layers for producing monovalent cation permselective membranes. Sep Purif Technol 278:119583
Funding
The authors received financial support from Arak University during this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seidypoor, A., Joudaki, E., Hosseini, S.M. et al. Double-layer electrodialysis cation exchange membrane by introducing chitosan/TiO2 thin-film nanocomposite on PVC-based substrate for Cu removal from water. Ionics 28, 3037–3048 (2022). https://doi.org/10.1007/s11581-022-04518-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04518-2