Abstract
Electrochemical modification of carbon-paste electrode (CPEs) by silver particles and its applicability for electroanalytical determination of 4-nitroaniline (4-NA) were reported in this study. Electrochemical surface modification was performed by cyclic voltammetry within the range from 600 to − 400 mV in solution containing 0.5 mmol L−1 AgNO3 dissolved in 0.1 mol L−1 of KNO3 at the scan rate of 10 mV s−1 by applying 1.5 cycles (six segments). Silver particles deposited onto surface of carbon-paste electrode (Ag-CPE) were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The proposed catalyst exhibits remarkably an electro-catalytic performance toward 4-nitroaniline reduction. The catalytic peak current obtained by differential pulse voltammetry (DPV) was linearly dependent on the 4-NA concentration over the range of 8.0 × 10−8 to 1.0 × 10−4 mol L−1 with a detection limit of 4.18 × 10−8 mol L−1. The proposed sensor was successfully applied for 4-nitroaniline determination in drinking water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
4-Nitroaniline is an aromatic nitro compounds largely used as an intermediate or precursor in the manufacture of various industrial and high-volume chemicals including pesticides [1, 2]. Thus, it has been reported as a contaminant in environment up to 100 mg L−1 [3] usually in agricultural soil from the application of such biosolid as a part of fertilizer [4] and from pesticide natural transformation [1, 5]. It has been cited in the list of priority pollutants and subjected for treatment [2] because of its chemical stability and persistence [6,7,8] along with toxicological effects to living organisms and human health even at small amounts [9, 10]. An important environmental concern has emerged for water analysis [11,12,13]. Untimely detection of aromatic nitro compounds is important to guard water resources and food supplies, to fortify against terrorist activity, and in the monitoring of detoxification processes [14]. Consequently, there are growing demands for field-deployable devices for reliable on-site monitoring of aromatic nitro compounds [15,16,17]. The 4-nitroaniline is widely detected using electrochemical methods by dint of their impressive advantages of simple operation, inexpensive, portable instrument, and high signal-to-noise ratio when they are compared with sophisticated methods [18].
In latest years, chemically modified electrodes (CMEs) have been used for the quantification of various organic and inorganic species after open-circuit accumulation [19, 20]. Many modifying agents have been used either as coatings on solid electrode surfaces or dispersed within a conductive matrix [21]. Thus, they are widely used in the voltammetric detection of organic compounds by dint of their efficiency and sensitivity. The design of new nanoscale materials has acquired ever-greater importance in recent years owing to their various applications. Among these materials, metallic particles are of great interest due to their numerous properties [22, 23]. Several methods defining the synthesis of silver particles in solution as well as by deposition on solid surfaces have been presented. These include chemical synthesis through the use of different reducing agents [24], electron beam irradiation or UV light [25], and electrochemical methods [26,27,28,29]. The latter methods provide an easy and rapid alternative for the synthesis of metallic particles.
In this paper, silver particles electrodeposited onto carbon-paste electrodes were used. Electrocatalytic studies of 4-nitroaniline reduction and their determination were investigated using cyclic voltammetry (CV), chronoamperometry, and differential pulse voltammetry (DPV) techniques. The experimental parameters affecting peak potential shifting and intensity were evaluated and the catalytic parameters of the electrode process were detailedly discussed. Finally, this electrode was successfully applied to determine trace amounts of 4-nitroaniline in real samples with satisfactory anti-interference ability.
Experimental section
Chemicals and reagents
All chemicals used in the present work were of analytical grade or of the highest purity available. Potassium nitrate, sodium hydroxide, silver nitrate, acetic acid, phosphoric acid, and boric acid were obtained from Merck (Darmstadt, Germany), Fluka (St. Gallen, Switzerland), and Riedel de Haen (Seelze, Germany) and were used as received. 4-Nitroaniline purchased from Sigma-Aldrich was dissolved in 0.1 mol L−1 Britton-Robinson Buffer (B-R; pH 2) to prepare stock solution of 1.0 × 10−3 mol L−1 4-nitroaniline. By diluting the stock solutions, the working standard solutions were prepared. Paraffin was used as the pasting liquid for the carbon-paste electrode (CPE). Carbon paste was supplied from Carbone Lorraine (Lorraine, France; ref. 9900). All these experiments were performed using distilled water, and at room temperature.
Apparatus
All electrochemical measurements in the present study were carried out with a voltalab potentiostat/galvanostat/EIS electrochemical analyzer (ZIVE Lab sp1, WonATech, Korea) controlled by the general purpose electrochemical systems data processing software (Smart Manager). The electrochemical cell was configured to work with three electrodes, using silver deposited onto carbon paste (Ag-CPE) as the working, platinum wire and Ag/AgCl/KCl (3 M) as counter and reference electrodes respectively. The pH meter (Radiometer, SensION; PH3, Leganes, Spain) was used for adjusting pH values.
Procedure of the modification of CPEs
CPEs were prepared according to the following procedure [30]. A portion of the homogeneous paste was firmly packed in the electrode cavity (laboratory made, 0.1256 cm2 geometric surface area) and was polished by smooth paper. A carbon bar was used to establish the electrical contact.
To deposit Ag particles onto CPE, it was soaked in an electrochemical cell containing 0.5 mmol L−1 of AgNO3 dissolved in 0.1 mol L−1 of potassium nitrates (KNO3) electrolyte solution. The Ag particles were fixed on the surface of the electrode with cyclic voltammetry between potential ranges from 600 to − 400 mV for 1.5 cycles (six segments). The prepared electrode was then transferred into a cell containing 0.1 mol L−1 Britton Robinson buffer (pH 2) and 4-nitroaniline. Cyclic voltammetry and differential pulse voltammetry were performed in the potential range of 200 to − 800 mV. Chronoamperometric measurements were performed at potential of − 500 mV. All experiments were performed in the atmospheric conditions.
Results and discussion
Electrochemical behavior of 4-nitroaniline
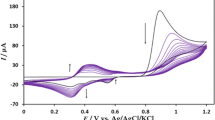
Figure 1a shows the individual CV results in B-R buffer solution (pH 2) containing 1.0 × 10−3 mol L−1 of 4-nitroaniline using (CPE) (a) and (Ag-CPE) (b) respectively. The two electrodes exhibited a reduction response toward 4-nitroaniline within the electrochemical window from 200 to − 800 mV. By comparing the behaviors of (CPE) and (Ag-CPE) electrodes, the cathodic peak potential of 4-nitroaniline was positively shifted from − 607 to − 230 mV when using the modified electrode. More than this potential shift of 370 mV, the reduction peak current was also increased at the Ag-CPE electrode compared to CPE.
a CV of 1.0 × 10−3 mol L−1 4-NA for CPE (A-a) and Ag-CPE (A-b) at scan rate of 100 mV s−1 in 1.0 × 10−1 mol L−1 B-R (pH 2). b, c, d, and e represent the influence of the experimental variables of the Ag electrodeposition (scan rate, potential range, cycle number, concentration of silver in electrolytic solution respectively) on DPV responses of 4-nitroaniline ([4-NA] = 10−4 mol L−1 in B-R buffer pH 2)
Effect of Ag electrodeposition conditions on 4-NA responses
The influence of the experimental conditions of Ag electrodeposition (scan rate, sweeping potential range, number of cycles, and concentration of silver in KNO3 solution) on the potential and intensity of the 4-nitroaniline reduction peak were studied.
Scan rate is one of the most important parameters. Their influence on the electrodeposition of silver particles onto (CPE) to reduce 4-nitroaniline was studied. The experiments were carried out in a solution of 0.1 mol L−1 KNO3 containing 5.0 × 10−4 mol L−1 of AgNO3 in the range from 1 to 30 mV s−1 as shown in Fig. 1b. The high intensity with positively shifted cathodic peak potentials was obtained at a scan rate of 10 mV s−1. Moreover, the range of electrodeposition potential was studied by testing a variety of sweeping potential ranges, which some of them is summarized in Fig. 1c. The result demonstrates that the (Ag-CPE) electrode prepared in a sweeping potential range of 600 to − 400 mV is efficient for the 4-nitroaniline detection. Similarly, the highest response was also improved when the electrode prepared for the total segments of six (Fig. 1d).
The deposition process was also depends on the concentration of reacting (electroactive) ions. Therefore, the effect of the latter on the response of 4-nitroaniline was studied in a concentration range of 1.0 × 10−5 to 1.0 × 10−3 mol L−1 of AgNO3 dissolved in cell containing 0.1 mol L−1 KNO3. The highest response was reached for 0.5 mmol L−1 AgNO3, above this concentration, a poor response for 4-nitroaniline is obtained (Fig. 1e). This behavior was attributed to the formation of a partial silver film on (CPE) [31].
Hence, for all succeeding experiments, 0.5 mmol L−1 AgNO3 under the scan rate of 10 mV s−1 with a total segments number of six in the sweeping potential range from 600 to − 400 mV were used to deposit silver particles onto carbon-paste electrode (CPE) surface.
Characterization of Ag-CP
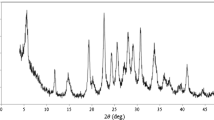
The procedure for modification of carbon-paste electrode by electrodeposition of metallic silver particles, as well as their characterization, has already been carried out in our laboratory by Farahi et al. [32]. The X-ray diffraction analysis of silver deposited on carbon paste (Fig. 2a) shows the formation of silver particles. In fact, the presence of three peaks of metallic silver at 2θ of 38.18, 46.62, and 66.42 affirms the metallic state of the coated silver. The SEM images (Fig. 2b) confirm that the carbon-paste surface becomes gray by formation of silver particles, indicating that they are deposited in aggregated form.
Catalytic study of Ag-CPE on 4-nitroaniline reduction
Effect of scan rate
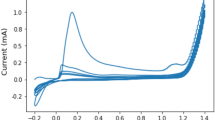
The scan rate effect on cathodic peak current of 4-nitroaniline was investigated at the (Ag-CPE) electrode under the concentration of 1.0 × 10−3 mol L−1 4-nitroaniline in 0.1 mol L−1 B-R buffer solution (pH 2). The reduction peak current of 4-nitroaniline increased gradually by increasing the scan rate (Fig. 3).
The relationship of current and scan rate was a nonlinear when the scan rate increased from 1 to 400 mV s−1. However, the perfect straight linear relationship between reduction peak current of 4-nitroaniline and the square root of scan rate (ν1/2) was confirmed according to the following equation with determination coefficient of 0.984:
This kinetic is expressed by the electronic transfer coefficient (α) and appears in the equation relating the peak intensity:
With C: concentration of 4-nitroaniline species in solution (mol L−1); A: area of the electrode (cm2); D: diffusion coefficient (cm2 s−1); and v: scan rate (Vs−1). The fact suggests that the reaction of 4-nitroaniline on the surface of (Ag-CPE) electrode is typical diffusion-controlled process [33]. Besides, the electrochemical reduction of 4-nitroaniline at the (Ag-CPE) electrode was still irreversible, which can be testified by the potential of 4-nitroaniline (Epc) varied with logarithm of scan rates (Log v). The reduction peak potentials shifted progressively toward the negative direction with the scan rate varied from 1 to 400 mV s−1. The fact can be reasonably explained from the limitation arising from charge transfer kinetics.
Further, to calculate the electronic transfer coefficient (α) and number of electrons transferred in the rate determining step (n), the Tafel slope (a) was calculated from the plot of peak potential (Epc) vs log scan rate (log υ) using the following equation:
As shown in Fig. 3, the reduction peak potential of 4-nitroaniline is proportional to log υ with a slope value of − 0.109 V/AgCl and the calculated Tafel slope value (a) is 0.218 V from Eq. (3). From the slope value (a), the transfer coefficient (α) and number of electron transferred in the rate determining step (n) are calculated using the equation:
where y is Tafel slope (a), α is transfer coefficient, n is number of electrons transferred in the rate determining step, and R, T, F are gas constant, temperature, and Faraday constant respectively. A value of 0.30 for α n is calculated using the above equation indicative of the overall four electrons process involved for the 4-nitroaniline electro reduction and the first electron transfer is the rate-determining step.
Effect of pH on peak potential
The voltammetric behavior of the 4-nitroaniline was characterized at various pH values using CV. Figure 4 presents the CVs of the modified electrode in solutions at various pH values ranging from 1.02 to 11.74. It is found that the peak potentials shifted to more negative values with the increase of buffer pH, implying that protons were involved in the electrode process. A good linear relationship was got between the formal peak potential (Ep) and buffer pH with the regression equation as Ep (mV) = − 123.6× − 24.03. The slope value of − 123.6 mV/pH is higher than the theoretical value of − 59 mV/pH at 25 °C and elucidated that the transfer-electron numbers were fewer than those of the proton numbers taking part in this reaction [13].
Chronoamperometric measurements
Catalytic reduction of 4-nitroaniline by the modified electrode was also studied by chronoamperometry. (Figure 5a) shows the chronoamperometric measurements for 4-nitroaniline. The figure represents the current-time profiles obtained by setting the working electrode potential at − 500 mV for various 4-nitroaniline concentrations.
a Chronoamperograms obtained for 4-NA concentrations at Ag-CPE of 1 (1), 0.5 (2), 0.1 (3), 0.01 (4), and 0.001 (5) mmol L−1at reduction potential (− 0.5 V). b Insets; plots of I versus t−1/2 obtained from chronoamperograms (1) to (5). c Plot of slopes of straight lines against 4-NA concentration and d dependence of IC/IL on t1/2
Chronoamperometry was also used to estimate the diffusion coefficient of 4-nitroaniline. For an electroactive material with diffusion coefficient D, the current response under diffusion control is described by the Cottrell equation [34]:
where Ip is the catalytic current of the suggested electrode in the presence of 4-nitroaniline, F is Faraday constant (96,498 C mol−1), A is the geometric surface area of the electrode (0.1256 cm2), D is the diffusion coefficient (cm2 s−1), C is the concentration of 4-nitroaniline (mol L−1), and t is the time elapsed (180 s). The plot of I vs. t−1/2 at various 4-nitroaniline concentrations was linear (Fig. 5b). From the slopes of the resulting straight lines and the 4-nitroaniline concentrations (Fig. 5c), the average value of D was calculated to be 2.91 × 10−6 cm2 s−1.
The value of the catalytic rate constant (kcat) between 4-nitroaniline and modified electrode can be evaluated using the obtained chronoamperograms and the following equation [35]:
where, IL and IC are the currents in the absence and presence of 4-nitroaniline, respectively.
ϒ = kC0t is the argument of error function. C0, k, and t are concentration of 4-nitroaniline in bulk solution, catalytic rate constant and elapsed time, respectively. When ϒ exceeds 2, the error function is almost equal to 1 and the equation can be summarized to:
Therefore, from the slope of the IC/IL versus t1/2 plot (Fig. 5d) and the above equation, the mean value of kcat was found to be approximately 6.43 × 10−4 mol−1 L s−1.
Analytical study
Calibration data and interference effect
Before establishing the calibration curve, we were interested in optimizing the various electrochemical variables (step, pulse, width, and period) that influence the determination of 4-nitroaniline at Ag-CPE using the DPV method. From the obtained results, it was found that the best response to 4-nitroaniline was recorded with 10 mVs−1, 140 ms, 470 mV, and 500 ms for step, pulse, width, and period, respectively.
On the other hand, the pH effect on cathodic current intensity of 4-nitroaniline was also evaluated. The results show that the peak intensity of 4-nitroaniline reduction increased when decreasing the value of pH solution. Therefore, the maximum intensity is obtained with pH = 1.34, which was used for further work.
Finally, in order to observe the effect of accumulation time, (Ag-CPE) was inserted into a cell containing 20 mL of the 4-nitroaniline at open potential for different times (0, 60, 120, 180, 240, 300, 360, 420, 600, and 900 s), and then the resulting responses of the prepared electrodes were evaluated to determine the optimum value. The increase of peak current with increase of accumulation time indicated that 4-nitroaniline can be accumulated at the surface of CPE electrode probably due to its increase surface activity. According to our findings, the most convenient value was obtained with an accumulation time of 180 s.
Calibration data were obtained for 4-nitroaniline solutions under optimum experimental conditions described obviously. Figure 6 shows some of the typical DPV voltammograms recorded on the (Ag-CPE) in two linear dynamic ranges of 8.0 × 10−8 − 1.0 × 10−6 and 5.0 × 10−6− 1.0 × 10−4 mol L−1 of 4-nitroaniline. The calibration plot is required for quantitative measurements of the 4-nitroaniline concentration at a given potential. The intensity of cathodic current increase with concentration of 4-NA with a determination coefficient of R2 = 0.994. The electrochemical sensing showed a detection limit (S/N = 3) of 4.18 × 10−8 mol L−1. The relative standard deviation (RSD.) for eight measurements was calculated as 2.44% for 4-nitroaniline concentration of 1.0 × 10−4 mol L−1. The LOD provided by (AC-CPE) is comparable than those obtained by the previous electrodes summarized in Table 1 [13, 21, 36,37,38,39].
In order to evaluate the effect of several species frequently found with 4-nitroaniline on the electrode response, a study involving these compounds was performed. The selectivity of the suggested sensor was investigated in electrolytic solution containing 1.0 × 10−5 mol L−1 of 4-nitroaniline spiked with different concentrations of dopamine, ascorbic acid, paracetamol, 4-nitrophenol, 2-nitrophenol, 4-aminophenol, and 2-nitroaniline.
The effect of representative potential interference on the determination of 4-nitroaniline was investigated, which were expressed as the recovery relating to the responses with and without interference compounds. The different concentrations of 4-nitrophenol were investigated and significantly interfered especially by the addition of tenfold resulting an increase in the peak current of 4-nitroaniline. The increase of current signal is probably due to the overlap of the 4-nitroaniline and 4-nitrophenol reduction peaks. It was to observed that the peak current of 4-nitroaniline was decreased by the addition of dopamine, ascorbic acid, and paracetamol. This decrease may be due to the adsorption of these compounds onto the electrode surface Ag-CPE, which is blocking the surface sites. Therefore, the potential range from 0.0 to − 600 mV was recommended to remove the effect of these interferences. However, 2-nitrophenol, 2-nitroaniline, and 4-aminophenol did not show interference with 4-nitroaniline detection, where the recovery rates were upper to 97%.
DPV measurements of 4-nitroaniline in water samples
In order to evaluate the practical application of the proposed modified (CPE), it was used to detect 4-nitroaniline in drinking water samples. No signal for 4-nitroaniline was observed when the sample was analyzed. Hence, the proposed method was applied to analyze water samples contaminated with 4-nitroaniline at different concentrations. The reduction peak of 4-nitroaniline was clearly displayed. The linear calibration curve of 4-nitroaniline was obtained in the concentration range of 1.0 × 10−6 mol L−1 to 1.0 × 10−5 mol L−1 for drinking water samples (Fig. 7). The coefficient of determination of the curve was (R2 = 0.981). The limit of detection (LOD) was found to be 1.38 × 10−7 mol L−1 with RSD of 2.2% and satisfactory recovery that exceeds 95%.
Conclusion
The electrochemical deposition of silver particles onto (CPE) was executed with cyclic voltammetry. The obtained electrode was used to determine 4-nitroaniline in B-R buffer solution (pH 2). Then, the electrochemical reduction of 4-nitroaniline was followed by cyclic voltammetry in the first time with determination of kinetic parameters. This study has demonstrated that (Ag-CPE) exhibits excellent electrocatalytic activity toward 4-nitroaniline reduction. Chronoamperometry is used for the determination of the diffusion coefficient. Differential pulse voltammetric (DPV) measurements exhibit two linear dynamic ranges of 8.0 × 10−8 − 1.0 × 10−6 and 5.0 × 10−6− 1.0 × 10−4 mol L−1 and detection limit of 4.18 × 10−8 mol L−1 for 4-nitroaniline reduction. Finally, the proposed modified electrode has been successfully applied for the determination of 4-nitroaniline in drinking water samples. Simplicity of preparation, good reproducibility, and low cost of the modified electrode as well as wide linear concentration range, low-detection limit, and good repeatability for 4-nitroaniline determination are the important advantages of (Ag-CPE).
References
Khan F, Pandey J, Vikram S, Pal D, Cameotra SS (2013) Aerobic degradation of 4-nitroaniline (4-NA) via novel degradation intermediates by Rhodococcus sp.strain FK48. J Hazard Mater 254–255:72–78
Sun JH, Sun SP, Fan MH, Guo HQ, Qiao LP, Sun RX (2007) A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. J Hazard Mater 148:172–177
Smith SR, Riddell-Black D (2007) Sources and impacts of past, current and future contamination of soil, in research project final report for defra project code SP 0547. Imperial College London:1–247
Smith SR (2009) Organic contaminants in sewage sludge (biosolids) and their significance for agricultural recycling. Philos Trans A Math Phys Eng Sci 367:4005–4041
Haderlein SB, Weissmahr KW, Schwarszenbach RP (1996) Specific adsorption of nitroaromatic explosives and pesticides to clay minerals. Environ Sci Technol 30:612–622
Khalid A, Arshad M, Crowley DE (2009) Biodegradation potential of pure and mixed bacterial cultures for removal of 4-nitroaniline from textile dye wastewater. Water Res 43:1110–1116
Kutty R, Purohit HJ, Khanna P (2000) Isolation and characterization of a Pseudomonas sp. strain PH1 utilizing meta-aminophenol. Can J Microbiol 46:211–217
Saupe A (1999) High-rate biodegradation of 3 and 4-nitroaniline. Chemosphere 39:2325–2346
Bhunia F, Saha NC, Kaviraj A (2003) Effects of aniline-an aromatic amine to some fresh water organisms. Ecotoxicology 12:397–404
Chung KT, Chen SC, Zhu YY, Wong TY, Stevens SE (1997) Toxic effects of some benzamines on the growth of Azotobacter vinelandii and other bacteria. Environ Toxicol Chem 16:1366–1369
Nair RS, Auletta CS, Schroeder RE, Johannsen FR (1990) Chronic toxicity, oncogenic potential, and reproductive toxicity of p-nitroaniline in rats. Fundam Appl Toxicol 15:607–621
Wang G, Zhang X, Yao C, Tian M (2010) Four-week oral toxicity study of three metabolites of nitrobenzene in rats. Drug Chem Toxicol 33:238–243
Laghrib F, Boumya W, Lahrich S, Farahi A, El Haimouti A, El Mhammedi MA (2017) Electrochemical evaluation of catalytic effect of silver in reducing 4-nitroaniline: analytical application. J Electroanal Chem 807:82–87
Hu S, Xu C, Wang G, Cui D (2001) Voltammetric determination of 4-nitrophenol at a sodium montmorillonite-anthraquinone chemically modified glassy carbon electrode. Talanta 54:115–123
Yosypchuk O, Karásek J, Vyskočil V, Barek J, Pecková K (2012) The use of silver solid amalgam electrodes for voltammetric and amperometric determination of nitrated polyaromatic compounds used as markers of incomplete combustion. Sci World J 2012:1–12. https://doi.org/10.1100/2012/231986
Vyskočil V, Barek J (2011) Electroanalysis of nitro and amino derivatives of polycyclic aromatic hydrocarbons. Curr Org Chem 15:3059–3076
Yosypchuk O, Barek J, Vyskočil V (2012) Voltammetric determination of carcinogenic derivatives of pyrene using a boron-doped diamond film electrode. Anal Lett 45:449–459
Wang H, Zhang A, Cui H, Liu D, Liu R (1998) Adsorptive stripping voltammetric determination of phenol at an electrochemically pretreated carbon-paste electrode with solid paraffin as a binder. Microchem J 59:448–456
Kalcher K, Kauffmann JM, Wang J, Svancara I, Vytras K, Neuhold C, Yang Z (1995) Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period 1990–1993. Electroanalysis 7:5–22
Li J, Liu S, Mao X, Gao P, Yan Z (2004) Trace determination of rare earths by adsorption voltammetry at a carbon paste electrode. J Electroanal Chem 561:137–142
Laghrib F, Lahrich S, Farahi A, Bakasse M, El Mhammedi MA (2018) Impregnation of silver in graphite carbon using solid reaction: electrocatalysis and detection of 4-nitroaniline. J Electroanal Chem 823:26–31
Starowicz M, Stypuła B, Banas J (2006) Electrochemical synthesis of silver nanoparticles. Electrochem Commun 8:227–230
Welch CM, Compton RG (2006) The use of nanoparticles in electroanalysis: a review. Anal Bioanal Chem 384:601–619
Sun L, Zhang Z, Mater HD (2003) A novel method for preparation of silver nanoparticles. Mater Lett 57:3874–3879
Fukushima M, Yanagi H, Hayashi S, Suganuma N, Taniguchi Y (2003) Fabrication of gold nanoparticles and their influence on optical properties of dye-doped sole gel films. Thin Solid Films 438-439:39–43
Dai X, Nekrassova O, Hyde ME, Compton RG (2004) Anodic stripping voltammetry of arsenic (III) using gold nanoparticles modified electrodes. Anal Chem 76:5924–5959
El-Deab MS, Okajima T, Ohsaka T (2003) Electrochemical reduction of oxygen on gold nanoparticle-electrodeposited glassy carbon electrodes. J Electrochem Soc 150:851–857
Welch CM, Banks CE, Simm AO, Compton RG (2005) Silver nanoparticles assemblies supported on glassy-carbon electrodes for the electroanalytical detection of hydrogen peroxide. Anal Bioanal Chem 382:12–21
Yin B, Ma H, Wang S, Chen S (2003) Electrochemical synthesis of silver nanoparticles under protection of poly(Nvinylpyrrolidone). J Phys Chem B 107:8898–8904
Hammani H, Boumya W, Laghrib F, Farahi A, Lahrich S, Aboulkas A, El Mhammedi MA (2017) Electro-catalytic effect of Al2O3 supported onto activated carbon in oxidizing phenol at graphite electrode, mater. Today Chem 3:27–36
Encarnacion BCM, Olga DR, Julia AMM (2007) Determination of lamotrigine by adsorptive stripping voltammetry using silver nanoparticle-modified carbon screen-printed electrodes. Talanta 74:59–64
Farahi A, Achak M, El Gaini L, El Mhammedi MA, Bakasse M (2015) Electrochemical determination of paraquat in citric fruit based on electrodeposition of silver particles onto carbon paste electrode. J Food Drug Anal 23:463–471
Zhang Y, Zhao Y, Yuan S, Wang H, He C (2013) Electrocatalysis and detection of nitrite on a reduced grapheme/pd nanocomposite modified glassy carbon electrode. Sens Actuator B 185:602–607
A.J. Bard, L.R. Faulkner, Electrochemical methods, fundamentals and applications, Wiley, New York, (2001), 163
AnuPrathap MU, Anuraj V, Satpati B, Srivastava R (2013) Facile preparation of Ni (OH)2-MnO2 hybrid material and its application in the electrocatalytic oxidation of hydrazine. J Hazard Mater 262:766–774
Zavar MHA, Heydari S, Rounaghi GH, Eshghi H, Toupkanloo HA (2012) Electrochemical behavior of para-nitroaniline at a new synthetic crown ether-silver nanoparticle modified carbon paste electrode. Anal Methods 4:953–958
Rameshkumar P, Ramaraj R (2014) Electroanalysis of nitrobenzene derivatives and nitrite ions using silver nanoparticles deposited silica spheres modified electrode. J Electroanal Chem 731:72–77
Zhao F, Liu L, Xiao F, Li J, Yan R, Fan S, Zeng B (2007) Sensitive voltammetric response of p-nitroaniline on single-wall carbon nanotube-ionic liquid gel modified glassy carbon electrodes. Electroanalysis 19:1387–1393
Ahmad R, Tripathy N, Ahn MS, Hahn YB (2017) Development of highly-stable binder-free chemical sensor electrodes for p-nitroaniline detection. J Colloid Interface Sci 494:300–306
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laghrib, F., Ajermoun, N., Hrioua, A. et al. Investigation of voltammetric behavior of 4-nitroaniline based on electrodeposition of silver particles onto graphite electrode. Ionics 25, 2813–2821 (2019). https://doi.org/10.1007/s11581-018-2735-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-018-2735-8