Abstract
The potential of the novel electro-chlorination system assembled with graphite anodes for its application in drinking water supply was explored. The process parameter optimization was carried out using response surface methodology (RSM) approach and the optimal conditions for highest yield of active chlorine were arrived. The effect of the process variables were investigated using Box-Behnken design. The experimentally observed results were correlated and integrated to derive a mathematical model. The derived RSM model predicted active chlorine production was validated using various statistical parameters i.e., coefficient of determination (R2), adjusted R2 (R2adj), and predicted R2 (R2pred). The experimental results were fitted well with the quadratic model suggested by the software and the R2 value obtained was 0.9828. The study concluded that active chlorine formation can be optimized and modeled using RSM approach and can be effectively implemented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorination is one of the most extensively adopted practice across the world to achieve pathogen-free drinking water. But shipping of chlorine in the gaseous phase is coupled with persistent health-related issues [1, 2]. Moreover, chlorine reacts with the natural organic matter (NOM) present in the water and gives rise to the formation of harmful disinfection by-products (DBPs), which are reported to be carcinogenic [3,4,5,6,7]. To resolve these issues associated with the conventional chlorination process, the water engineers are thinking of other treatment alternatives for onsite generation of active chlorine that would be regulated with optimized control on its dosing [8]. Earlier studies have suggested that onsite chlorine generation system is one of the most competent alternatives due to its effectiveness and environment-friendly handling from the view point of safety and health-related issues to the associated problems of chlorine [9]. Additionally it has been reported that the DBP formation potential reduced to about 50% from electrochemical modes of water treatment [10].
The raw water to be treated is forced to pass through an electro-chlorination cell assembled with 12 electrodes through which the desired voltage is supplied [11]. The electrochemical cell performance efficiency is influenced by different parameters among which electrode material plays the most imperative role. Generally, dimensionally stable anodes (DSA) are the most widely used in large-scale industrial productions of hypochlorite [12]. Platinum and doped diamond electrodes are the other most extensively used electrodes in addition to DSA [13, 14]. But the formation of platinum oxide film at high anodic potentials reduces the platinum activity [15, 16]. Moreover, for industrial-scale productions of sodium hypochlorite, these electrodes choice are very expensive. The high cost of these electrodes also adds to a higher capital cost which is still one of the unsettled barriers for its widespread application in the water treatment industry. Graphite was chosen in this study as a choice of electrode material because of its inexpensiveness in contrast with commercially available costly metals [17, 18]. Earlier studies have reported that coating carbon-based materials with noble materials is a viable and cost-effective way for decreasing the raw material cost [19, 20]. Thus, prevailing studies on coating with metal alloys has marked the beginning of a new era in fabricating cost-efficient electrodes and has thereby attracted a great deal of attention [21].
From the view point of the above limiting issues, a research study is undertaken with the objective of optimizing a novel electro-chlorination process assembled with cheap graphite electrodes to assess its potential towards active chlorine generation for disinfection purpose [22]. Optimization of this electro-chlorination system settle on optimal values for process parameters such as current density, chloride concentration, electrolysis time, and pH in order to improve the disinfection efficiency, reduce the raw material cost, and thereby lessen the formation of carcinogenic DBPs. The current efficiency and power consumption by the electro-chlorination system was considered in this study.
Designs of experiment (DOE) methods are the most readily used optimization process which includes response surface methodology (RSM), factorial design, and mixture design which has been taken up in the present study. RSM is an assortment of various statistical and mathematical methods which are helpful for improving, developing, and optimizing a process. RSM is beneficial for reducing the number of experimental trials and useful for evaluating the interactions between the various process parameters [23,24,25,26,27]. RSM has been used for the first time, for optimization of the process parameters in a novel electro-chlorination system designed for onsite drinking water treatment. RSM only takes into consideration the effects of the operational parameters on the removal process [25, 28, 29]. In the previous studies, RSM has also been used to optimize the electrochemical treatment of industrial paint wastewater, textile dye wastewater, electrochemical removal of mercury ions from wastewater, sodium from fermented food composts, chromium from industrial wastewater, and chromium contaminated waters [30,31,32,33,34,35,36].
The linear, quadratic, and the interaction effects of the process parameters on the response were scrutinized with the help of empirical models built using RSM as described in [27, 37,38,39]. The main objective of the study include the exploration of the interaction of the focal effects, current density, chloride concentration, electrolysis time, and pH on efficient active chlorine productions used for onsite drinking water disinfection. The statistical competence of the developed models was further validated using analysis of variance (ANOVA). The model fit results were used to develop the model equation for any unique combination of the process factor levels for efficient active chlorine productions. The obtained optimal values of the process factors were further validated for comparison of active chlorine produced as suggested by the RSM model and as obtained by the experimental procedure.
Materials and methods
Laboratory-scale reactor setup
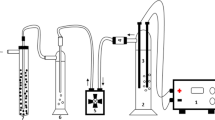
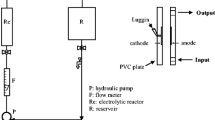
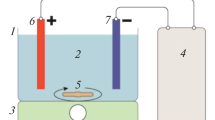
An electrochemical cell was constructed with transparent poly (methyl methacrylate) material. The cell consisted of six graphite anodes and six stainless steel cathodes and the corresponding surface area of a single anode and cathode was 32.97 and 24.78 cm2, respectively. A DC power supply was used to provide a constant current source. Sampling ports were placed alongside the height of the column for water analysis. Before the inception of every experiment, the electrochemical cell was thoroughly cleansed with deionized water. The experiments were carried out in a batch mode where the raw water was continuously electrolyzed for 50 min. All the experiments were performed at room temperature that was maintained at 25 °C. A schematic illustration of the experimental setup is shown in Fig. 1.
The tap water supplied at the consumer’s end in IIT(ISM) campus was simulated by adding chloride salts. Varying amounts of reagent-grade sodium chloride salts were used for preparation of sample water. The electrolysis experiments were carried out at different concentrations of chloride ions, the range of chloride concentration varying from 0 to 50 mg/l respectively. Elevated concentration levels of chloride ions was avoided keeping in view for reducing the raw material cost of procuring reagent grade salts.
Experimental design and optimization of process parameters
The concurrent productions of active chlorine using the designed electro-chlorination system were optimized via RSM packages in Design Expert 9 (USA). The experimental runs were designed in agreement with the Box-Behnken design with bounds of four process parameters. The four independent process parameters were coded at four levels between − 1 and + 1, where the respective process parameters namely current density (A), chloride concentration (B), electrolysis time (C), and pH (D) were evaluated in the range of 0.1–3 mA/cm2, 10–50 mg/l, 10–30 min, and 7–10 respectively. An earlier experiment was done in continuation of the present investigation for determining the range of values of the necessary process factors (A, B, C, D) in accordance with literatures. The independent process factor variables along with their coded levels are shown in Table 1. The experimental design runs of Box-Behnken design along with their responses are shown in Table 2. The first four columns designate the number of runs and the various experimental combinations as arranged by the Box-Behnken design. The process performance was evaluated by analyzing the active chlorine production by the electro-chlorination system. The total experimental runs were 30 and were carried out in randomized order on the basis of requirement as carried out in many design procedures. Second-order models were used for correlating the response with the process factors. Quadratic response surface designs or such second-order models are customarily stated as [37, 40, 41]:
where y represents the expected response; ßo represents the intercept or the constant regression coefficient; ßi and ßii represents the linear and pure quadratic regression coefficients, respectively, referring to coded independent process factor xi; while ßij indicates the interaction coefficient in relation to the coded factors xi and xj. Least squares estimation was used for determining the regression coefficients; multiple linear regression (MLR) analysis was used for statistically formulating the quadratic response surface models [23].
ANOVA technique was used for statistically investigating the competence of the fitted second-order models [24]. The ANOVA technique mathematically divides the total variation obtained within the experimental results into two primary sections namely (a) treatment variation concerning to the variance in the process response because of the computed regression or model and (b) inexplicable or uncontrolled variation relating to the variance arising in the measurements because of the experimental error [40]. The variance ratio, i.e., the ratio of the mean square due to regression or treatment effects to the mean square due to experimental error or residual is generally termed as the F ratio; thus, at a particular confidence level (95% confidence level, in the present case), a model term is considered significant if the corresponding F statistic level surpasses the computed critical F value [40, 42]. Other ways for estimating the significance of the model terms is by using the P value test statistic; if a P value is less than 0.05, it implies that the corresponding model term, or in other words, its associated coefficients are significant [40, 42].
For the optimization process, a section in the software model looked for different combinations in the process factor levels that simultaneously satisfy the desired necessities on each of the factors and the corresponding responses. The desired response was set as maximum for active chlorine productions that is required for onsite drinking water disinfection.
Analytical methods
Argeno-titrimetric method (method no. 4500 Cl− B) and the N-diethyl-p-phenylenediamine (DPD) ferrous titrimetric method (method no. 4500-Cl F) was used for determining the concentration of the dissolved chloride ions and active chlorine, respectively, where DPD is used as an indicator with ferrous ammonium sulfate (FAS) as the titrant, as described in standard methods [43]. The pH of the solution was buffered to a slightly alkaline range (pH 8.5 ± 0.5). The reagents were procured from Merck, Germany. All the experiments were performed at room temperature.
Estimation of power consumption and current efficiency
The active chlorine formation during electro-chlorination was carried out by a process of two electron transfer. Current efficiency, CE (%) and power consumption (Wh/g) was calculated as per the following formulas [44]:
where Vol is the solution volume in liters, ΔC is the difference in the active chlorine concentration (M), n is the number of electrons involved in the chlorine formation reaction, F is Faraday’s constant (96,485 C/mol), i is the applied current in amperes, and t is the electrolysis time in seconds.
Quality control and precisions
The analysis of the samples was carried out in triplicate for the precision of the measurements undertaken. The difference in values between two samples was analyzed and cross-verified. When the relative percentage difference (RPD) between two parallel samples surpassed more than 5%, the samples were re-analyzed again. The final value was taken as the average value of all the three readings.
Results and discussions
Effects of various operational parameters on the performance of reactor
The key effects and the interaction effects of the process factors on the response, namely active chlorine formation, were appraised using suitable response surface (RS) plots, wherein the variations in the response as functions of two independent process factors were delineated, keeping the other two process factors at their respective central levels [42]. These plots are exhibited in Fig. 2. Thus, the RS plots shown in Fig. 2 explicate the variations in active chlorine formation as functions of current density (A) and chlorides (B), A and time (C), A and pH (D), B and C, B and D, and C and D, respectively.
Effect of current density and chloride concentration
The influence of current density and chloride concentration on active chlorine formation are plotted in Fig. 2a. It was found that, there was a progressive increase in the active chlorine formation with the sequential increase in both current density and chloride concentration. Experiments were carried out in the laboratory scale at a range of lower current densities varying from 0.1 to 2.5 mA/cm2. The formation of active chlorine was seen to vary proportionally till 2.5 mA/cm2. Surprisingly, it was observed that the chloride conversion rate decreased with the further increment in current density beyond 2.5 mA/cm2. This may be explained due to the fact that high current density corrodes graphite electrodes [45]. Moreover, in the earlier studies, it was confirmed that formation of harmful by-products occurs at high current density conditions [45,46,47]. Earlier researchers have reported that high current density leads to increased formation of inorganic by-products due to higher concentration of ozone [48]. Moreover, the current efficiency falls with the augment in current density [44, 47]. So, in the present study, all the experiments were carried out at a range of lower current densities in order to enhance the current efficiency and prevent the formation of harmful by-products. The active chlorine production rate increased proportionally by augmenting chloride concentration levels in electrolyte as shown in Fig. 2a. Some early researchers have reported that the active chlorine formation increases with the increase in chloride concentration [14, 45, 49, 50]. But in the present study, the experiments were carried out at low levels of chloride concentration, in order to achieve economically attractive cost.
Effect of current density and electrolysis time
It is seen from Fig. 2b that the active chlorine formation amplified with the increase in current density and electrolysis time. But from Fig. 2b, it can be concluded that the current density plays a more noteworthy effect than electrolysis time in the onsite water disinfection process. The total chlorine formation was found to be increased proportionally with electrolysis time in Fig. 2b, d, f. It was found that at an electrolysis time of 30 min and a maximum chloride conversion of 57.3% was attained, whereas a conversion rate of 28.3 and 41% was observed at 10 and 20 min, respectively. The results of present investigation is in agreement with the earlier studies where it has been reported that maximum amount of active chlorine was 1.2 mg/l in 1 h with platinum mesh anodes from an electrolyte concentration of 0.01 M NaCl [51]. In other research works, it was reported that maximum amount of active chlorine approximately 1.65 mg/l was produced in 30 min from less than 10 mg/l of chloride ions [52]. In the present study, the electrolysis was carried out for 50 min because it has been found that beyond an electrolysis time of 30 min, there was a fall in the current efficiency, and intense oxygen evolution was seen to occur.
Effect of current density and pH
As can be seen in Fig. 2c, pH has a negligible effect in comparison to current density for the optimal productions of active chlorine. In the earlier works carried out by Kodera et al. [17], it was proved that the peak current augmented with a change in pH to a slightly alkaline range. But after the completion of the overall electrolysis reaction, the pH change remained unaltered due to the neutralization effect.

The formation of oxidation by-products, i.e., chlorate were minimal under alkaline pH conditions [53]. Thus, it can be concluded that in comparison to pH, current density has a much more driving force on the response.
Effect of chlorides, electrolysis time, and pH
It is observed from Figs 2d, e that active chlorine formation depends more on chloride concentration in comparison to electrolysis time and pH. But as illustrated in Fig. 2f, electrolysis time has a slightly more significant effect as compared to pH in the formation of active chlorine. Thus, it can be concluded that current density, chloride concentration, and the electrolysis time are the vital significant factors and with a judicious selection of the upper and lower limits of the process parameter levels, sufficient amount of active chlorine can be produced effectively in an onsite electro-chlorination system.
Optimization of the experimental conditions
Instantaneous active chlorine productions were conducted for obtaining the optimum process factor conditions for maximized response using the Design Expert® 9.0.10, by the use of the respective models in Eq. (5). The upper limit of the process parameters was judiciously selected for reducing time, cost, and electrical energy consumption. The optimization program was used for setting the highest desirability and then different numerical combinations were looked for, maximizing the model functions. The optimization results are depicted in Figs. 3 and 4 which shows that the optimized conditions for maximized responses were obtained at a current density of 2.064 mA/cm2, chloride concentration of 43 mg/l, electrolysis time of 21 min, and at a pH of 8.59, respectively. These combinations were obtained at a desirability of 1.000. At the optimized conditions, active chlorine formation of 2.22 mg/l was predicted using the model Eq. (5). The obtained optimum conditions were further validated by an additional experiment that was performed to corroborate the active chlorine concentration. This confirmatory run validated the accuracy of the model showing an active chlorine concentration of 2.15 mg/l as compared with the respective value of 2.22 mg/l that was obtained using the model Eq. (5). Thus, the experimentally determined response levels conformed satisfactorily with the model assumed theoretical values thereby corroborating the correctness and precision of the response surface models.
Performance analysis of electro-chlorination cell
Electro-chlorination is considered as an energy driving process, so the current efficiency and power consumption for treating 3 l of water on a laboratory scale was calculated. It was found that for an active chlorine formation of 2.22 mg/l at a chloride concentration of 43 mg/l, current density of 2.064 mA/cm2, electrolysis time of 21 min, and at a pH of 8.5, the CE (%) and power consumption of the designed electro-chlorination system was 68.9% and 6.572 (Wh/g), respectively. The appreciable CE (%) and low-power consumption can be explained by the phenomena that effect of low current density (2.064 mA/cm2) in our experiments, decreased the power consumption and enhanced the current efficiency by hindering highly aggressive reactions like evolution of oxygen that spontaneously takes place at high current and voltage conditions [44].
Development and analysis of response surface models
Response surface designs were tailored to fit the experimental results obtained from the design runs conducted apropos of the configured Box-Behnken design. This resulted to an equation (Eq. 5) in coded forms, where, A, B, C, and D represented current density, chloride concentration, electrolysis time, and pH. Accordingly, the response, i.e., formation of active chlorine (Y) can be computed using Eq. (5):
The analysis of variance (ANOVA) results obtained for the formation of active chlorine are summarized in Table 3. The F statistic values corresponding to the formation of active chlorine was 57 clearly indicating that the model is statistically significant. In this case A, B, C, AD, A2, B2, and C2 are the significant model terms with a P value less than 0.05. Values that are greater than 0.1000 indicates that the model terms are not significant. The precision warranted by the quadratic models and the statistical adequacy of their fit were further confirmed using other statistical parameters, such as coefficient of determination or R2, adjusted R2 statistic (R2adj), predicted R2 (R2pred), coefficient of variation (CV %), standard deviation (Std. dev.), and adequate precision “Adeq Precision” [37]. The values of these model statistics are listed in Table 4. The “Predicted R2 value” of 0.9278 is in reasonable agreement with the “Adj R2 value” of 0.9655; i.e., the difference is less than 0.2. “Adeq Precision” specifically measures the signal to noise ratio. A ratio greater than 4 is desirable. In the present study, a ratio of 26.491 indicates an adequate signal that can be used for navigating the design space. Actual values shown in Fig. 5 are the measured response for a run assembled by Box-Behnken design and the predicted response values evaluate the models and are produced by using the approximate functions. The values of the correlation coefficient R2 and R2adj, were obtained as 0.9828 and 0.9655 for active chlorine formations. The normal % probability versus externally studentized residual graphs for response active chlorine (mg/l) yielded moderately straight lines (Fig. 6), thus showing normal distribution of the data. Normal distribution was indicated in the residual plots and there was no visible problem with normality.
Validation of the model
A t test is a statistical hypothesis test in which the test statistic follows a student’s t distribution under the null hypothesis. The test is used for determining the significant differences between two sets of data. In a null hypothesis, we consider that the set A is statistically equal to the set B. A t test was performed to determine the bias trend of the model. The tstat (0.190) for the model was less than the tcrit (2.04) indicating that the model biasness is insignificant. It may thus be concluded that the model developed is significant since the observed values are statistically equal to the predicted values (Table 5).
Conclusions
The optimization of active chlorine productions in a novel electro-chlorination system was deliberated for examining the efficacy and economy of onsite drinking water disinfection in the water supply system. The study was carried out with cheap, easily affordable graphite anodes and stainless steel cathodes. The process parameters governing efficient active chlorine productions were thoroughly evaluated and statistically optimized using consistent, flexible empirical models laid down by RSM. The empirical models developed by Design Expert 9.0.10 software for active chlorine productions satisfactorily fitted with the experimental observed values. The statistically optimized process parameter settings offered appreciable active chlorine productions of 2.22 mg/l at a chloride concentration of 42 mg/l in the electrolyte. The experimentally determined response levels conform satisfactorily with the model assumed theoretical values thereby corroborating the correctness and precision of the developed response surface models. Optimization of the process deliberated that active chlorine formation was found to be dependent on current density, electrolysis time and chloride levels in the water. The present investigation addresses a new outlook of the onsite electro-chlorination system with easily affordable graphite anodes where the salt requirement will be reduced significantly, and hence could serve as effective alternative drinking water disinfection means for onsite active chlorine productions.
References
Cantor KP, Lynch CF, Hildesheim M, Dosemeci M, Lubin J, Alavanja M, Craun G (1998) Drinking water source and chlorination byproducts I. Risk of bladder cancer. Epidemiology 9(1):21–28. https://doi.org/10.1097/00001648-199801000-00007
White GC (2010) Handbook of chlorination and alternative disinfectants. van Nostrand Reinhold, New York, pp 212–287
USEPA (1986) Guidelines for carcinogen risk assessment. U.S. Environmental Protection Agency. EPA, Washington, DC (/600/8-87/045)
USEPA (1999) Guidelines for carcinogen risk assessment. U.S. Environmental Protection Agency. EPA, Washington, DC (NCEA-F-0644) (revised draft) risk assessment forum
USEPA (2002) Integrated risk information system (electronic database). US. Environmental Protection Agency. EPA, Washington, DC 〈http://www.epa.gov/iris〉
Lee SC, Guo H, Lam SMJ, Lau SLA (2004) Multi-pathway risk assessment on disinfection by-products of drinking water in Hong Kong. Environ Res 94(1):47–56. https://doi.org/10.1016/S0013-9351(03)00067-7
Kumari M, Gupta SK, Mishra BK (2015) Multi-exposure cancer and non-cancer risk assessment of trihalomethanes in drinking water supplies—a case study of eastern region of India. Ecotoxicol Environ Saf 113:433–438. https://doi.org/10.1016/j.ecoenv.2014.12.028
Kristiana I, Gallard H, Joll C, Croué JP (2009) The formation of halogen-specific TOX from chlorination and chloramination of natural organic matter isolates. Water Res 43(17):4177–4186. https://doi.org/10.1016/j.watres.2009.06.044
Rajeshwar KIJG, Ibanez JG, Swain GM (1994) Electrochemistry and the environment. J Appl Electrochem 24(11):1077–1091
Venczel LV, Arrowood M, Hurd M, Sobsey MD (1997) Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Appl Environ Microbiol 63(4):1598–1601
Butterfield IM, Christensen PA, Curtis TP, Gunlazuardi J (1997) Water disinfection using an immobilised titanium dioxide film in a photochemical reactor with electric field enhancement. Water Res 31(3):675–677. https://doi.org/10.1016/S0043-1354(96)00391-0
Hayfield PCS (1998) Development of the noble metal/oxide coated titanium electrode. Platin Met Rev 42(2):46–55
Kraft A (2007) Doped diamond: a compact review on a new, versatile electrode material. Int J Electrochem Sci 2(5):355–385
Patil RS, Juvekar VA, Naik VM (2014) A polarity switching technique for the efficient production of sodium hypochlorite from aqueous sodium chloride using platinum electrodes. Ind Eng Chem Res 53(50):19426–19437. https://doi.org/10.1021/ie503084m
Kuhn AT, Wright PM (1973) The behaviour of platinum, iridium and ruthenium electrodes in strong chloride solutions. J Electroanal Chem Interfacial Electrochem 41(3):329–349
Patil RS, Juvekar VA, Naik VM (2011) Oxidation of chloride ion on platinum electrode: dynamics of electrode passivation and its effect on oxidation kinetics. Ind Eng Chem Res 50(23):12946–12959. https://doi.org/10.1021/ie200663a
Kodera F, Umeda M, Yamada A (2005) Determination of free chlorine based on anodic voltammetry using platinum, gold, and glassy carbon electrodes. Anal Chim Acta 537(1):293–298. https://doi.org/10.1016/j.aca.2005.01.053
Murata M, Ivandini TA, Shibata M, Nomura S, Fujishima A, Einaga Y (2008) Electrochemical detection of free chlorine at highly boron-doped diamond electrodes. J Electroanal Chem 612(1):29–36. https://doi.org/10.1016/j.jelechem.2007.09.006
Song S, Liang Y, Li Z, Wang Y, Fu R, Wu D, Tsiakaras P (2010) Effect of pore morphology of mesoporous carbons on the electrocatalytic activity of Pt nanoparticles for fuel cell reactions. Appl Catal B Environ 98(3):132–137. https://doi.org/10.1016/j.apcatb.2010.05.021
Yi L, Liu L, Liu X, Wang X, Yi W, He P, Wang X (2012) Carbon-supported Pt–Co nanoparticles as anode catalyst for direct borohydride-hydrogen peroxide fuel cell: electrocatalysis and fuel cell performance. Int J Hydrog Energy 37(17):12650–12658. https://doi.org/10.1016/j.ijhydene.2012.06.065
Seetharaman S, Balaji R, Ramya K, Dhathathreyan KS, Velan M (2014) Electrochemical behaviour of nickel-based electrodes for oxygen evolution reaction in alkaline water electrolysis. Ionics 20(5):713–720. https://doi.org/10.1007/s11581-013-1032-9
Saha, J, Gupta, SK (2017) A novel electro-chlorinator using low cost graphite electrode for drinking water disinfection. Ionics 23(7):1903–1913
Chen MJ, Chen KN, Lin CW (2005) Optimization on response surface models for the optimal manufacturing conditions of dairy tofu. J Food Eng 68(4):471–480. https://doi.org/10.1016/j.jfoodeng.2004.06.028
Karacan F, Ozden U, Karacan S (2007) Optimization of manufacturing conditions for activated carbon from Turkish lignite by chemical activation using response surface methodology. Appl Therm Eng 27(7):1212–1218. https://doi.org/10.1016/j.applthermaleng.2006.02.046
Sharma S, Malik A, Satya S (2009) Application of response surface methodology (RSM) for optimization of nutrient supplementation for Cr (VI) removal by Aspergillus lentulus AML05. J Hazard Mater 164(2):1198–1204. https://doi.org/10.1016/j.jhazmat.2008.09.030
Aghav RM, Kumar S, Mukherjee SN (2011) Artificial neural network modeling in competitive adsorption of phenol and resorcinol from water environment using some carbonaceous adsorbents. J Hazard Mater 188(1):67–77. https://doi.org/10.1016/j.jhazmat.2011.01.067
Pal S, Mukherjee S, Ghosh S (2014) Optimum phenol sorption in peat by the response surface method. Environ Geotechnics 1(3):142–151. https://doi.org/10.1680/envgeo.13.00017
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product optimization using designed experiments. John Wiley & Sons
Ravikumar K, Pakshirajan K, Swaminathan T, Balu K (2005) Optimization of batch process parameters using response surface methodology for dye removal by a novel adsorbent. Chem Eng J 105(3):131–138. https://doi.org/10.1016/j.cej.2004.10.008
Körbahti BK, Aktaş N, Tanyolaç A (2007) Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J Hazard Mater 148(1):83–90. https://doi.org/10.1016/j.jhazmat.2007.02.005
Körbahti BK (2007) Response surface optimization of electrochemical treatment of textile dye wastewater. J Hazard Mater 145(1):277–286. https://doi.org/10.1016/j.jhazmat.2006.11.031
Körbahti BK, Tanyolaç A (2008) Electrochemical treatment of simulated textile wastewater with industrial components and Levafix blue CA reactive dye: optimization through response surface methodology. J Hazard Mater 151(2):422–431. https://doi.org/10.1016/j.jhazmat.2007.06.010
Madaria PR, Mohan N, Rajagopal C, Garg BS (2004) Application of carbon aerogel for electrolytic removal of mercury from aqueous solutions. CSIR
Baek K, Shin HJ, Lee HH, Jun YS, Yang JW (2002) Statistical modeling of electrochemical removal of sodium in fermented food composts. Korean J Chem Eng 19(4):627–631. https://doi.org/10.1007/BF02699308
Rana P, Mohan N, Rajagopal C (2004) Electrochemical removal of chromium from wastewater by using carbon aerogel electrodes. Water Res 38(12):2811–2820. https://doi.org/10.1016/j.watres.2004.02.029
Rana-Madaria P, Nagarajan M, Rajagopal C, Garg BS (2005) Removal of chromium from aqueous solutions by treatment with carbon aerogel electrodes using response surface methodology. Ind Eng Chem Res 44(17):6549–6559. https://doi.org/10.1021/ie050321p
Chakraborty S, Dasgupta J, Farooq U, Sikder J, Drioli E, Curcio S (2014) Experimental analysis, modeling and optimization of chromium (VI) removal from aqueous solutions by polymer-enhanced ultrafiltration. J Membr Sci 456:139–154. https://doi.org/10.1016/j.memsci.2014.01.016
Fradj AB, Hamouda SB, Ouni H, Lafi R, Gzara L, Hafiane A (2014) Removal of methylene blue from aqueous solutions by poly (acrylic acid) and poly (ammonium acrylate) assisted ultrafiltration. Sep Purif Technol 133:76–81. https://doi.org/10.1016/j.seppur.2014.06.038
Dang TTH, Li CW, Choo KH (2016) Comparison of low-pressure reverse osmosis filtration and polyelectrolyte-enhanced ultrafiltration for the removal of co and Sr from nuclear plant wastewater. Sep Purif Technol 157:209–214. https://doi.org/10.1016/j.seppur.2015.11.019
Gardiner WP, Gettinby G (1998) Experimental design techniques in statistical practice: a practical software-based approach. Elsevier. https://doi.org/10.1533/9780857099785
Cojocaru C, Zakrzewska-Trznadel G, Jaworska A (2009) Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. Part 1: optimization of complexation conditions. J Hazard Mater 169(1):599–609. https://doi.org/10.1016/j.jhazmat.2009.03.145
Zuorro A, Fidaleo M, Lavecchia R (2013) Response surface methodology (RSM) analysis of photodegradation of sulfonated diazo dye reactive green 19 by UV/H2O2 process. J Environ Manag 127:28–35. https://doi.org/10.1016/j.jenvman.2013.04.023
Federation WE (2012) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC
Choi J, Shim S, Yoon J (2013) Design and operating parameters affecting an electrochlorination system. Ind Eng Chem Res 19(1):215–219. https://doi.org/10.1016/j.jiec.2012.08.004
Khelifa A, Moulay S, Hannane F, Benslimene S, Hecini M (2004) Application of an experimental design method to study the performance of electrochlorination cells. Desalination 160(1):91–98. https://doi.org/10.1016/S0011-9164(04)90021-5
Bergmann MEH, Koparal AS (2005) Studies on electrochemical disinfectant production using anodes containing RuO2. J Appl Electrochem 35(12):1321–1329. https://doi.org/10.1007/s10800-005-9064-0
Badruzzaman M, Oppenheimer J, Adham S (2009) Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J Membr Sci 326(2):392–399. https://doi.org/10.1016/j.memsci.2008.10.018
Rajab M, Heim C, Letzel T, Drewes JE, Helmreich B (2015) Electrochemical disinfection using boron-doped diamond electrode–the synergetic effects of in situ ozone and free chlorine generation. Chemosphere 121:47–53. https://doi.org/10.1016/j.chemosphere.2014.10.075
Kraft A, Blaschke M, Kreysig D, Sandt B, Schröder F, Rennau J (1999) Electrochemical water disinfection. Part II: hypochlorite production from potable water, chlorine consumption and the problem of calcareous deposits. J Appl Electrochem 29(8):895–902. https://doi.org/10.1023/A:1003654305490
Kraft A, Stadelmann M, Blaschke M, Kreysig D, Sandt B, Schröder F, Rennau J (1999) Electrochemical water disinfection part I: hypochlorite production from very dilute chloride solutions. J Appl Electrochem 29(7):859–866. https://doi.org/10.1023/A:1003650220511
Kerwick MI, Reddy SM, Chamberlain AHL, Holt DM (2005) Electrochemical disinfection, an environmentally acceptable method of drinking water disinfection? Electrochim Acta 50(25):5270–5277. https://doi.org/10.1016/j.electacta.2005.02.074
Mezule L, Denisova V, Briedis A, Reimanis M, Ozolins J, Juhna T (2015) Disinfection effect of electrochemically generated chlorine on surface associated Escherichia coli in a drinking water system. Desalin Water Treat 53(13):3704–3710. https://doi.org/10.1080/19443994.2013.873742
Krstajić N, Nakić V, Spasojević M (1991) Hypochlorite production II. Direct electrolysis in a cell divided by an anionic membrane. J Appl Electrochem 21(7):637–641. https://doi.org/10.1007/BF01024853
Acknowledgements
The authors thank the financial support from Indian Institute of Technology (ISM), Dhanbad, funded by the Ministry of Human Resource Development (MHRD), Government of India, New Delhi, for carrying out this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Study on a novel electro-chlorination reactor using low-cost graphite anodes and stainless steel cathodes for drinking water treatment.
• The study was conducted with electrolytes at low chloride concentration occurring naturally in the surface water.
• The optimization process was carried out using designs of experiment-based response surface methodology (RSM).
• Box-Behnken design was used for evaluating the impacts of the process factors on the response.
• The experimentally determined response levels conformed satisfactorily with the model assumed theoretical values at an R2 value of 0.98.
Rights and permissions
About this article
Cite this article
Saha, J., Gupta, S.K. Application of response surface methodology for optimization of an onsite electro-chlorinator for drinking water treatment. Ionics 24, 3237–3248 (2018). https://doi.org/10.1007/s11581-017-2430-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2430-1