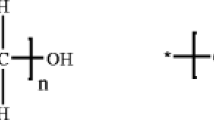Abstract
A novel PEO-based blends solid polymer electrolytes doping liquid crystalline ionomers (LCI), PEO/PMMA/LiClO4/LCI, and PEO/LiClO4/LCI were prepared by solution casting technology. Scanning electron microscope (SEM) and energy-dispersive spectroscopy (EDS) analysis proved that LCI uniformly dispersed into the solid electrolytes and restrained phase separation of PEO and PMMA. Differential scanning calorimetry (DSC) results showed that LCI decreases the crystallinity of blends solid polymer electrolytes. Thermogravimetric analysis (TGA) proved LCI not only improved thermal stability of PEO/PMMA/LiClO4 blends but also prevent PEO/PMMA from phase separation. Infrared spectra results illustrated that there exists interaction among Li+ and O, and LCI that promotes the synergistic effects between PEO and PMMA. The EIS result revealed that the conductivity of the electrolytes increases with LiClO4 concentration in PEO/PMMA blends, but it increases at first and reaches maximum value of 2.53 × 10−4 S/cm at 1.0 % of LCI. The addition of 1.0 % LCI increases the conductivity of the electrolytes due to that LCl promoting compatibility and interaction of PEO and PMMA. Under the combined action of rigidity induced crystal unit, soft segment and the terminal ionic groups in LCI, PEO/PMMA interfacial interaction are improved, the reduction of crystallinity degree of PEO leads Li+ migration more freely.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to unique pore molecular space structure of polyethylene oxide (PEO), PEO not only provides extremely high density of electron-donating group but also possess flexible polyether chain segment. It can dissolve cation effectively by cage effects to form complexes with lithium salt. This makes PEO the best option of matrix material of polymer electrolyte [1, 2]. The high crystallinity of PEO leads to a low conductivity of solid polymer electrolyte [3]; therefore, decreasing crystallinity of PEO becomes a main task to improve ion conductivity of solid polymer electrolyte.
Since their discovery in 1973 [4], solid-like polymer electrolytes (SPEs) have been extensively investigated. In 1991, Xu Xi [5] pointed out that the crystallization of PEO was inhibited by hydrogen bonding complexation between poly(methyl methacrylate-co-methacrylic acid) [P(MMA-co-MAA)] and PEO when the mole fraction of MAA in P(MMA-co-MAA) is above 0.5.
Gelpolymerelectrolytes (GPEs) based on a co-polymer matrix of methoxy-poly(ethyleneglycol) methacrylate and hexadecal-poly(ethylene glycol) methacrylate were synthesized, which possess excellent thermal stability and exhibit relatively high ionic conductivity of 0.59 × 10−3 S · cm−1 at 303 K [6]. S. Radhakrishnan [7] verified that the existence of PMMA limited the crystallization process of PEO by disturbing the regular molecular arrangement of PEO, so the high crystallinity of PEO could be weakened. For PEO/LiClO4 polymer electrolyte, reduction of PEO crystallinity benefits to Li+ migration from one ether bond to another ether bond, which own to the much easier segment movement in amorphous region. Although PMMA could weaken PEO crystallinity effectively, a new method to prevent phase separation between PEO and PMMA should be carried out.
In 1999, C.T. Imrie [8] found a new side-group liquid crystalline polymer electrolyte system in which the ionic conductivity is insensitive to T g (278 K), and sub-T g conductivities was 10−5S · cm−1. Ganesh Chandra Nayak [9] noted that liquid crystalline polymers (LCP) could reinforce polymer blends which are incompatible in nature, but for its rigid structure there occurred interfacial slippage at the polymer-LCP interface, so in order to bind the two polymer phases together, the rigid structure of LCP could be improved. The study of liquid crystalline ionomer (LCI) containing a sulfonate group was initiated in 2000 [10], which a serious phase separation problem in polar-nonpolar polymer blends was resolved by doping LCI. Qu Wenzhong [11] prepared a main chain liquid crystalline ionomers (LCI) with sulfonic group and proved LCI could improve the compatibility of poly (butylene terephthalate) (PBT) and polypropylene (PP) blends. The same conclusion was reached by Xu Xinyu [12] with three side-chain liquid crystalline ionomers (SLCIs) using to the same PBT/PP systems, which indicated that SLCIs lead to finer and better dispersion of PP polymer in the blends. Li Yuanming [13] discovered that LCI could play the same role in PA1010/PP blends. Stoeva Z [14] prepared a discotic liquid crystal triblock copolymer consisting of LCP block capped at both ends by blocks of PEO doped with lithium perchlorate in order to improve Li+ conductivity by suppressing PEO crystallinity degree, but phase-separated phase separation could be prevented efficiently.
In this work, a novel PEO-based blends solid polymer electrolytes doping liquid crystalline ionomers (LCI), PEO/PMMA/LiClO4/LCI and PEO/LiClO4/LCI, were prepared by solution casting technology to investigate the effection of LCI on ionic conductivity and thermal behavior of electrolytes. It has been studied on the influence of main-chain liquid crystalline ionomers (LCI) containing sulfonate groups on properties of polymer electrolyte by mixing PMMA, PEO separately with LCI, and the results showed that low content of LCI has a very good compatibility with the two matrix and, besides, ionic conductivity and mechanical properties of polymer electrolyte can be enhanced. For LCI, a high rigidity block interact with PEO of crystal state, a long soft block will combine with PMMA of amorphous structure; in addition, an existence of small amount sulfonate group will promote the polarity of polymer than other groups, such as carboxyl [15, 16]. So by comparing PEO/PMMA/LiClO4/LCI and PEO/LiClO4/LCI systems, to investigate the influence of LCI on electrolyte properties is necessary.
Experimental
Materials
PEO (Mw = 6 × 105) and PMMA (Mw = 1.2 × 105) were all purchased from Aldrich Chemicals. LiClO4 was produced by Tianjin Jinke institute of precision chemistry. Above materials were all deposited in vacuum dryer. Dichloromethane and NMP were all from Sinopharm Chemical Reagent Company. The materials were used after purification. LCI was prepared according to the literature [17], and Fig. 1 shows the structural formula of LCI.
Preparation of solid polymer electrolyte (SPE)
Prior to the preparation of polymer electrolytes, PEO, PMMA, LCI, and LiClO4 were dried in vacuum oven for 24 h at 40 °C in order to eliminate trace amounts of water in the material. PEO and PMMA were dissolved with a certain ratio in dichloromethane 30 g L−1 of polymer by weight. The solution was stirred at room temperature until the mixture turned to pellucid solution. After LiClO4 and LCI were dissolved in N-methyl-2-pyrrolidone (NMP) with a certain ratio, they were mixed with PEO/PMMA blends and pure PEO solution, and then casted on a tetrafluoroethylene mold after the mixture solution became homogeneous and transparent. The solvent was quickly evaporated and further dried in vacuum drying oven for 48 h at −0.08 MPa, and NMP was distilled through dichloromethane. The film thickness is kept at about 20 μm.
Instruments for characterization
The ionic conductivities were obtained by impedance spectroscopy using CHI604D in the frequency range from 10 Hz to 100 MHz with signal amplitude of 10 mV. IR spectra were determined on a IR Prestige-21 spectrometer in the region of 400 to 4000 cm−1. Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed under a continuous nitrogen flow using a TA Instruments Q50 and Q20DSC at a heating rate of 20° and 10 °C min−1. The morphology of the nanostructures was observed by a SU8010 field emission scanning electron microscope (FESEM), and the elemental composition of the samples was detected by the energy-dispersive X-ray spectrometry (EDS) equipped on the SU8010 FESEM.
Results and discussion
SEM characterization of solid polymer electrolytes
Three phase zones are obvious in Fig. 2: (1) PEO crystalline region of near circle, without LiClO4 crystal and micropore structure; (2) PEO/PMMA blends region with LiClO4 crystal; (3) PMMA microporous region without LiClO4 crystal. Because LiClO4 has an inhibitory effect on crystallization of PEO [18], and high-molecular-weight PEO is easy to form spherical crystallization, it can be deduced that micro-area 1 is area of PEO crystallization, and microporous region is a characteristic for PMMA. When content of LCI is higher, the area 2 of PEO/PMMA blends increases with the decreasing of PMMA microporous. It illustrates that PEO and PMMA disperse more uniform with the action of LCI.
Figures 3 and 4 are EDS analysis results of PEO/LiClO4/LCI and PEO/PMMA/LiClO4/LCI blends. Cl atom and C atom were provided, respectively, by LiClO4 and PEO; O atom was provided by PEO and LiClO4 in PEO/LiClO4/LCI blends. It can be seen from Table 1 that Na and S content both increases after the addition of LCI. C atom is provided by PEO and PMMA, O atom is provided by PEO, PMMA, and LiClO4 in PEO/PMMA/LiClO4/LCI blends. From Table 2, we can infer the same conclusion. It illustrates that LCI made PEO/PMMA mixing sufficient. It does provide support for LCI enhanced PEO/PMMA compatibility.
FTIR spectra characterization of solid polymer electrolytes
The FTIR spectra of composite polymer electrolytes are shown in Fig. 5. 1063–1193 cm−1 wavenumber is the stretch vibration absorption spectra of ether bond [19, 20]. The absorption peak shifts to low wave number direction with the addition of LiClO4, illustrating that there is a coordination effect between Li+ and O, with vibrational C-O energy levels decreasing, vibrational frequency dropping and wave number falling. The result is in accordance with literature [21]. In the wave number region between 3200 and 3500 cm−1, no apparent H2O absorption peak was observed, and it means that H2O absorption effection of PEO was interrupted effectively by waterless operation, so that the effection of H2O residue to conductivity can be eliminated.
Figure 6 shows the FTIR spectra of PEO/LiClO4/LCI electrolytes with different LCI contents. 1060–1146 cm−1 wavenumber range is the stretch vibration absorption spectra of ether bond in amorphous phase PEO. With the increasing of LCI content, no apparent change of peaks position was observed.
Figure 7 shows the FTIR spectra of PEO/PMMA/LiClO4/LCI blends with different LCI contents. The absorption peak of C-O bonds of PMMA occurs at 1193 cm−1, and with the LCI content increasing, the absorption peak of C-O bonds of PEO at 1061–1150 cm−1 turns to a broad peak, which illustrates that LCI promotes the interaction between PEO and PMMA.
Thermal analysis
Heating and cooling DSC curves of electrolytes are shown in Figs. 8 and 9. Table 3 lists the detailed data. Melting range (ΔT m) is inversely proportional to perfection of crystallization, ΔTc is proportional to crystal growth rate, and X c is crystallinity degree. It can be seen pure PEO owns a comparatively perfect crystal structure, narrow particle size distribution and higher crystallinity. The existence of PMMA and LiClO4 disrupts the crystal structure of PEO [22–25], with particle size distribution and crystal growth rate getting larger, crystallinity degree decreasing. LCI exists as crystal at room temperature, and its addition will improve perfection of crystallization, but because of its compatibility to PEO/PMMA and plasticization to PEO, crystallization keeps decreasing. As the content of LCI is increased, the compatibility and plasticization become more apparent.
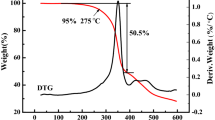
In both Figs. 10 and 11, there does not occur a weight loss between 80 and 250 °C, which proved that H2O and NMP have been removed thoroughly in vacuum drying oven [26]. There exists two phases of PEO in the blends: crystal state and amorphous state. In Fig. 10, The decomposition temperature of amorphous state PEO is at 300–350 °C, while crystal state PEO is at 350–430 °C. The addition of LCI improved the thermal stability, which decomposition temperature of both phase all raised. In Fig. 11, with the addition of PMMA, content of amorphous state PEO increased, and there occurs two obvious peaks at 380 °C (decomposition temperature of PMMA) [27] and 400 °C (decomposition temperature of PEO) without LCI addition. When LCI was added into the blends, the two peaks combined to one peak at 395 °C, which proved LCI prevented PEO and PMMA from phase separation.
Electrical performance analysis of solid polymer electrolytes
Figure 12 shows the dependence of conductivity of electrolyte with different PEO/PMMA ratios on LiClO4 content. It can be seen that the conductivity of these three kinds of electrolyte increases with the increasing of LiClO4 content, and the conductivity of PEO:PMMA = 70:30 group is higher than the other two groups. Conduction of electrolyte mainly depends on migration of Li+, and lithium charge carrier number increases with Li+ content increasing.
The relationships between LCI content and SPE conductivity with different PEO:PMMA:LiClO4 ratios are shown in Fig. 13. It can be seen with the LCI content increasing, the conductivity increases and reaches the maximum 2.53 × 10−4S/cm at PEO:PMMA:LiClO4:LCI = 70:30:10:1. When LCI content is higher than 1 %, the conductivity of PEO:PMMA:LiClO4 = 70:30:10 system decreases sharply while other systems change little. There maybe two contributions of LCI to conductivity going up: LCI plays compatibilizer in SPE distributing on the phase interface to decrease the interfacial tension, improves the state of interface and phase morphology, so as to promote movement of polymer chain; Li+ can be transmitted by sulfonate groups of LCI. But LCI exists as crystal at room temperature, and high content of LCI will enhance the crystallization PEO, and the higher PEO content, the higher crystallization of PEO.
Figure 14 shows the conductivity contrast of PEO/LiClO4/LCI and PEO/PMMA/LiClO4/LCI blends with different LCI contents. It can be seen in PEO/LiClO4/LCI blends, the electrolytes conductivities decrease linearly with the increase of LCI contents. The reason may be as follows: LCI as crystal at room temperature, when it was thorough mixed with PEO, improved crystallization property of PEO, thus causing the decreasing of electrolytes conductivity. But in PEO/PMMA/LiClO4/LCI blends, with the LCI content increasing, the conductivity increases at first and reaches the maximum 3.16 × 10−4S/cm, which draws the same conclusion as a repeated experiment. Here, the conclusion can be also made that LCI improved the electrolytes conductivity by promoting the interaction between PEO and PMMA.
Figure 15 shows the temperature dependence of ionic conductivity of SPE with different LCI contents. It can be seen the conductivity increases with the temperature rising. Thermal motion leads to relaxation and migration of Li+, the closer the controlled temperature to Tg, the faster the relaxation and migration of Li+, and the higher the conductivity.
Mechanism of compatibility
PEO owns a high crystal degree for its strong polarity, which constrains the molecular chain movement, so that Li+ migration from one oxygen atom to another oxygen atom in PEO gets difficult. PMMA as an amorphous polymer, when it blends with PEO, the regular molecular arrangement will be disrupted, but for the reason of PEO/PMMA poor compatibility, phase separation occurs. LCI consisting of rigidity induced crystal unit, soft segment and the terminal ionic groups, when it used as a compatibilizer, rigidity-induced crystal unit will improve the thermal stability and mechanical properties of the blends, meanwhile, its strong polar imide structure leads a better compatibility with PEO; soft segment in LCI will leads a better compatibility with PMMA; sulfonic acid group could form hydrogen bonds with oxygen atoms not only in ether of PEO, but also in ester of PMMA side chains (Fig. 16).
Under the combined action of three functional groups in LCI, PEO/PMMA interfacial interaction is improved obviously, under the traction of soft segment and the terminal ionic groups of LCI, flexible molecular chains of PMMA can be easily inserted into the crystal state molecular chains of PEO, the regular arrangement of oxyethylene chain gets disrupted, the degree of crystallinity reduces significantly, with the improving of molecular chain activity ability, lithium ions transfer more freely.
Conclusion
This work prepared novel PEO-based blends solid polymer electrolytes doping liquid crystalline ionomers, PEO/PMMA/LiClO4/LCl, and PEO/LiClO4/LCl. SEM photos and EDS results prove LCI uniformly scattered in electrolytes and restrain phase separation of PEO/PMMA by enhancing PEO/PMMA compatibility. Infrared spectra suggest that there exists coordination interactions between Li+ and O, and LCI promotes the interaction between PEO and PMMA. DSC results show that LCI decreases the crystallinity degree by promoting compatibility of PEO/PMMA. TGA analysis proves LCI not only improved thermal stability of PEO/PMMA/LiClO4 blends but also prevents PEO/PMMA from phase separation. The results of EIS show that the conductivity of the electrolytes increases with the concentration of LiClO4 in PEO/PMMA blends, but with the addition of LCI, it increases at first and reaches maximum 2.53 × 10−4S/cm at 1.0 % of LCI content. under the combined action of rigidity induced crystal unit, soft segment and the terminal ionic groups in LCI, PEO/PMMA interfacial interaction are improved, the reduction of crystallinity degree of PEO leads Li+ migration more freely.
References
Nazri GA, Pistoia G (2008) Lithium batteries: science and technology. Springer Science & Business
Scrosati B, Garche J (2010) J Power Sources 195(9):2419
Scrosati B, Croce F, Persi L (2000) J Electrochem Soc 147(5):1718
Fenton D, Parker J, Wright P (1973) Polymer 14(11):589
Xu X, Wang Q (1991) Chem J Chin Univ 12(3):413
Cai F, Zuo X, Liu XM (2013) Electrochim Acta 106:209
Radhakrishnan S, Venkatachalapathy P (1996) Polymer 37(16):3749
Imrie CT, Ingram MD, Mchattie GS (1999) Adv Mater 11(10):832
Nayak GC, Das CK (2016) Liquid Crystalline Polymers: LCP Based Polymer Blend Nanocomposites. Springer International Publishing, p251
Li Y, Zhang BY, Feng Z (2002) J Appl Polym Sci 83(13):2749
Qu WZ, Xu XY, Chu HZ, Zhang BY (2011) Polym Mater Sci Eng 27(2):107
Xu XY, Zhou ZL (2015) Appl Mech Mater 751:21–25
Li YM, Zhang BY, Wang J (2002) J Funct Polym 15(1):1
Stoeva Z, Lu Z, Ingram MD (2013) Electrochim Acta 93:p279
Xia Y, Wang S, Ma N (2014) China Synth Resin Plast 31(2):25
Donald AM, Windle AH, Hanna S (2006) Liquid Crystalline Polymers: Liquid crystalline polymers in blends and composites (Cambridge University Press), p483
Zhang AL (2002) Main-Chain Liquid Crystalline Ionomer and Composite Materials. Ph.D., Northeastern University, China
Liu Q, Pan C, Shen S (2006) Chin J Nonferrous Met 16(2):377
Wang Y, Li M, Rong J (2013) Colloid Polym Sci 291(6):1541
Su YL, Liu HZ, Guo C (2003) Mol Simul 29(12):803
Ramesh S, Yuen TF, Shen CJ (2008) Spectrochim Acta A Mol Biomol Spectrosc 69(2):670
Ghelichi M, Qazvini NT, Jafari SH (2013) J Appl Polym Sci 129(4):1868
Chen N, Yan LT, Xie XM (2013) Macromolecules 46(9):3544
Schwahn D, Pipich V, Richter D (2012) Macromolecules 45(4):2035
Shi W, Yang J, Zhang Y (2012) Macromolecules 45(2):941
Angulakshmi N, Thomas S, Nahm KS (2011) Ionics 17(5):407
Toshimi H, Takashi K, James EB (1985) Macromolecules 18:1410
Acknowledgments
Liaoning Provincial Key Laboratory for Polymer Catalytic Synthesis Technology (Document No.36 by DST, Liaoning Province [2010].); Advanced Polymer Materials Engineering Laboratory in Liaoning province (2012.5); and Shenyang Science and Technology plan project (F14-231-1-28) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Al., Cao, Fy., Na, Gz. et al. A novel PEO-based blends solid polymer electrolytes doping liquid crystalline ionomers. Ionics 22, 2103–2112 (2016). https://doi.org/10.1007/s11581-016-1732-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1732-z