Abstract
The LiFe0.98M0.02PO4/C (M = Ni, V, and Mn) composite materials were synthesized by solid state reaction using Fe2O3 as a mixed iron source and citric acid as a carbon source. The crystal structure features and particle morphology were characterized by X-ray diffraction (XRD) and scanning electron microscope (SEM). The electrochemical properties of samples were characterized by charge-discharge testing, cyclic voltammetry (CV), and electrochemical impedance spectra (EIS). It is found that Ni2+, V5+, and Mn2+ doping do not destroy the olivine structure of LiFePO4/C but can stabilize the crystal structure and decrease average particle size. Electrochemical test results indicate Ni2+ and V5+ doping decrease charge transfer resistance and enhance Li+ ion diffusion velocity and thus improve cycling and high-rate capability of the LiFePO4/C. Especially, the LiFe0.98V0.02PO4/C shows the best cycling stability and rate capability among all the doped samples. The maximum discharge capacities of LiFe0.98V0.02PO4/C at 0.1, 0.2, 0.5, 1.0, and 2.0C are 152, 140.9, 130.3, 119.8, and 105.9 mAh/g, respectively,which are significantly higher than other samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, lithium-ion battery has quickly occupied the portable electronics market and is under development for electric vehicles (EVs), hybrid electric vehicles (HEVs), and plug-in hybrid electric vehicles (PHEVs) [1]. Widespread attentions have been paid upon the development of cathode material. Compared with LiNiO2, LiCoO2, and LiMn2O4, the most promising cathode material, olivine-type LiFePO4 presents several advantages, such as low material cost, no toxicity, high theoretical capacity (170 mAh/g), thermal stability at a rather high temperature, safety, and so on. However, LiFePO4 suffers from two drawbacks of poor ionic conductivity and poor electronic conductivity, which are the major limitations of its large-scale application such as in electric vehicles [2]. To overcome the problems, several methods have been developed, such as coating electronically conductive additives (carbon or metal) [3, 4], doping with supervalent cation [5, 6], and minimizing the particle size [7].
It has been reported that carbon coating is an effective way to increase the electrochemical performance of these materials as well as to avoid the formation of Fe3+ oxidation state. However, the carbon coating method obviously helps nothing in the lattice electronic conductivity or chemical diffusion coefficient of lithium within the crystal. On the other hand, metal ion doping of LiFePO4 is a convenient and effective way to improve the electrochemical performance of LiFePO4. The previous research proves that doping metal ions at Li-site can hinder the diffusion of Li ions [8, 9], which leads to poor electrochemical performance, so considerable effort is paid to the Fe-site doping. The doping at Fe-site can effectively stabilize the crystal structure and also improve the transportation of electrons and probably Li ion. First-principle calculation shows that metal cations doping at Fe-site of LiFePO4 facilitate the diffusion of Li ions, and thus, the electronic and ionic conductivity can be improved [10]. It is worth mentioning that first-principle method has been demonstrated crucial in the study of electrode materials for practical lithium-ion battery. Up to now, many metal ions, for example, Mg2+, Co2+, Nb5+, and so on, were reported as doping ions.
However, to the best of our knowledge, the way of considering both the aspects of metallic element doping and carbon coating to improve the performance of LiFePO4 has been reported less. In this study, the LiFe0.98M0.02PO4/C (M = Ni, V, and Mn) composite materials were synthesized by solid state reaction, using Fe2O3 as an iron source and citric acid as a carbon source. Our aim is to improve the electrochemical performance, especially its high-rate lithium intercalation/de-intercalation properties, and study the mechanism why the capability is changed by doping metal ions.
Experimental
Material synthesis
The stoichiometric amounts of Fe2O3, Li2CO3, NH4H2PO4, and doping material (C4H6NiO4 · H2O,NH4VO3 or Mn(CH3COO)2 · 4H2O) were added the proper acetone. Then, the mixture was ball-milled for 5 h. An appropriate amount of citric acid was added to deionized water to form a saturated solution, which was as the reducing agent and coated carbon. The carbon content is about 5 % in the obtained powders. After ball-milling, the mixture was slowly introduced into the citric acid solution under stirring for 10 min to get a rheological body.
Later, the mixture was calcinated in a tube furnace at 350 °C for 5 h with flowing nitrogen gas then sintered at 650 °C for 12 h and cooled down to room temperature in the tube furnace under the same atmosphere. LiFePO4/C and LiFe0.98M0.02PO4/C (M = Ni, V, and Mn) composite materials were obtained.
Material characterization
XRD measurements were collected on a Rigaku Rint-2000 X-ray diffractometer with Cu Kα radiation in the 2θ range of 10° ≤ 2θ ≤ 90° to identify the structures of the synthesized materials. The morphology of the sample powders was observed by scanning electron microscopy (SEM) with FEI, Nova NANOSEM-450.
Electrochemical characterization
Electrochemical properties of cathodes were evaluated using CR2032 coin cell. Cells were composed of a cathode and a lithium metal anode separated by a polypropylene microporous film (Celgard 2300).
Cathode electrodes were fabricated by blending the active material with conductive carbon black and polyvinylidene fluoride (PVDF) in the weight ratio of 85:8:7 while N-methyl-pyrrolidone (NMP) was used as solvent. Then, the slurry was cast on aluminum foil and dried at 110 °C for 10 h in vacuum. The obtained electrode film was subsequently pressed and punched into a circular disk. LiPF6(1M) in a 1:1:1 (v/v/v) mixture of dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), and ethylene carbonate (EC) was used as an electrolyte. The coin cells were assembled in an argon-filled glove box. The cells were cycled at different C-rates between 2.4 and 4.2 V with a land battery testing system at room temperature. The current value for different C-rates was calculated from the theoretical capacity of LiFePO4 (1C-rate = 170 mAh/g). Cyclic voltammetry (CV) was conducted by a CHI660a electrochemical analyzer, operated at 0.1 mV/s between 2.4 and 4.2 V. Electrochemical impedance spectroscopy (EIS) was also performed on this apparatus with a frequency range from 100 to 0.01 Hz.
Results and discussion
Structure and morphology analyses
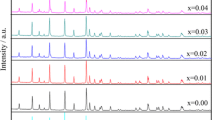
Figure 1 shows the XRD patterns of LiFePO4/C and doped LiFe0.98M0.02PO4/C (M = Ni, V, and Mn) materials. The crystal phases of the samples are in full accord with the ordered olivine structure indexed orthorhombic Pnma (JCPDS Card No.40-1499), and no extra reflection peak from impurity is observed, indicating that a small amount of doping metal ions does not destruct the lattice structure of LiFePO4. Citric acid is decomposed into carbon through high temperature which is believed to be present as amorphous carbon or low degree of crystallinity [11]. Table 1 shows the corresponding structural parameters and unit cell volume of the samples. The lattice parameters and unit cell volume slightly decrease after doping metal ions. Compared with the LiFePO4/C, the LiFe0.98V0.02PO4/C presents the less lattice parameters (a = 0.59994 nm, b = 1.0306 nm, and c = 0.46841 nm) and the smaller unit cell volume (v = 0.28962 nm3), which is possible to improve the lithium ion diffusion rate. The results are attributed to the smaller ionic radii of Ni2+ (0.070 nm), V5+ (0.054 nm), and Mn2+ (0.067 nm) compared with those of Fe2+ (0.078 nm) and indicate that Fe2+ ions should have been partially substituted by metal ions [12, 13].
Figure 2 shows the SEM of LiFePO4/C and doped LiFe0.98M0.02PO4/C (M = Ni, V, and Mn) materials. The morphology of LiFePO4/C and LiFe0.98M0.02PO4/C powders is very similar, and doping metal ions (Ni, V, and Mn) have little influence on the morphology of LiFePO4/C, as shown in Fig. 2a, b, c, d. The average crystal size of sample particles is uniform, although there are slight agglomeration and a small quantity of fragments. The unique morphology and size are due to the precursor of adding citric acid, which is decomposed into a coating layer on the LiFePO4 particle surface, protecting LiFePO4 from oxidation and agglomeration, during the following high-temperature processing. It is believed that the unique morphology is conducive to shorten the lithium diffusion distance and make it possible to achieve better electrochemical performance [14].
Electrochemical characteristics
Figure 3 shows the initial charge-discharge curves at 0.1C at room temperature for all samples. All the samples exhibit flat voltage plateaus around 3.5 V, which is the main characteristic of the two-phase reaction of the lithium extraction and insertion between LiFePO4 and FePO4 [15, 16]. There is a slight difference in the charge and discharge plateau voltages that can be attributed to the polarization of electrode. The initial discharge capacities of samples (a, b, c, and d) are 142.1, 131.5, 150.9, and 125.3 mAh/g, respectively. From the above results, we can find doping vanadium can improve the initial discharge capacities, which indicates low polarization of doping vanadium cathode due to the better electron conductivity and Li+ diffusion [17]. They are perfectly proved by the electrochemical tests latter.
Figure 4 shows the C-rate performances of all samples. It is clear that C-rate performances of LiFePO4/C are greatly enhanced after doping nickel and vanadium, especially high rate performance. The maximum specific capacity of LiFe0.98V0.02PO4/C at 0.1, 0.2, 0.5, 1.0, and 2.0C are 152, 140.9, 130.3, 119.8, and 105.9 mAh/g, respectively, which are significantly higher than other samples. Moreover, the cycle stability of LiFe0.98V0.02PO4/C is much superior to other samples. After 10 cycles at 0.1, 0.2, 0.5, 1.0, and 2.0C, the discharge specific capacity of LiFe0.98V0.02PO4/C is 148.2, 140.8, 129.1, 119.6, and 104.6 mAh/g, and the capacity retention is 98.21, 100.85, 99.31, 100.25, and 98.77 %, respectively. The main reason leading to the decrease of the cycle stability and C-rate performances is that the material structure has changed with charge and discharge number increasing[18, 19]. However, the appropriate vanadium doping plays a significant role in stabilizing the crystallite structure. Therefore, it is concluded that LiFe0.98V0.02PO4/C possesses the best C-rate and cycle performances among all the samples.
Figure 5 shows the cyclic voltammetry (CV) curves of all the samples. Each of the curves has one pair of redox peaks which correspond to the electrochemical intercalation/de-intercalation reactions of lithium ions. This is corresponding to the voltage plateaus in Fig. 3. It can be seen that each of the redox peak profiles of LiFe0.98M0.02PO4/C (M = Ni, V, Mn), especially doping vanadium, are more symmetric than those of LiFePO4/C. The potential separation between the two peak profiles is 0.392, 0.384, and 0.395 V for doping nickel, vanadium, and manganese, respectively, whereas the potential separation of LiFePO4/C is 0.406 V, higher than that of the doping metal ions samples. Moreover, the LiFe0.98V0.02PO4/C has the largest peak currents. This clearly implies the enhancement of the Li+ diffusion ability [20–22]. The decreased redox peak potentials separation and increased peak currents demonstrate that the reversibility and reactivity of LiFePO4/C with doping vanadium were greatly enhanced.
The electrochemical impedance spectra (EIS) are used to further analyze the effect of doping metal ion on the electrode reaction impedance. Before EIS tests, the cells are cycled for two cycles at 0.1C rate. The electrodes were conducted at 50 % of discharge or charge state to avoid the huge potential change brought by small current alternation during EIS measurements [17], and the results were fitted using Zview-Impedance 2.80 software. Figure 6 shows the impedance spectra and simplified equivalent circuit model (inset) of LiFePO4/C and LiFe0.98M0.02PO4/C. Usually, an intercept in the high-frequency region of the Z’ real axis corresponds to the ohmic resistance (Rs), which represents the resistance of the electrolyte and electrode material. The semicircle in the middle-frequency range indicates the charge transfer resistance (Rct) [23, 24]. The inclined line in the low frequency represents the Warburg impedance (Zw), which is associated with lithium ion diffusion in the LiFePO4 particles.
The lithium ion diffusion coefficients (DLi+) of LiFePO4/C and LiFe0.98M0.02PO4/C are calculated according to the following equation [25]:
Here, R is the gas constant, T is the absolute temperature which is 298.15 in this present work, and n is the number of electrons per molecule during oxidization, F is Faraday’s constant, A is the area of the cathode/electrolyte interface, C is the concentration of lithium ion [26], and σ is the Warburg factor which is associated with Z’.
Herein, Rs is the electrolyte resistance, Rct is the charge transfer resistance, and ω is the angular frequency in the low-frequency region. Figure 7 shows the relationship plot between Z’ and reciprocal square root of the angular frequency (ω −1/2) in the low-frequency region [27]. The diffusion coefficient of lithium ion was calculated in Eqs. (1) and (2), listed in Table. 2. We can find that, compared with the other samples, LiFe0.98V0.02PO4/C possesses the lowest charge transfer resistance of 28.026 Ω and the higher diffusion coefficient of lithium ion of 1.408 × 10−13 cm2 S−1. It will facilitate the charge and discharge process at a high C-rate condition. This is in accordance with the analysis of C-rate performances, which resulted from V5+ doping leads to the elevated electrochemical activity of LiFePO4/C.
Conclusions
The LiFe0.98M0.02PO4/C (M = Ni, V, and Mn) composite materials were synthesized by solid state reaction using Fe2O3 as an iron source and citric acid as a carbon source, and all the raw materials are inexpensive and with good operability. High valence state and small radius Ni2+, V5+, and Mn2+ are used to dope LiFePO4. Doping Ni2+ and V5+ can enhance the electrochemical performance of LiFePO4/C, especially doping V5+. The maximum discharge capacity of LiFe0.98V0.02PO4/C at 0.1, 0.2, 0.5, 1.0, and 2.0C are 152, 140.9, 130.3, 119.8, and 105.9 mAh/g, respectively, which are significantly higher than the others. Moreover, the cycle stability of LiFe0.98V0.02PO4/C is much superior to other samples. This is because doping V5+ can stabilize the crystal structure, decrease charge transfer resistance, and enhance Li+ ion diffusion velocity thus improve cycling and high-rate capability of the LiFePO4/C.
References
Amine K, Liu J, Belharouak I (2005) High-temperature storage and cycling of C-LiFePO4/graphite Li-ion cells. Electrochem Commun 7:669–673
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Ravet N, Chouinard Y, Magnan JF, Besner S, Gauthier M, Armand M (2001) Electroactivity of natural and synthetic triphylite. J Power Sources 97–8:503–507
Mi CH, Cao GS, Zhao XB (2005) Low-cost, one-step process for synthesis of carbon-coated LiFePO4 cathode. Mater Lett 59:127–130
Chung SY, Bloking JT, Chiang YM (2002) Electronically conductive phospho-olivines as lithium storage electrodes. Nat Mater 1:123–128
Saiful Islam M, Driscoll DJ, Craig AJ, Slater PR (2005) Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem Mater 17:5058–5092
Yamada A, Chung SC, Hinokuma K (2001) Optimized LiFePO4 for lithium battery cathodes. J Electrochem Soc 148(3):A224–A229
Khang H, Michelle J (2011) Tailoring native defects in LiFePO4: insights from first-principles calculations. Chem Mater 23(11):3003–3013
Ouyang CY, Shi SQ, Wang ZX, Li H, Huang XJ, Chen LQ (2004) The effect of Cr doping on Li ion diffusion in LiFePO4 from first principles investigations and Monte Carlo simulations. J Phys: Condens Matter 16(13):2265–2272
Hong L, Zhaoxiang W, Liquan C, Xuejie H (2009) Research on advanced materials for Li-ion batteries. Adv Mater 21(45):4593–4607
Jianjun S, Guangjie S, Meiwu S, Zhipeng M (2013) The effect of doping Co on the electrochemical properties of LiFePO4/C nanoplates synthesized by solvothermal route. Solid State Ionics 253:39–46
Deyu W, Hong L, Siqi S, Xuejie H, Liquan C (2005) Improving the rate performance of LiFePO4 by Fe-site doping. Electrochim Acta 50(14):2955–2958
Zeheng Y, Jianfeng X, Lihua Z, Weixin Z (2014) An improved solid-state reaction route to Mg2+-doped LiFePO4/C cathode material for Li-ion battery. Ionics 20:169–174
Yourong W, Yang Yifu H, Xin YY (2009) Electrochemical performance of Ru-doped LiFePO4/C cathode material for lithium-ion batteries. J Alloys Compd 481:590–594
Masaya T, Tobishima S, Koji T, Yoji S (2001) Characterization of LiFePO4 as the cathode material for rechargeable lithium batteries. J Power Sources 97–8:508–511
Jiangfeng N, Morishita M, Yoshiteru K, Masaharu W, Nobuhiko T, Tetsuo S (2010) Hydrothermal preparation of LiFePO4 nanocrystals mediated by organic acid. J Power Sources 195:2877–2882
Shi SJ, Tu JP, Mai YJ, Zhang YQ, Gu CD, Wang XL (2012) Effect of carbon coating on electrochemical performance of Li1.048Mn0.381Ni0.286Co0.286O2 cathode material for lithium-ion batteries. Electrochim Acta 63:112–117
Liang F, Yaochun Y, Yongnian D, Bin Y, Wenhui M (2012) Preparation of porous structure LiFePO4/C composite by template method for lithium-ion batteries. Solid State Ionics 214:31–36
Hsieh Chien T, Ling CI, Yu CW (2012) Synthesis of iron phosphate powders by chemical precipitation route for high-power lithium iron phosphate cathodes. Electrochim Acta 83:202–208
Yanpu L, Enshan H, Lingzhi Z, Ling Z (2014) The effect of MgO coating on Li1.17Mn0.48Ni0.23Co0.12O2 cathode material for lithium ion batteries. Solid State Ionics 255:113–119
Chongwei L, Jing A, Ruisong G, Yong L, Li L (2013) Enhanced electrochemical performance of LiFePO4/C cathode material modified with highly conductive TiN. J Alloys Compd 563:33–38
Honggui D, Shuangling J, Liang Z (2012) Nest-like LiFePO4/C architectures for high performance lithium ion batteries. Electrochim Acta 78:633–637
Zhipeng M, Guangjie S, Guiling W, Zhang Ying D, Jianping D (2014) Effects of Nb-doped on the structure and electrochemical performance of LiFePO4/C composites. J Solid State Chem 210:232–237
Jiali L, Rongrong J, Xiaoya W, Huang Tao Y, Aishui Y (2009) The defect chemistry of LiFePO4 prepared by hydrothermal method at different pH values. J Power Sources 194:536–540
Molenda J, Ojczyk W, Marzec J (2007) Electrical conductivity and reaction with lithium of LiFe1−yMnyPO4 olivine-type cathode materials. J Power Sources 174:689–694
Zhou YK, Wang J, Hu Y, O’Hayre R (2010) A porous LiFePO4 and carbon nanotube composite. Chem Commun 46:7151–7153
Denis YWY, Christopher F, Wolfgang W, Wolfgang W (2007) Study of LiFePO4 by cyclic voltammetry batteries and energy storage. J Electrochem Soc 154:A253–A257
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, G., Han, E., Zhu, L. et al. Preparation of LiFe0.98M0.02PO4/C cathode material for lithium-ion battery. Ionics 21, 319–324 (2015). https://doi.org/10.1007/s11581-014-1308-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1308-8