Abstract
Polyaniline-modified tin oxide and tin oxide nanoparticles were synthesized using a solution route technique. The obtained pristine products were characterized with X-ray diffraction, thermogravimetric analysis, scanning electron microscopy, and optical absorption spectroscopy. Thermogravimetric analysis results showed that the polyaniline-modified SnO2 nanoparticles exhibit higher thermal stability than the SnO2 nanoparticles. Scanning electron microscopy analysis on the as-synthesized powders showed spherical particle in the range of 50–100 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Semiconductor nanoparticles have been studied widely from both experimental and theoretical viewpoints because of their potential applications in the optoelectronic industry, as catalysts, and in solar energy conversion [1–3]. Tin oxide is an n-type semiconductor with a large band gap and is well-known for its applications in dye-based solar cells [4] and gas sensors [5]. Recently, attention has been focused on its possible application in electrochemical devices [6]. Rising demand for power sources with high power density has motivated an immense interest in electrochemical supercapacitors in recent years with projected applications in electric vehicles, burst power generation, memory back-up devices, digital communications, and other related devices which require high pulse power. Supercapacitors have high power density, excellent reversibility, and long cycle life as compared to batteries [7].
In recent years, inorganic–organic nanocomposites have turn into a most attractive subject of research because of their specific physical and chemical properties [8]. Semiconductor–polymer nanocomposites are a particularly promising new field of development of advanced materials in science and technology. The properties of these nanocomposites are quite different from those of the component materials because of interfacial interactions between nanostructured semiconductors and polymers. The properties of these materials can easily be tuned for desired applications through the variation of particle size, shape, and distribution of nanoparticles.
Polypyrrole, polyaniline, and polythiophene have been suggested for applications as active electrode materials in primary and secondary batteries and in supercapacitors [9–11]. Among these polymers, polyaniline has attracted attention because of its environmental stability, good electrical conductivity, and easy synthesis [2]. A synergistic effect in polyaniline–inorganic nanocomposites, particularly, in polyaniline–metal oxide composites, has been applied in electrode materials for supercapacitor. SnO2 has the benefit of its environmentally benign nature and low cost. It is necessary to prepare and investigate composites combining excellent properties of both polyaniline (PANI) and SnO2 for electrochemical applications.
Several methods were reported in the literature for the synthesis of PANI–inorganic composite powders and thin films, for example, ex situ intercalation process, drop cast method and in situ self-assembly, and hydrothermal and co-precipitation methods [1, 12–15]. However, to synthesize bulk amounts of fine particles of organic–inorganic powders requires low temperature and short duration of synthesis. The solution co-precipitation method is suitable to synthesize nanosize PANI–SnO2 particles [1]. The present work reports on the synthesis of PANI–SnO2 nanoparticles by a co-precipitation method using citric acid as a carrier.
Experimental
Synthesis of PANI nanoparticles
Aniline was distilled under vacuum; 0.1 M aniline and 1 g citric acid were dissolved in 250 mL of 0.1 M hydrochloric acid and kept below 4 °C for 30 min, and 0.125 M ammonium persulfate (APS) was dissolved in 50 mL of aqueous 0.1 M hydrochloric acid and added to the above solution. The mixed solution was stirred for 2 h, and subsequently, the solution was kept unstirred for further 22 h. The green precipitate was filtrated and washed with a large amount of water, 0.1 M hydrochloric acid, and acetone. The obtained product was dried at 50 °C for 12 h.
Synthesis of SnO2 nanoparticles
Citric acid (0.1 M) and 0.1 M of tin chloride (SnCl2·2H2O) were dissolved in 250 mL of distilled water under mixing with a magnetic stirrer for 4 h. Hydrogen peroxide (H2O2) was added to the above solution to oxidize tin ions into tin oxide, and the solution turns into a white-colored suspension of SnO2. The suspension was aged at 90 °C for 48 h. The white precipitate was filtrated and washed with a large amount of water and C2H5OH. It was dried at 100 °C for 8 h and then calcinated at 300 °C for 4 h.
Synthesis of polyaniline-modified SnO2 nanoparticles
The white-colored suspension of SnO2 was prepared using the solution route technique described above. It was mixed with 0.1 M aniline and kept below 4 °C in an ice bath. After 30 min, 0.125 M of APS solution was added to the mixture, and the ice bath was removed after a green-colored solution was obtained. The reactant mixture was stirred for 22 h and subsequently aged at 90 °C for 48 h. The green precipitate was filtrated and washed with large amounts of water and acetone. It was dried at 100 °C for 8 h and then calcinated at 250 °C for 4 h. A color change from green to brown was observed after calcination.
Characterization techniques
Crystallographic information of the samples was obtained using an X-ray powder diffractometer STADI P (STOE) with Ge(111) monochromatized Cu-Kα radiation (λ = 1.54187 Å). Diffraction data were collected over the 2θ range of 20° to 80°. Fourier transform infrared (FTIR) absorption spectra of the nanoparticles were measured using a Bruker IR spectrometer of 4 cm−1 resolution in the 600–4,000-cm−1 range. The morphologies of the resulting products were characterized using a scanning electron microscope (NanoNova SEM of FEI). For thermogravimetric analysis (TGA) measurements, an instrument TGA 7 (Perkin-Elmer) operating in dynamic mode (heating rate = 10 °C/min under helium gas) was employed. Optical absorption spectra of equal amounts of SnO2 and polyaniline-modified SnO2 nanoparticles dispersed in ethanol were recorded at room temperature in the wavelength region 200–800 nm using a Shimadzu UV–Vis–NIR (Model UV-3100) spectrophotometer. Electrochemical properties of SnO2 nanoparticles were analyzed by using a three-electrode cell with a platinum counter electrode and a silver wire in a solution saturated with KCl as the reference electrode. The working electrode, prepared by mixing 80 wt.% of active material, 15 wt.% of acetylene black, and 5 wt.% of polyvinylidene fluoride, was coated onto a glassy carbon electrode with a diameter of 5 mm. An aqueous 2.0-mol/L solution of H2SO4 was used as the electrolyte. Cyclic voltammetric (CV) measurements were carried out between the potential limits of −0.5 and 1.0 V against Ag/AgCl using a custom-built potentiostat. CV curves were recorded at dE / dt = 10 mV s−1.
Results and discussion
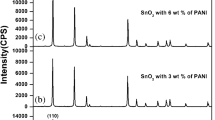
Data on phase purity and crystallographic information on the SnO2 and polyaniline-modified SnO2 nanoparticles were obtained using powder X-ray diffraction; XRD patterns of nanoparticles are shown in Fig. 1. All diffraction patterns of SnO2 and polyaniline-modified SnO2 nanoparticles show characteristic peaks of the tetragonal rutile phase. The XRD patterns are in good agreement with the standard data of the tetragonal rutile phase with a = 4.741 Å and c = 3.182 Å in close agreement with the standard data file (ICDD # 01-070-6995). No impurity phases were observed. The observed broadening of diffraction peaks in polyaniline-modified SnO2 indicates incorporation of polyaniline in tin oxide [5].
FTIR spectra of PANI and polyaniline-modified SnO2 nanocomposite are shown in Fig. 2. PANI exhibits peaks at wavenumbers 1,582, 1,492, 1,302, 1,142, and 805 cm−1 [2]. The peaks at 1,582 and 1,492 cm−1 correspond to C = N- and C = C-stretching modes of the benzenoid rings. The peaks at 1,302 and 1,142 cm−1 are attribute to the C–N-stretching mode of benzenoid rings. The peak at 805 cm−1 is assigned to C–H out-of-plane bending vibrations. All characteristic peaks of PANI can be observed in the FTIR spectrum of polyaniline-modified SnO2 nanocomposite. However, some peaks of modified PANI have shifted slightly due to interaction with tin oxide.
TGA curves of PANI, SnO2, and polyaniline-modified SnO2 precursor are shown in Fig. 3. The weight losses happened in two steps. The first weight loss was 0.69 mg in the temperature range 30–97 °C; this weight loss could be attributed to evaporation of water from the PANI. The second weight loss is 0.62 mg in the temperature range 97–250 °C; it is attributed to decomposition of citric acid. The respective first weight loss was 0.79 mg in SnO2 in the temperature range 30–195 °C and 0.546 mg in polyaniline-modified SnO2 in the temperature range 30–122 °C. This first weight loss corresponds to evaporation of water from the metal oxides. The second weight loss was 2.03 mg in SnO2 in the temperature range 195–455 °C and 2.914 mg in polyaniline-modified SnO2 in the temperature range 122–595 °C. This second weight loss can be attributed to decomposition of citric acid in SnO2 and in polyaniline-modified SnO2 to decomposition of citric acid and polyaniline. PANI decomposes between 97 and 250 °C, but the SnO2+ PANI composite decomposes slowly from 120 to 600 °C. This result implies that PANI interacted strongly with the surface SnO2, enhancing the thermal stability of polyaniline.
Figure 4 shows the UV–Vis absorption spectra of the SnO2 and polyaniline-modified SnO2 nanocomposite and PANI. The UV–Vis spectra show absorption peaks at 265 nm for SnO2 and 270 nm for PANI-modified SnO2 and 350 nm for PANI. The absorption peak shift from 265 to 270 nm indicates the presence of polyaniline in the composite and a possible increase in π-conjugation of the polymer chains when they are interacted strongly with the surface SnO2. The optical band gap value E g can be determined from the optical absorption spectra using Tauc’s relation [16]:
where α is the absorption coefficient, h·ν is the photon energy, and A is a constant. For a direct transition, band gap semiconductor E g can be obtained from the relation
To calculate direct band gap E g, one can plot (α·h·ν)2 vs. h·ν and extrapolate the linear portion of it to α = 0 value yielding the corresponding band gap energy. Plots of (α·h·ν)2 vs. h·ν for SnO2 and PANI-modified SnO2 are shown in Fig. 5. The calculated band gap energy value for SnO2 is 4.0 eV and for PANI-modified SnO2 is 4.2 eV.
For indirect transitions, which require photon assistance, the absorption coefficient has the following dependency on the photon energy
where E p is the energy of the photon associated with the transition, and A and B are constants depending on the band structure. The band gap energies for indirect transitions were obtained from the plots of (a·h·ν)1/2 vs. photon energy (h·ν) as shown in Fig. 6. The obtained value for SnO2 is 3.75 eV and for PANI-modified SnO2 is 4.0 eV.
The position of the absorption edge values was calculated by extrapolating the linear portions of the α vs. h·ν plot as shown in Fig. 7 to zero absorption value. The calculated absorption edge value for SnO2 is 3.8 eV and for PANI-modified SnO2 4.3 eV. The absorption edge, direct band gap, and indirect band gap energies are higher for PANI-modified SnO2 than for plain SnO2. The widening of band gaps occurs with the modification of polyaniline in SnO2 matrix [1]. Compared to the bulk energy gap (3.6 eV), the higher band gap energy is due to quantum confinement effects of SnO2 nanoparticles.
SEM images of PANI and PANI-modified SnO2 nanocomposite are shown in Fig. 8. Polyaniline particles (Fig. 8a) are of spherical shape with diameter in the range from 50 to 100 nm. In Fig. 8b, a doll-like microporous structure of PANI/SnO2 is observed. According to the SEM images, it appears to be very likely that the nanostructured SnO2 particles are embedded within PANI chains. This implies that the composite is highly microporous and thus capable of increasing the liquid–solid interfacial area providing more locations for the insertion and extraction of ions, ensuring a higher heterogeneous reaction rate.
The capacitance behavior of SnO2 and PANI-modified SnO2 nanoparticles as electrode materials has been studied using 2 M H2SO4 electrolyte solution within a potential window of − 0.5 to 1.0 V vs. Ag/AgCl by standard cyclic voltammetry. Cyclic voltammograms of SnO2 nanoparticles and PANI-modified SnO2 nanoparticles are shown in Fig. 9. The CV curves are more or less rectangular in shape within the measured potential window, indicating good charge propagation within the electrode. Less intense anodic and cathodic peaks were observed in CVs of SnO2 nanoparticles.
The CV of the PANI-modified SnO2 nanocomposite (Fig. 9b) presents a large pseudocapacitive background current, but it also reveals visible current peaks (P1/P2, P3/P4). The ideal capacitor performance requires that redox processes occur continuously (causing pseudocapacitive behavior). Negative to 0.20 V PANI is in its entirely neutral state, designated as leucomeraldine (LE). At 0.20 V, LE undergoes partial oxidation resulting in the emeraldine (EM) state. If the potential becomes more positive, EM undergoes further oxidation yielding pernigraniline (PE). The oxidation and reduction processes are accompanied by doping and dedoping of counter anions. Since these processes are reversible, charge storage in PANI provides a pseudocapacitance performance [8]. The shape of the curves (Fig. 9b) reveals that the capacitance feature of the PANI-modified SnO2 nanocomposite electrode is mainly related to the redox mechanism, and in Fig. 9b, two processes of the PANI redox transitions are clearly observed. The anodic and cathodic peaks (P1/P2) correspond to the LE/EM transition. Similarly, the anodic and cathodic peaks are attributed to the EM/PE transition. The occurrence of current peaks (albeit weak ones) is due to the polydispersity of PANI. In case of a complete absence of a most likely chain length (a maximum in a distribution function), chain segments showing conjugation ranging from very short to very long ones would be present resulting in a correspondingly broad distribution of redox peaks actually merged into a capacitive-like current. Apparently, PANI as prepared here does not show this extreme distribution, instead a chain length correlated with the peak positions dominates resulting in the observed behavior.
Conclusions
PANI-modified SnO2 and SnO2 nanoparticles were successfully synthesized by a solution route technique using citric acid as a carrier. Scanning electron microscopy analysis of the as-synthesized powders showed spherical particles with sizes in the range of 50–100 nm. Electrochemical results indicate that the PANI-modified SnO2 composite had a higher background current with apparent cathodic and anodic peaks.
References
Dutta K, De SK (2007) Mater Lett 61:4967
Haili P, Changting H, Jinhua C, Bo L, Yafei K, Xiaohua Z (2010) J Solid State Electrochem 14:169
Barsan N, Weimar U (2003) J Phys Condens Matter 15:R813
Srivastava DN, Chappel S, Palchik O, Zaban A, Gedanken A (2002) Langmuir 18:4160
Deshpande NG, Gudage YG, Ramphal Sharma, Vyas JC, Kim JB, Lee YP (2009) Sens Actuators B 138:76.
Zhu J, Lu Z, Aruna ST, Aurbach D, Gedanken A (2000) Chem Mater 12:2557
Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Kluwer Academic/Plenum, New York
Zhong-Ai H, Yu-Long X, Yao-Xian W, Li-Ping M, Yu-Ying Y, Zi-Yu Z (2009) Mater Chem Phys 114:990
Liang HC, Chen F, Li RG, Wang L, Deng ZH (2004) Electrochim Acta 49:3463
Aurian-Blajeni B, Beebe X, Rauh RD, Rose TL (1989) Electrochim Acta 34:795
Kim YT, Tadai K, Mitani T(2005) J Mater Chem 15:4914.
Ma X, Wang M, Li G, Chen H, Bai R (2006) Mater Chem Phys 98:241
Ram MK, Yavuz O, Lahsangah V, Aldissi M (2005) Sens Actuators B 106:750
Huiling T, Yadong J, Guangzhong X, Junsheng Y(2010) J Mater Sci Technol 26:605.
Lina G, Yingqiang Z, Xueliang H, Shurong W, Shoumin Z, Shihua W (2007) Sens Actuators B 120:568
Thutupalli GM, Tomlin SG (1976) J Phys D: Appl Phys 9:1639
Acknowledgments
One of the authors (VSRC) thanks the Alexander von Humboldt-Foundation for a fellowship. The authors gratefully acknowledge the valuable contributions by Maik Schlesinger and Michael Mehring’s group in the structural analysis using XRD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Channu, V.S.R., Holze, R. Synthesis and characterization of a polyaniline-modified SnO2 nanocomposite. Ionics 18, 495–500 (2012). https://doi.org/10.1007/s11581-011-0648-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-011-0648-x