Abstract
The present work reports the effect of light on the open-circuit voltage of a photoelectrochemical cell (PEC) formed of TiO2 photoanode, Pt cathode and Na2SO4 (0.35 M) aqueous solution as electrolyte. The studies included the measurements of the electromotive force (EMF) during the light-off and light-on cycles for the PEC involving photoanode that was made of both oxidised and reduced TiO2 thin films. These specimens were formed by oxidation of the titanium metal at high and low oxygen activities. This was achieved by the imposition of the gas phase of two different compositions, including air, p(O2) = 21 kPa, and the hydrogen–water vapour mixture, \(p({\rm O}_2) = 10^{-10}\) Pa, at 1,123 K and subsequent cooling to room temperature. The determined data indicate that the PEC formed of the oxidised specimen exhibits larger EMF and a substantially better stability in time. It is, therefore, concluded that the TiO2 obtained in air exhibits superior performance-related properties compared to the reduced specimen. The obtained experimental EMF data are considered in terms of the effect of light on the reactivity of TiO2 with oxygen and water and the related charge transfer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interest in TiO2 as a photosensitive compound has been generated since Fujishima and Honda first observed that TiO2 is a promising photoelectrode material for water splitting [1]. The research on its photoelectrochemical properties aims at the modification of its performance as a photoelectrode in order to increase the energy conversion efficiency (ECE) of TiO2-based photoelectrochemical cells (PEC) [2–13].
The main advantage of TiO2 over other photosensitive semiconductors reported in the literature so far lies in its resistance to corrosion and photocorrosion in aqueous environments. At the same time, its main disadvantage is a large band gap (3 eV), which allows for only a small fraction of the solar energy spectrum to be absorbed. Therefore, in order to achieve a high-performance PEC with a sufficiently high ECE, there is a need to reduce the band gap. This, consequently, requires the modification of its properties in order to achieve the functional properties that are desired for high-performance photoelectrodes.
The main research effort to reduce the band gap is through the incorporation of aliovalent ions, including W, Ta, Nb, Zn, In, Li and Ge [12]; Pb [11]; Mo and Cr [3]; Cr [8]; C [8] and N [7]. So far, the most spectacular effect has been achieved by Khan et al. [8], who reported that exposure of titanium metal to a natural gas flame leads to the formation of a carbon-doped TiO2 layer, of which the band gap is reduced to 2.3 eV, leading to an increase of the ECE to 8.4% [8]. The benchmark of the US Department of Energy, which is required for commercialisation, is ECE > 10% [14].
Most of the ECE data on electrochemical properties reported in the literature have been considered in terms of the I–V characteristics. So far, however, little is known about the dynamics of the TiO2-based PEC during prolonged performance. The information about the PEC dynamics is important in order to assess its performance stability. The present work, which aims at addressing this issue, will study the open cell voltage of the TiO2-based PEC during prolonged exposure to light. Specifically, the present work will study the dynamics of the electromotive force (EMF) of the PEC, involving a TiO2 photoanode and platinum cathode. The experimental part of the present study will be preceded by a brief outline of the band model for the TiO2-Pt system, the photoreactivity of the TiO2 surface with water and the related charge transfer.
TiO2-based PEC
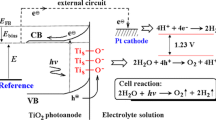
The concept of photo-electrochemical hydrogen generation, which is based on the splitting of the water molecule on the surface of a photo-electrode using solar energy, is shown in Fig. 1. The electrochemical chain of the photo-electrochemical device based on a TiO2 photo-anode is shown in Fig. 2. The experimental approaches to assess the electrochemical properties of this chain include the determination of the effect of light on cell voltage and current-voltage characteristics. The latter can be used for the evaluation of the ECE.
An essential part of the PEC is the semiconducting photoanode, which consists of an n-type TiO2 semiconductor. The most commonly applied cathode is platinum (there is a general assumption that Pt is resistant to corrosion in water). The band model of both TiO2 and Pt is schematically represented in Fig. 3a. The TiO2/Pt system exhibits the following specific features:
-
1.
Work function (WF) of platinum is substantially larger than that of TiO2.
-
2.
The surface layer of TiO2 exhibits an upward band bending, which is reflective of the negative surface charge formed as a result of oxygen chemisorption. This process takes place at the TiO2/O2 interface when TiO2 is exposed to air. The WF of both TiO2 and Pt involves a potential drop related to the adsorbed water molecules.
The galvanic cell is formed when the two phases (TiO2 and Pt) are immersed in an aqueous electrolyte. The resulting band model of the cell electrodes, which are internally connected by the electrolyte, is shown in Fig. 3b. The immediate consequence of immersing the electrodes in an aqueous electrolyte is adsorption of water, leading to the potential drop over water molecules within the adsorbed layer.
Schematic representation of the band model and the distribution of electrical potential for the Pt–TiO2 system involving chemisorption and the potential drop related to the dipole moment of water molecules: a electrically separated electrodes of Pt-based cathode and TiO2-based photoanode, b both electrodes connected galvanically in the presence of water and the absence of light, c the effect of light on the charge distribution in the presence of both light and water. \({\mathit \Phi}_{\mathrm{Pt}}\) and \({\mathit \Phi}_{\mathrm{TiO}_2}\) are WFs of platinum and titanium dioxide, respectively; χ is external WF; \({\mathit \Phi}_{\mathrm{S}}\) is the WF component related to band bending and \({\mathit \Delta}{\mathit \Phi}_{\mathrm{H}_2\mathrm{O}}\) represents WF changes related to the adsorption of water
Exposure of TiO2 to light results in intrinsic ionisation over the band gap, leading to the formation of electrons (majority carriers) and holes (minority carriers):
The effect of light on surface electrical properties of TiO2 may be considered in terms of a split of the Fermi energy level, E F, into a quasi-Fermi energy of electrons, \((E_{\mathrm{F}}^\ast)_n\), and a quasi-Fermi energy of electron holes, \((E_{\mathrm{F}}^\ast)_p\). While the effect of light on the chemical potential of the predominant charge carriers (electrons) is insignificant, light results in a substantial change of the chemical potential of the minority charge carriers (electron holes). The excess minority charge carriers (holes) give rise to a photo-voltage leading, in consequence, to:
-
1.
Increased potential drop over the adsorbed water molecules layer which are ionised according to the following reaction:
$$\label{eq:water-ionisation} \mathrm{H}_2\mathrm{O}_{(\mathit{ads})} + h^\bullet \rightarrow \mathrm{H}_2\mathrm{O}_{(\mathit{ads})}^+ $$(2) -
2.
The upward band bending related to oxygen chemisorption is reduced due to the transition between singly ionised atomic oxygen species, stable in dark conditions, and singly ionised molecular species, stable under illumination. This effect will be discussed in detail below.
A schematic representation of the effect of light on the WF of TiO2, compared to Pt, is shown in Fig. 3c.
When the photovoltage is sufficiently large and the PEC is externally short-circuited leading to a current flow through the PEC circuit, water molecules split into hydrogen ions and gaseous oxygen:
Gaseous oxygen evolves at the photo-anode and the hydrogen ions migrate to the cathode through the internal circuit (electrolyte), where the reduction of hydrogen ions to gaseous hydrogen takes place:
The overall reaction of the photo-electrochemical process is as follows:
Reaction 5 takes place when the EMF of the photo-electrochemical cell is 1.23 V (in practice, this value must be higher due to energy losses caused by polarisation effects). The considerations in the present work will be limited to the effect of light on the open circuit PEC voltage. Consequently, the present work considers the case when the electrodes of PEC are not connected externally and the process of water photolysis does not occur.
Experimental
The thin films of undoped TiO2 were prepared by the oxidation of spectrally pure titanium metal discs (0.25 mm thick and 28 mm in diameter). Its impurities included: 95 ppm Cr, 75 ppm Si, 70 ppm Fe, 45 ppm Ni and 40 ppm Al. Two thin film specimens were prepared.
Thin film #1 was formed by oxidation of the titanium disk at 1,123 K in air (p(O2) = 21 kPa) for 15 min. The film thickness, determined by the weight difference before and after oxidation (assuming 100% of theoretical density), was 2 μm. The scanning electron microscope micrograph of the specimen is shown in Fig. 4. This specimen was used for the determination of the EMF changes as a function of time, which are shown in Fig. 5a.
After completing the first experiment, specimen #1 was annealed at 1,123 K in reduced conditions (\(p({\rm O}_{2}) = 10^{-10}\) Pa) for 15 min. The gas phase with the low oxygen activity was imposed by an argon, hydrogen and water vapour mixture flowing at a rate of 25 mL/min. This treatment resulted in an increase of the film thickness by ~200 nm (also determined by the change of weight). The obtained specimen (#2) was used for the second experiment, including the measurements of EMF as a function of time, which is shown in Fig. 5b.
A schematic representation of the oxygen nonstoichiometry within the studied specimen, including the Ti metal support and the TiO2 thin film, is shown in Fig. 6. As can be expected, the O/Ti ratio for the TiO2 thin film processed in oxidised conditions is larger than that formed in reduced conditions. One should also expect that the film exhibits a concentration gradient of oxygen between the outermost surface layer and oxide/metal boundary. The gradient is reflected schematically by the O/Ti ratios in Fig. 6. The outermost surface exhibits the rutile phase, which is the thermodynamically stable phase of TiO2 [15]. The nonstoichiometry of the surface layer is consistent with the effect of oxygen activity on point defect structure, represented by defect diagram of TiO2 [16].
Before the installation of the specimen as a photoanode, one side of the TiO2 layer was removed by polishing to achieve a good electrical contact with the electrode holder. The specimen was then sealed inside the sample holder, which is shown in Fig. 7. The cathode was made of platinum covered with the platinum black layer. The electrolyte was formed of a 0.35-M Na2SO4 aqueous solution.
The light source used was a 150-W halogen lamp (NEC Dichro). The photoanode area exposed to light was ~1 cm2. The EMF was monitored as a function of time, during light-on and light-off cycles, using a Hewlett-Packard 34401A multimeter.
Results
The changes of the EMF during light-on and light-off cycles, over a period of 500 h (20 days), are shown in Fig. 5. The data for specimens #1 and #2 are shown in Fig. 5a and b, respectively.
As seen in Fig. 5a, the following effects can be observed for specimen #1:
-
1.
The initial EMF in dark conditions is ~100 mV.
-
2.
The initial exposure to light results in a sudden EMF increase to ~540 mV, which then slowly decreases.
-
3.
Prolonged exposure to light (S1) results in a decrease in the EMF with a rate of ~0.4 mV/h. The final EMF after 75 h is ~500 mV.
-
4.
Imposition of the first light-off cycle leads to a drop of the EMF to ~100 mV.
-
5.
The second exposure to light results in a sudden EMF increase to ~500 mV, which then slowly decreases.
-
6.
Prolonged exposure to light (S2) results in a decrease of EMF with a rate 0.2 mV/h over 170 h. The final EMF is ~470 mV.
-
7.
Imposition of the second light-off cycle leads to a drop of the EMF to ~200 mV.
-
8.
The third exposure to light results in a sudden EMF increase to ~480 mV.
-
9.
Prolonged exposure to light (S3) results in a further decrease of EMF with a rate of ~0.2 mV/h.
The EMF changes, determined by the initial and final values in light-off and light-on cycles for the PEC involving the TiO2 specimen #1 as photoanode, are shown in Fig. 8a. As seen, these changes have a tendency to decrease with time.
The experiment with specimen #2 (Fig. 5b), involving light-on and light-off cycles, resulted in the following effects:
-
1.
The light-off EMF has a tendency to decrease from 180 to ~30 mV over 50 h.
-
2.
Imposition of the first light-on cycle results in a sudden increase of the EMF to ~540 mV and a subsequent slow decrease.
-
3.
Prolonged exposure to light (S1) over 280 h results in a decrease of EMF with an initial rate of 1 mV/h and a final rate of 0.3 mV/h. The final EMF value after 280 h is ~240 mV.
-
4.
Imposition of the second light-off cycle results in a drop of the EMF, which assumes a stable value at ~60 mV.
-
5.
Prolonged exposure to light in the second light-on cycle, S2, results in a sudden EMF increase to ~380 mV, a subsequent rapid drop to 280 mV and a final slower decrease at a rate of 0.5 mV/h over 60 h. The final EMF is ~230 mV.
As seen in Fig. 8b, the extreme values of EMF in the light-on and light-off conditions have a substantially stronger tendency to decrease than in the case of the oxidised specimen.
Discussion
The EMF of the studied PEC is related to the potential of both the photoanode (E A ) and the cathode (E C ):
Assuming that the changes of the potential of the platinum electrode, which was kept in dark conditions, can be neglected, the observed dynamics of the EMF is reflective of the effect of light on the electrical potential of TiO2 and the electrical phenomena at the TiO2/H2O interface. Therefore, the reactions at the TiO2/H2O interface may be considered in terms of surface chemisorption/desorption equilibria, involving both water and oxygen, and the related charge transfer.
Reactivity and photoreactivity of TiO2 with oxygen and water
Oxidation of n-type TiO2 at lower and moderate temperatures, at which the lattice is quenched, may be considered in terms of the following adsorption and chemisorption equilibria:
Equilibrium 7 represents the formation of physically adsorbed neutral molecular oxygen species. The formation of these species is not related to charge transfer. Therefore, these species have no effect on electrical properties. However, these species may then be ionised and dissociated according to the following equilibria:
Donor-type defects, such as oxygen vacancies, are the most likely active sites for oxygen chemisorption on the TiO2 surface [17]. The rightwards shifts of equilibria 8–10 lead to the negative surface charge formed by chemisorbed oxygen species, which is compensated by positive space charge in the surface layer that is represented by the upward band bending (Fig. 3a). The associated electric field plays an important role in the separation of light-induced charge. The charge separation is essential in the reduction of recombination-related energy losses.
Both the singly ionised molecular and atomic oxygen species may be formed at room temperature; however, the atomic form is substantially more stable in dark conditions [18]. Consequently, the singly ionised atomic oxygen species are much more difficult to remove from the surface than the singly ionised molecular species. The doubly ionised atomic species are stable only in the crystal field when incorporated into the lattice [18].
The extensive studies by using WF measurements [19–23] have shown that, in dark conditions, oxygen preferentially reacts with the TiO2 surface according to the reaction in Eq. 9 leading to the formation of singly ionised atomic species. This reaction leads to enhanced n-type properties of the TiO2 surface layer. However, the imposition of light leads to an increase of the concentration of electron holes (minority charge carriers) in the TiO2 surface layer exposed to illumination. Therefore, light results in the conversion of TiO2 surface layer from the n-type to the p-type. In consequence, the light is the driving force for the following processes:
-
1.
The equilibrium in Eq. 9 is shifted to the left resulting, initially, in the formation of singly ionised molecular species and, ultimately, leading to oxygen desorption from the TiO2 surface. This conclusion is consistent with the observation of Bourasseau et al. [19–23].
-
2.
Increased water chemisorption.
In dark conditions, the reactivity of TiO2 with water may be considered in terms of water adsorption, leading to the formation of the adsorbed water molecules:
The light-induced electron holes then react with the adsorbed water molecules leading to water splitting into H + ions and oxygen gas:
The reaction in Eq. 12 requires that the photon energy is ≥ 1.23 eV. Alternatively, the reaction between water molecules and electron holes leads to the formation of OH ∗ radicals [24, 25]:
It has been shown that the active sites for water chemisorption on TiO2 are titanium vacancies [26]. At the same time, the reaction in Eq. 8 will be favoured at the cathodic site of the PEC (Pt), where electrons are available. Thus formed oxygen species will react with H + ions leading to the formation of hydrogen peroxide according to the following reaction [25]:
Theoretical model
The formation of the EMF within the PEC formed of TiO2, Pt and an aqueous electrolyte is schematically shown in Fig. 9. Its upper part, representing the light-off cycle, includes the distribution of the electrical potential within the PEC, involving its components:
-
1.
Platinum. The Pt is acting as a lead material contacting the TiO2 photoanode with the electrometer.
-
2.
TiO 2 photoanode. The distribution of the electrical potential within TiO2 involves the following effects:
-
(a)
The contact potential difference formed between Pt and TiO2. Its value remains constant during the experiments in dark conditions.
-
(b)
Constant electrical potential within the bulk of the TiO2 phase.
-
(c)
The electrical potential within the space charge of the boundary layer for TiO2. Exposure of the TiO2 surface to light leads to:
-
1.
Shifts of oxygen chemisorption equilibria 7–10 to the left, leading ultimately to oxygen desorption
-
2.
Reduction of the negative surface charge by protons that are formed according to reaction 12 or 13 and penetrate the surface layer of the TiO2 photoanode
-
3.
Changes in the potential drop over the dipoles of water molecules adsorbed on TiO2
-
1.
-
(a)
-
3.
Electrolyte. Constant electrical potential within the electrolyte, at the absence of charge transport within the PEC.
-
4.
Platinum surface layer. The potential drop over the dipoles of water molecules adsorbed on Pt.
The lower part of Fig. 9 represents the effect of light on the distribution of electrical potential within the PEC. As seen, the EMF is the result of the following effects:
-
1.
Platinum. The electrical potential distribution of the Pt lead material, contacting the TiO2 photoanode with the electrometer, is the same as in the light-off cycle.
-
2.
TiO 2 photoanode. The potential distribution within the bulk phase of TiO2 is the same as that in the light-off cycle. The effect of light on the local split of the Fermi energy is shown in Fig. 3c. The resulting charge separation is expected to lead to a potential gradient within the bulk phase and the boundary layer.
-
3.
Surface layer. The light-induced decrease of the quasi-Fermi energy related to electron holes leads to an increase in the concentration of water molecules on TiO2, which are the donors of electrons. This, consequently, results in an increase of the potential drop across this layer.
-
4.
Electrolyte. The electrical potential within the electrolyte remains constant.
-
5.
Platinum surface. The potential drop over the dipoles of water molecules adsorbed on Pt is the same as in the light-off cycle.
As seen in Fig. 9, the imposition of light results in a substantial increase of the potential drop over the adsorbed water molecules. This potential drop is responsible for the observed increase in the EMF.
Effect of the reactivity and photoreactivity of TiO2 on EMF
The observed EMF changes during the light-on and light-off cycles for both oxidised and reduced specimens, which are observed in Fig. 5a and b, respectively, indicate the following effects:
-
1.
Dark EMF. The initial dark EMF is determined by the processing conditions of the two electrodes, TiO2 and Pt, and the related chemisorption equilibria established after these electrodes are immersed in water. The change of the EMF in the dark is reflective of the shifts of these chemisorption equilibria, which are represented by Eqs. 2 and 8–10. As seen in Fig. 5a and b, the observed EMF changes depend on the oxygen content in the studied specimens:
-
(a)
Oxidised specimen. As seen in Fig. 5a, the EMF changes observed during the first light-off cycle are insignificant, indicating that the surface potential of the TiO2 specimen, which has been oxidised in air, and that of the Pt surface remain stable.
-
(b)
Reduced specimen. As seen in Fig. 5b, the EMF changes during the first light-off cycle for the reduced specimen are substantial (175–10 mV). These changes, which are determined by the surface potential of the TiO2 specimen, indicate that the reduced surface has a strong tendency to oxidise. Therefore, it is logical to expect that the observed initial changes of the EMF for the reduced specimen are reflective of the lack of stability of this specimen in the applied experimental conditions.
-
(a)
-
2.
Light-on. As seen in Fig. 5, the imposition of light results in an increase of the EMF in all light-on cycles. This effect is consistent with the model shown in Fig. 9, according to which the EMF increase is related to chemisorption of water, expressed by Eq. 2, and the related charge transfer between TiO2 and water molecules in the adsorbed layer. As seen, however, the character of the EMF changes depends on the oxygen content in the specimen:
-
(a)
Oxidised specimen. The imposition of light leads to a sudden EMF increase by ~650 mV, which is related to an increase in the concentration of chemisorbed water species. The following slow drift of the EMF (0.4–0.2 mV/h) indicates that the driving force for the generation of light-induced EMF is diminishing. The latter effect may be considered in terms of blocking the surface by the increasing population of chemisorbed oxygen species, which cannot be removed in the open-cell conditions.
-
(b)
Reduced specimen. The imposition of light in this case leads to a sudden EMF increase by a similar value that is observed for the oxidised specimen (~650 mV), thus indicating that the oxidation potential of the outermost surface layer is similar to that of the oxidised specimen. The following strong drift of the EMF (1–0.3 mV/h) is related to lower oxygen activity in the specimen. Specifically, the reduced specimen has stronger affinity to oxygen than the oxidised specimen.
-
(a)
-
3.
Dark EMF subsequent to the light-on cycle. As seen in Figs. 5 and 8, termination of illumination results in a sudden decrease of EMF. This effect has a tendency to diminish. Assuming that this diminution is related to oxygen chemisorption, the experimental data are consistent with the theoretical reactivity model between TiO2 and oxygen.
The comparison of the character of the observed EMF changes indicates that the performance of the TiO2 as a photoanode is closely related to its oxidation state. As seen, the performance of the oxidised TiO2 photoanode is superior to that of reduced TiO2 in terms of the magnitude of the EMF and its stability. The driving force for the photoelectrochemical water splitting is the EMF, which is larger for the oxidised specimen. Moreover, the EMF in the latter case exhibits better stability.
Conclusions
The present work studied the effect of oxygen activity for the TiO2-based photoanode on the open circuit voltage (EMF) of a PEC formed of the TiO2 photoanode, platinum cathode and aqueous solution (0.35 M Na2SO4) as an electrolyte. It has been shown that the EMF is closely related to oxygen content in the photoanode. Specifically, the obtained experimental data indicate that the oxidised TiO2 exhibits a superior performance as a photoanode to that of the reduced TiO2 in terms of its better EMF stability during long-term exposure to light.
References
Fujishima A, Honda K (1972) Nature 238:37–38
Karakitsou KE, Verykios XE (1993) J Phys Chem 97:1184–1189
Rahman MM, Krishna KM, Soga T, Jimbo T, Umeno M (1999) J Phys Chem Solids 60:201–210
Wilke K, Brauer HD (1999) J Photochem Photobiol A Chem 121:49–53
Bak T, Nowotny J, Rekas M, Sorrell CC (2002) Int J Hydrogen Energy 27:19–26
Barnes PRF, Randeniya LK, Murphy AB, Gwan PB, Plumb IC, Grey IE, Li C (2004) TiO2 photo-electrodes for water splitting prepared by flame pyrolysis. In: Intern conf on materials for hydrogen energy, Sydney, 27 August 2004
Nakamura R, Tanaka T, Nakato Y (2004) J Chem Phys B 108:10617–10620
Khan SUM, Al-Shahry M, Ingler WB (2002) Science 297:2243–2244
Fujishima A, Kohayakawa K, Honda K (1975) J Electrochem Soc 122:1487–1489
Morisaki H, Watanabe T, Iwase M, Yazawa K (1976) Appl Phys Lett 29:338–340
Houlihan JF, Madacsi DP, Walsh EJ, Mulay LN (1976) Mater Res Bull 11:1191–1197
Houlihan JF, Hamilton JR, Madacsi DP (1979) Mater Res Bull 14:915–920
Guruswarmy V, Bockris JO’M (1979) Solar Energy Mats 1:441–449
Service RF (2002) Science 297:2189–2190
Wu Q, Hou Y, Wu L, Fu X, Wang X (2007) Mater Chem Phys 102:53–59
Bak T, Nowotny J, Nowotny MK (2006) J Phys Chem B 110:21560–21567
Menterey M, Mankovits A, Minot C (2003) Surf Sci 524:49–62
Bielanski A, Haber J (1979) Catal Rev Sci Eng 19:1–41
Bourasseau S, Martin JR, Juillet F, Teichner S (1973) J Chim Phys 70:1472–1477
Bourasseau S, Martin JR, Juillet F, Teichner S (1974) J Chim Phys 71:122–126
Bourasseau S, Martin JR, Juillet F, Teichner S (1974) J Chim Phys 71:1018–1024
Bourasseau S, Martin JR, Juillet F, Teichner S (1974) J Chim Phys 71:1025–1027
Bourasseau S (1973) Variation du travail d’extraction thermoelectrique du dioxyde de titane pulveruent, soumis a un rayonnement ultraviolet. Ph.D. thesis, L’Universite Claude Bernard-Lyon
Linsebigler AL, Lu G, Yates JT (1995) Chem Rev 95:735–758
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Chem Rev 95:69–96
Nowotny J, Bak T, Nowotny MK, Sheppard LR (2007) J Phys Chem B 110:18492–18495
Acknowledgements
The present work was supported by the Australian Research Council, Mailmasters Pty Ltd, Brickworks Pty Ltd, Avtronics (Australia) Pty Ltd, and Rio Tinto Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
The present work was performed when the authors were with the UNSW Centre for Materials Research in Energy Conversion.
Rights and permissions
About this article
Cite this article
Bak, T., Nowotny, J., Nowotny, M.K. et al. Dynamics of TiO2-based photoelectrochemical cell. Ionics 15, 671–679 (2009). https://doi.org/10.1007/s11581-009-0346-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0346-0