Abstract
Polyethylene oxide (PEO)–potassium hydroxide (KOH)-based alkaline solid polymer electrolyte films have been prepared by using methanol as solvent. The highest room temperature ionic conductivity of (2.1 ± 0.5) × 10−8 S cm−1 was achieved for the composition of 70 wt% PEO:30 wt% KOH. The addition of plasticizer, ethylene carbonate, propylene carbonate, or polyethylene glycol to the highest conductivity of PEO–KOH system helps to increase the ambient ionic conductivity to the order of 10−6–10−4 S cm−1. The log σ vs 1/T plot of PEO–KOH showed a small conductivity decrease at 50–60 °C range. The small decrease and the hysteresis that occur during the heating–cooling cycle was overcome by the presence of the plasticizer. X-ray diffraction observation supports the conductivity results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid polymer electrolytes based on the poly(ethylene oxide) (PEO)–alkali metal salt complex have been studied extensively since ion conduction was reported in 1973 [1]. The main advantages of the PEO polymeric electrolytes are their good mechanical properties, ease of fabrication into thin films of desirable sizes, and the ability to form good electrode/electrolyte contact. However, PEO–salt/dopant solvent-free polymer electrolyte has low conductivity in the range of 10–7 to 10−8 S cm−1 at room temperature [2–4]. This is below the required value for electrochemical device applications [5]. To enhance the conductivity in PEO-based polymer electrolytes, a lot of efforts have been used, such as plasticization, copolymerization, blending, and addition of ceramic fillers/additives [6–8]. In the plasticization method, a small amount of a low-molecular-weight plasticizer (mostly liquids) markedly improves the ionic conductivity.
The present work is to study the effect of plasticizers on the ionic conductivity of the PEO–potassium hydroxide (KOH) polymer electrolytes. The anhydrous alkaline solid polymer electrolyte (ASPE) were prepared using methanol as solvent. The highest conductivity of PEO–KOH system was then mixed with plasticizers such as ethylene carbonate (EC), propylene carbonate (PC), or polyethylene glycol (PEG). The impedance spectroscopy and X-ray diffraction (XRD) techniques were used to characterize the conductivity and structural properties of ASPE films, respectively.
Experimental description
Films of PEO as host polymer electrolytes were prepared using the solution cast technique. The starting materials, PEO (M w = 600,000, Aldrich), KOH (Merck), and methanol (Merck). The plasticizers used were EC (M w = 88.06), PC (M w = 102.9), and PEG (Mw = 200), which were procured from Aldrich, Huntsman, and Aldrich, respectively. One gram PEO was dissolved in 50 ml methanol and stirred for 24 h. Different amounts of KOH was then mixed to the solution and stirred for 12 h. For the plasticized films, EC, PC, or PEG was mixed with the highest conductivity of the PEO–KOH system and stirred again for another 12 h. The solution obtained was cast onto glass plate and allowed to evaporate slowly. Then, the samples were kept in a dry place with 24% relative humidity for ∼3 months to ensure that the films were fully dried. The ionic conductivities of the samples were measured at temperatures ranging from 25 to 120 °C using HIOKI 3531 LCR bridge interfaced to a computer. XRD measurement has been carried out using Siemens D5000.
Results and discussion
Conductivity–composition
The composition dependence of the KOH concentrations in PEO films at room temperature is shown in Fig. 1. The conductivity of pure PEO is (8.6 ± 2.3) × 10−11 S cm−1 at room temperature, while the conductivity of films increases up to the maximum of (2.1 ± 0.5) × 10−8 S cm−1 with additional of 30 wt% KOH (sample named P-30K). This increase is attributed to the increasing number of charge carriers [9–11]. Further additions of KOH, however, decrease the conductivity. The decrease in conductivity after 30 wt% KOH concentration is due to ion pairing at high concentrations of KOH. The ion pairing will reduce the number of mobile ions [12]. Compared to the highest conductivity of the polyvinyl alcohol–KOH system [13], the conductivity of the PEO–KOH system is lower by one order.
The highest conductivity of the PEO–KOH system (P-30K) was then mixed with different plasticizers such as EC, PC, or PEG as shown in Fig. 2. The conductivity of P-30K (plasticizer-free) increases sharply with the addition of 10, 20, and 30 wt% EC. However, thereafter, the increase in conductivity became slower on further addition of EC. The EC plasticized system reached (1.3 ± 0.4) × 10−4 S cm−1 before the film showed a problem because of mechanical strength or becoming glue-like. In the PC plasticized system, with a small percentage of PC (10 wt%) was added, the conductivity also increased sharply. On further additions of PC, the increase in conductivities was very slow. The highest conductivity attained by this system (P-30K-50PC) is (1.8 ± 0.2) × 10–5 S cm−1 before the sample showed mechanical problems. On the other hand, in the PEG-plasticized system, the highest conductivity value achieved is (3.4 ± 0.3) × 10−6 S cm−1 for the sample with 60 wt% PEG. The addition of PEG higher than 60 wt% will reduce the physical properties of films. It can be clearly seen that the system with EC is more conductive compared to PC and PEG plasticizers. The conductivity values can be represented in the order of EC > PC > PEG.
Conductivity–temperature
Figure 3 shows the heating–cooling plot (log σ vs 1/T) for the highest PEO–KOH systems, i.e., P-30K. During heating, a small decrease in conductivity is shown at the 50-60 °C temperature region. The decrease appears to be due to the loss of residue solvent (methanol, boiling point = 64–67 °C) from the electrolytes and its absence during the cooling cycle.
For the plasticized systems, Fig. 4 shows the heating–cooling plot of its highest conductivity samples, i.e., P-30K-70EC, P-30K-50PC, and P-30K-60PEG, respectively. During heating, the P-30K-70EC sample (Fig. 4a) showed a small increase in conductivity at 40 °C and a sudden increase at 50–70 °C. However, during cooling, the conductivity did not follow the same track as during heating instead creating a hysteresis. A small increase in conductivity at 40 °C in P-30K-70EC could be due to the melting of EC (melting point = 37–38 °C), and the hysteresis observed could be due to the different melting/freezing temperatures during the heating/cooling cycles. It can also be seen that the conductivity was maintained almost in the same order. The plot of samples P-30K-50PC (Fig. 4b) and P-30K-60PEG (Fig. 4c) showed no hysteresis. The pattern of the heating–cooling cycles from this work is quite similar to Nicotera et al. [14] using a PEO-based polymer electrolyte.
X-ray diffraction
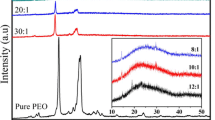
XRD measurement was used to analyze the effect of KOH and plasticizers on conductivity with respect to pure PEO. XRD patterns of the PEO–KOH–EC/PC/PEG system are shown in Fig. 5. For the pure PEO film, the XRD pattern shows characteristic peaks at 2θ between 18 and 22°. At P-30K, the intensity of PEO characteristic peaks decreases. This is due to the decrease in degree of crystallinity of the polymer with increasing concentration of KOH and due to complexation of PEO with KOH. The crystallinity of the plasticized samples also decreases. The P-30K-70EC sample is the most amorphous in nature. However, at higher PEG concentration, i.e., P-30K-60PEG, new peaks appeared at 2θ = 30, 31, 35, 40, and 50°. This explains why the conductivities in the plasticized sample are highest for the EC and lowest for the PEG sample. It has been explained that ionic transport only occurs in the amorphous regions of the polymer and is governed by segmental motion of the polymer chain [15, 16].
Conclusion
PEO mixed with KOH has a conductivity of the order 10−8 S cm−1. On adding EC, PC, or PEG as the plasticizer, the ionic conductivity enhances up to 10−6–10−4 S cm−1. The differences in conductivity in the order of EC > PC > PEG are mostly controlled by the crystalline properties of films. The heating–cooling plot of the PEO–KOH system shows two regions with a small decrease in conductivity at the 50–60 °C temperature region. By using plasticizers, the decrease in conductivity can be prevented.
References
Fenton DE, Parker JM, Wright PV (1973) Polymer 14:589
Dias FB, Plomp L, Veldhuis JBJ (2000) J Power Sources 88:169
Quartarone E, Mustarelli P, Magistris A (1998) Solid State Ionics 110:1
Song JY, Wang YY, Wan CC (1999) J Power Sources 77:183
Wieczorek W, Florjanczyk Z, Stevens JR (1995) Electrochim Acta 40:2251
Mitra S, Kulkarni AR (2002) Solid State Ionics 154–155:37
Kumar M, Sekhon SS (2002) Eur Polym J 38:1297
Jaipal Reddy M, Sreekanth T, Subba Rao UV (1999) Solid State Ionics 126:55
Srivastava N, Chandra A, Chandra S (1995) Phys Rev B 52:225
Mohamad AA, Mohamed NS, Alias Y, Arof AK (2002) J Alloys Compd 337:208
Mohamad AA, Mohamed NS, Yahya MZA, Othman R, Ramesh S, Alias Y, Arof AK (2003) Solid State Ionics 156:171
Srivastava N, Chandra S (2000) Eur Polym J 36:421
Mohamad AA, Arof AK (2006) Ionics 12:57
Nicotera I, Ranieri GA, Terenzi M, Chadwick AV, Webster MI (2002) Solid State Ionics 146:143
Maurya KK, Srivastava N, Hashmi SA, Chandra S (1992) J Mater Sci 27:6357
Mohamed NS, Zakaria MZ, Ali AMM, Arof AK (1997) J Power Sources 66:169
Acknowledgments
The authors would like to thank Prof. A.K. Arof (Universiti Malaya) for the experimental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohamad, A.A., Haliman, H., Sulaiman, M.A. et al. Conductivity studies of plasticized anhydrous PEO-KOH alkaline solid polymer electrolyte. Ionics 14, 59–62 (2008). https://doi.org/10.1007/s11581-007-0154-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-007-0154-3