Abstract
Thin solid polymer electrolytes based on polyethylene oxide (PEO) and silver triflate (AgCF3SO3) dispersed with various concentrations of aluminum oxide (Al2O3) nanoparticles have been prepared by solution casting technique. These thin polymer films are found to have thickness of the order of 30 to 100 μm. The X-ray diffraction (XRD) patterns have indicated the amorphous nature of the polymer electrolyte. The differential scanning calorimeter (DSC) traces showed slight change in the glass transition temperature (T g) whereas the degree of crystallization (X c) decreases markedly due to the addition of alumina nanoparticles. Fourier transform infrared (FTIR) spectral analysis of all these samples has revealed the presence of absorption bands around 1,000 cm−1; thus indicating the complexation of silver ions with oxygen in PEO. Employing the Wagner’s polarization technique as the standard method, the total ionic transference number for the complexed polymer electrolyte was found to be approximately unity thereby revealing that the significant contribution to electrical conduction was due to ions only.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Ionic conductivity in alkali metal salt complexes of polyethylene oxide (PEO) as discovered by P.V. Wright in 1973 has received considerable attention because of their potential application in electrochemical devices [1–4]. Though many kinds of polymer electrolytes have since been studied [5–7], PEO still remains as the most promising solid polymer host to date [8, 9]. PEO is known to exhibit good mechanical stability up to its melting point and excellent processability for making thin films. Though it exhibits good stability, its high concentration of crystalline phase results in low ionic conductivity, which may be improved by the addition of an inert filler to the polymer complex [10]. In general, incorporation of inert fillers such as silica, alumina or others to the polymer complex improves the transport properties, crystallization resistance, and stability of the electrode electrolyte interface [11].
The present work describes the structural, thermal, and transport analyses of polyethylene oxide (PEO)–silver triflate (AgCF3SO3) polymer electrolyte system dispersed with Al2O3 nanoparticles of various compositions. X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, differential scanning calorimeter (DSC), and transference number measurements have been employed for the characterization of synthesized solid polymer electrolytes.
Polyethylene oxide PEO (M W = 5 × 106), silver triflate, AgCF3SO3 and Al2O3 nanoparticles of particle size 40–47 nm were obtained from Aldrich and used as such. AgCF3SO3 salt and Al2O3 nanoparticles were dried in a vacuum desiccator at appropriate temperatures before use. Commercially available, PEO and AgCF3SO3 (O/M = 50/1) were initially mixed in a flat bottomed glass beaker using acetonitrile as the common solvent. The mixture was stirred magnetically for about 4 h. Al2O3 nanoparticles were first dispersed in acetonitrile solution and then the suspensions were thoroughly mixed until it became homogeneous. For the formation of thin films, the suspension was poured into clean dry petri dishes and kept in a vacuum oven at 333 K for solvent evaporation. Aluminum oxide concentrations of 1, 2, 3, 4, and 5 wt% were used in this investigation for the synthesis of various thin film samples of PEO50–AgCF3SO3–x wt% Al2O3 nanocomposite polymer electrolytes followed by subsequent drying for the removal of moisture and acetonitrile. Drying was carried out for about 4 h. After the removal of moisture and acetonitrile, dry thin film samples of thickness value ranging from 30 to 80 μm were prepared and stored in a vacuum desiccator for further studies. Air wedge technique was preferred for the thickness measurement [12].
The complexation of the films of PEO50–AgCF3SO3–x wt%Al2O3 (where x = 0, 2, and 5 wt% Al2O3) has been investigated through XRD studies using a Siefert model SF60 XRD system with Cu–Kα1 radiation at room temperature. The differential thermograms were recorded in the temperature domain 173–373 K using a NETZSCH DSC 204 instrument at a heating rate of 5 K min−1 in nitrogen atmosphere. All the patterns were recorded by loading the sample in an aluminum crucible. The FTIR spectral studies on all the thin film samples containing PEO and AgCF3SO3 corresponding to an O/M ratio 50/1 and various concentrations of Al2O3 nanoparticles were carried out using a Perkin Elmer RX1 IR spectrometer in the wave number region 3,000 to 400 cm−1. The ionic transference number, t ion, was measured using Wagner’s polarization technique on a cell configuration of Ag|polymer electrolyte|C. A constant DC voltage of 300 mV was applied across the blocking electrodes of the cell and the current passing through the cell was monitored as a function of time for about 4 h to let the samples get polarized. A KEITHLEY model 614 electrometer was employed for the accurate measurement of current as a function of time.
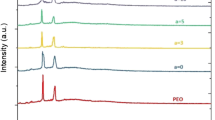
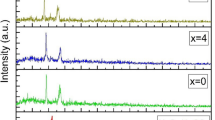
XRD patterns observed for the freshly prepared solid polymer films of PEO and those PEO50–AgCF3SO3 electrolytes complexed with various concentrations of Al2O3 nanoparticles (x = 0, 2, and 5 wt%) are shown in Fig. 1. The appearance of peaks between 2θ = 18 and 30 for pure PEO and the complexed polymer system corresponds to characteristic peaks of the polymer. The incorporation of the Al2O3 nanoparticles from 2 to 5 wt% has revealed a corresponding decrease in the intensities of the observed peaks thus pointing toward the complexation between PEO and the salt. It is also seen from Fig. 1 that the XRD pattern obtained for the complexed polymer has not revealed new peaks, suggesting the absence of any new crystalline complexes[13]. The degree of crystallization of the polymer electrolyte film filled with Al2O3 nanoparticles decreases with increasing mass fraction of aluminum oxide, which in turn increases the amorphous phases of the polymer film.
Thermal properties of PEO, PEO50–AgCF3SO3, and Al2O3 blended polymer electrolytes at various weight percentages were studied using DSC to observe the changes in the glass transition temperature and degree of crystallinity. Figure 2 shows the DSC thermograms for pure PEO and PEO50–AgCF3SO3–x wt% Al2O3 (x = 0, 1, 2, 3, 4, and 5 wt%) in the temperature domain 173 to 373 K. Pure PEO exhibits the melting peak (T m) at 340.1 K, which agrees well with the standard value. On complexation of pure PEO with AgCF3SO3, two exothermic peaks were observed. The peak at 339.6 K corresponds to the melting point of PEO and the other peak at 330.9 K may be assigned to the melting point of complexed polymer electrolyte. It is evident that the glass transition temperature T g increases markedly from 211.1 K (pure PEO) to 219.5 K by the addition of silver triflate confirming the fact that ion–dipole interaction reduces the segmental motion of PEO. On the other hand, the incorporation of Al2O3 nanoparticles to the polymer complexed electrolyte influences slightly on the glass transition temperature [5, 6].The glass transition temperature T g, melting enthalpy ΔH m, and the degree of crystallinity X c estimated for all the samples are listed in Table 1.
Though T g was not affected much by the addition of Al2O3 nanoparticles, the degree of crystallinity (X c, %) decreases from 99.2% for pure PEO was observed to fall to 52.4% for the Al2O3 blended complexed polymer electrolyte. Considering the area of the melting peak as the melting enthalpy ΔH m of the polymer electrolytes, the degree of crystallinity can be calculated from the following equation.
where ΔH m (J/g) is the melting enthalpy of a polymer sample measured by DSC and ΔH m Φ (J/g), the melting enthalpy of 100% crystalline PEO material [7]. The decrease in the degree of crystallinity and a slight change in the T g increases the flexibility of the polymer chain and plays a vital role in the enhancement of ionic conductivity [8].
Figure 3 shows the FTIR spectra of pure PEO and PEO50–AgCF3SO3–x wt% Al2O3 samples. The strong absorption band appearing in the region 2,800–2,950 cm−1 in pure PEO corresponds to symmetric and asymmetric C–H stretching modes of the CH2 group, and those bands around 1,344 cm−1 may be assigned to the asymmetric CH2 wagging mode of PEO. Additional bands at 1,280 cm−1 (asymmetric and symmetric) and 1,242 cm−1 (asymmetric) CH2 twisting modes have also been observed. The band observed around 1,150 cm−1 suggests the C–O–C asymmetric stretching mode of pure PEO. Complexation of silver triflate with PEO was confirmed based on the appearance of peaks at 1,030 and 638 cm−1 corresponding to free triflate ion γs (SO3) and δs (SO3), respectively [14, 15]. No unique absorption bands have been observed on addition of Al2O3 nanoparticles. Generally, PEO on complexation with any metal salts does not affect its gauche conformation. The spectral features observed between 950 and 840 cm−1 for all the samples including pure PEO suggest that the symmetrical rocking mode of CH2 group has not been affected by complexation with AgCF3SO3 and with the addition of Al2O3 nanoparticles, thus indicating that a gauche conformation exists in O–[(CH2)2]–O [16].
The total ionic transference number data for thin solid polymer electrolyte films employed in the cell configuration Ag|polymer electrolyte|C, were evaluated using Wagner’s polarization technique [17,18]. After the complete polarization of the cell, the ionic transference number, t ion, has been obtained from the equation
where the initial current, I i, is the sum of ionic and electronic currents and the final current, I f, is just the electronic current alone. All the experimentally evaluated transference number t ion values for the various compositions are listed in Table 2. It is quite evident from Table 2 that the t ion values obtained from the transport number measurements are approximately unity and hence the charge transport in these thin polymer electrolyte films is highly ionic while their electronic contribution to the total conduction process is negligible.
To conclude, the solid polymer electrolyte material PEO50–AgCF3SO3, prepared in the form of thin films through solution casting method, has been found to show a decrease in crystallinity with the increasing Al2O3 nanoparticles content, which has been confirmed by the XRD and DSC analysis. The complexation of salt with the polymer have been confirmed using FTIR studies and transference number measurements showed that the PEO50–AgCF3SO3–x wt%Al2O3 nanocomposite polymer electrolytes are silver ion conductors.
References
Mac Glashan GS, Andreev YG, Bruce PG (1999) Nature 398:792
Andreev YG, Bruce PG (2000) Electrochim Acta 45:1417
Watanabe M, Uchida H, Emori M (1998) J Phys Chem B 102:3129
Fenton DE, Parker JM, Wright PV (1973) Polymer 14:589
Rhoo HJ, Kim HT, Park JK, Hwang TS (1997) Electrochim Acta 42:1571
Choe HS, Carroll BG, Pasquariello DM, Abraham KM (1997) Chem Mater 9:369
Starkey SR, Frech R (1997) Electrochim Acta 42:471
Lee HS, Yang XQ, McBreen J, Xu ZS, Skotheim TA, Okamoto Y (1994) J Electrochem Soc 141:886
Prosini PP, Fujieda T, Passerini S, Shikano M, Sakai T (2000) Electrochem Commun 2:44
Qian X, Gu N, Cheng Z, Yang X, Wang E, Dong S (2001) Electrochim Acta 46:1829
Ji K-S, Moon H-S, Kim J-W, Park J-W (2003) J Power Sources 117:124
Suthanthiraraj SA, Sheeba DJ (2005) Indian J Phys 79:807
Li Z, Su G, Wang X, Gao D (2005) Solid State Ion 176:1903
Deepa M, Sharma N, Agnihotry SA, Chandra R (2002) J Mater Sci 37:1759
Frech R, Giffin GA, Castillo FY, Glatzhofer DT, Eisenblatter J (2005) Electrochim Acta 50:3963
Hashmi SA, Kumar A, Maurya KK, Chandra S (1990) J Phys D Appl Phys 23:1307
Wagner JB, Wagner C (1957) J Chem Phys 26:1597
Saikia D, Kumar A (2003) Indian J Pure Appl Phys 41:961
Acknowledgements
The financial assistance received from the University Grants Commission, New Delhi under the program “University with Potential for Excellence”, in the form of a research project, is gratefully acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suthanthiraraj, S.A., Sheeba, D.J. Structural investigation on PEO-based polymer electrolytes dispersed with Al2O3 nanoparticles. Ionics 13, 447–450 (2007). https://doi.org/10.1007/s11581-007-0131-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-007-0131-x