Abstract
Sequencing of the ribosomal ITS region was used to resolve the relationship among USA collections of the morphological species Fomitiporia robusta. F. robusta corresponding to European collections was not found and its occurrence in the USA is regarded as questionable. Birch-growing fungus from mid-western and eastern United States known as Phellinus bakeri is a closely related polypore, but because of the absence of typical F. robusta here, it should be considered a separate species. Oak-growing pileate fungus from the south-eastern USA, also known as Fomes calkinsii, is a distinct species, rather distant from the European F. robusta. New combinations Fomitiporia bakeri and F. calkinsii are proposed. Notes on other similar species are provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On the basis of DNA sequence data, a number of authors (e.g., Wagner and Fischer 2001, 2002) divided the rich polypore group of Phellinus s.l. (Hymenochaetaceae) into several subgroups, currently considered as distinct genera by most authors. One of the best defined is the Phellinus punctatus-robustus complex that includes species sharing subglobose, thick-walled, and dextrinoid basidiospores. It was redefined as Fomitiporia Murrill, with Phellinus punctatus (Fr.) Pilát as type species (Fiasson and Niemelä 1984) and confirmed by Fischer (1996). Murrill originally established this genus for Fomitiporia langloisii Murrill (Murrill 1907) which has for a long time been considered a synonym of Fomitiporia punctata (Fr.) Murrill, until Decock et al. (2007) demonstrated on the basis of phylogenetic analysis and type studies that it represents a distinct species on its own, and re-established Fomitiporia langloisii.
About 30 species of Fomitiporia are now recognized (www.indexfungorum.org, Decock et al. 2005), and more than 10 of them were described in the past 5 years. Yet, many others will be probably discovered using multigenic phylogenetic tools. Morphology is poorly discriminative within the genus because most species lack setae and have relatively uniform hyphae and spores. Nonetheless, phylogenetic species recognition methods based on DNA sequence often revealed the existence of species complex in Fomitiporia. Specific habitat and ecology data provide the first and the simplest implications for the existence of a potentially new species that can be then confirmed by DNA sequencing. This combined approach was successful in the identification of several resupinate, F. puncata-like polypores: F. mediterranea Fischer (Fischer 2002), F. tenuis, F. aethiopica (Decock et al. 2005), F. langloisii Murr., and F. maxonii Murr. (Decock et al. 2007).
In this article, we focused on the Fomitiporia robusta species complex in the USA. Fomitiporia robusta (P. Karst.) Fiasson & Niemelä is a very characteristic species in Europe, quite common in Central Europe but only scattered in the warmer Mediterranean region (Kotlaba 1984). It grows almost exclusively on living oaks and introduced Robinia (Kotlaba 1984, p. 39). In the American literature, however, the image of F. robusta appears somewhat blurred, as contradictory distribution and substrate records are available. Lowe (1957) and Overholts (1953) reported the species as widespread across the United States, growing on every substrate possible. Gilbertson and Ryvarden (1987), however, reported this only in the southernmost central and western states (Louisiana, Texas, New Mexico, California), in the region of southern oak–pine forest, mostly on oaks.
It is surprising that the oak-growing F. robusta is not mentioned in the recent distribution lists of the eastern states, where forests similar to European woods prevail. Nevertheless, we can only confirm this as our numerous mycological excursions in eastern USA—from Maine to Virginia—between 2001 and 2009 did not yield any F. robusta-like species on oak (Vlasák 2004). In the Great Smoky Mountains, though, we could quite frequently find an oak-growing F. robusta-like, but slightly different in appearance, polypore. We obtained another specimen from northern Florida where it is, according to collectors’ comments, also common. Searching the literature, we discovered Fomes calkinsii (Murrill) Sacc. & D. Sacc., an oak-growing, hymenochaetoid, pileate polypore with dextrinoid spores (Ryvarden 1985). Overholts (1953) considered F. calkinsii a good species that differs from Fomes robustus P. Karst. (syn. Phellinus robustus) in many, although rather subtle, macro- and microanatomical features. He mentions its occurrence only on oak and beech, in south-central and eastern USA, including North Carolina, Tennessee and Florida where our collections also came from. Lowe (1957), on the other hand, includes Fomes calkinsii into F. robustus, even though he acknowledges some microscopical differences. Gilbertson and Ryvarden (1987) do not mention this species at all, while Larsen and Cobb-Poulle (1990) regard it as conspecific with Phellinus robustus.
Four original specimens of F. calkinsii collected in 1886 and 1887 by W.W. Calkins in northern Florida (including the type) are preserved in NYBG herbarium with photos available on the Internet, and their similarity with our collections is striking. Also, we have inspected and sequenced a collection of M.A. Donk from 1970: an oak-growing, Phellinus robustus-like polypore, kindly provided by the University of Tennessee Herbarium. This collection was from the Great Smoky Mountains, similar to our oak collections.
On the basis of ecological and morphological characteristics of F. calkinsii, and comments on its microanatomy made by L.O. Overholts (1953) and J.L. Lowe (1957), we have concluded that it is identical with our collections from the Great Smoky Mountains and northern Florida, even though we have not studied NYBG herbarium collections directly.
We have also compared our F. robusta/calkinsii collections to other similar species from the F. robusta group, namely the recently described F. dryophila Murrill (Decock et al. 2007) and conifer-growing and birch-growing types. Semipileate F. dryophila is a well-defined species that grows abundantly on live oak (Q. virginiana Miller) in the very south of Florida. Its morphology is rather similar to F. robusta in early development but sequences show only a distant relationship (Decock et al. 2007). Conifer-growing Fomitiporia robusta-like fungi, usually named F. hartigii (Allesch. & Schnabl) Fiasson & Niemelä or F. tsugina Murrill are common in the eastern as well as western USA. They are distinctly different from the true F. robusta, as was shown, for example, by Fischer and Binder (2004), and are treated only marginally in this paper. Some comments on the identity of American collections are included in the “Discussion”. Betula-specific, F. robusta-like polypore Phellinus bakeri (Murrill) A. Ames was described by W. A. Murrill (Murrill 1908) from Wisconsin, and its range was reported as “Winsconsin, Missouri and westward” (Murrill 1914). Because of the substrate, P. bakeri was sometimes mistaken for Phellinus igniarius (L.) Quél. Overholts (1920) stressed some morphological and type-of-rot differences between P. bakeri and P. igniarius (both as Fomes) and described three collections from eastern USA. According to our field experience, this fungus is common in eastern states where Betula lenta L. and/or B. nigra L. grow (Vlasák 2004), but in the Great Smoky Mountains it also colonizes B. allegheniensis. P. bakeri was often identified as a birch-specific variety of P. robustus (Lowe 1957). Gilbertson and Ryvarden (1987) do not mention it at all, but Larsen and Cobb-Poulle (1990) regard it as a good species.
In this article, we present the results of our collections of P. calkinsii, including ribosomal RNA sequence data that demonstrate unequivocally that it is a distinct species, rather distant from F. robusta. We also show that the birch-growing P. bakeri is much more similar to European F. robusta judging by ribosomal RNA sequence, but still different, in our opinion. Comments on some other pileate Fomitiporia species occurring in the USA are added.
Materials and methods
Specimens studied
Fresh material was collected by the first author and his son (collections signed -J) in 2001–2009 across eastern USA (Table 1); the European material studied for the comparison was retrieved from the private herbarium of the first author (http://mykoweb.prf.jcu.cz/polypores) where are also preserved all specimens collected in the USA. Duplicates of most of the specimens are also preserved in Prague Museum Herbarium (PRM). The collection of M.A. Donk 35560 is preserved at the University of Tennessee Herbarium (TENN). Herbarium acronyms follow Holmgren and Holmgren (1998).
The microscopic inspections were performed in Melzer’s reagent. A total of 30 basidiospores from each specimen were measured.
Sequencing
About 20 mg of basidiocarp context material was disintegrated for 30 s in a ball mill (MM301 RETSCH), and DNA was isolated using CTAB/NaCl extraction buffer as described by Murray and Thompson (1980), followed by repeated extractions with chloroform, and isopropanol precipitation. Crude DNA was dissolved in 100 μl of water with 5 μg of RNaseA, incubated for 30 min at 37°C and purified with GENOMED JetSorb silica kit. The resulting DNA solution was ten times diluted and 1 μl was used as a template for amplification with ITS1 and ITS4 primers (White et al. 1990) in 25 μl of reaction mixture, using 55°C annealing temperature. Then, 0.5 μl of the amplified DNA reaction mixture was sequenced using ITS1 and ITS4 primers. The sequencing was performed in the Genomics laboratory of Biology Centre, Academy of Sciences of the Czech Republic, České Budějovice, on an ABI 3730xl DNA analyzer, using BigDye Terminator 3.1 kit. The sequences obtained in this study were deposited at GenBank (Table 1).
Most of the sequences contained some heterozygous sites due to genetically different nuclei (alleles) present in polykaryotic tissues. In about 30% of specimens (but none of Fomitiporia calkinsii), insertions/deletions were detected in one genome compared to the other, which led to overlapping peaks in parts of the sequence scanner output. These sectors had to be re-sequenced with additional primers ITS2 and ITS3 (White et al. 1990) that anneal behind the heterozygous region. Ribosomal RNA sequence of such collections had to be published under two different accession numbers referring to individual alleles.
Phylogenetic analysis
The sequences were aligned by Clustal X and manually curated. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei 1987) and the evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al. 2004). All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). There were a total of 628 positions in the final dataset.
Results
ITS sequence analysis
All five sequenced Quercus/Carya-growing F. robusta-like collections from the Great Smoky Mountains and Northern Florida (Table 1) gave a very similar sequence of 694-bases-long ITS-5.8S region with only one variable position. Preliminary BLAST search at GenBank (Altschul et al. 1990) demonstrated rather low homology with other Fomitiporia species (F. robusta in particular). Recently described European F. mediterranea M. Fisch. (Fischer 2002) and F. punicata Y.C. Dai, et al. (Dai et al. 2008) from China came through as the closest relatives in BLAST search, but with only 89% similitude. Therefore, we also sequenced our own collections of European F. robusta as well as American F. robusta-like polypore growing on birch, Phellinus bakeri, both from Pennsylvania and the Great Smoky Mt., and F. dryophila Murrill, another oak-growing, pseudopileate species from Florida (Decock et al. 2007) (Table 1). Phylogenetic analyses of the sequences obtained and of others retrieved from the GenBank were conducted in MEGA4 (Tamura et al. 2007). The results demonstrated that Quercus/Carya-growing, pileate F. robusta-like collections from eastern USA form a well-separated clade (Fig. 1) that justifies the establishment of Fomitiporia calkinsii (Murrill) comb. nov.
Evolutionary relationships of 28 Fomitiporia species based on sequence comprising ITS1, 5.8S and ITS2. The optimal tree with the sum of branch length = 0.15814583 is shown.The bootstrap consensus tree inferred from 1,000 replicates is taken to represent the evolutionary history of the taxa analyzed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances are in the units of the number of base substitutions per site. Accession numbers with * represent sequences retrieved from GenBank. Other GenBank accession numbers are in Table 1
Ribosomal RNA sequence of birch-growing Phellinus robustus (bakeri) is much more similar to the European F. robusta. Yet, two sequenced specimens (Table 1) from distant and very different localities and different substrates show typical sequence features, not present in F. robusta: four insertions/deletions 1–4 bases long and four transitions/transversions in ITS2, where all sequenced F. robusta have identical sequence, and four insertions/deletions 1–5 bases and also four other changes in ITS1, where F. robusta sequenced specimens show only transitions/transversions in five other positions. The sequence difference in BLAST search of about 8–9% in ITS1 and 6–7% in ITS2 between P. robustus (bakeri) and F. robusta is the same as between, for example, Fomitiporia hartigii and F. mediterranea (Fig. 1). Taking into consideration that in the distribution area of P. robustus (bakeri) the oak-growing F. robusta does not occur at all, we approve the notion of Larsen and Cobb-Poulle (1990) that the birch-growing fungus should be considered a good species for which we suggest the name Fomitiporia bakeri (Murrill) comb. nov.
For the comparison, we have also sequenced four of our collections, semipileate or resupinate, of American conifer-growing Fomitiporia robusta (tsugina, hartigii?) from different conifer substrates in the east and the west of USA. All these sequences are essentially identical with the previously published two sequences of American F. hartigii (Fischer and Binder 2004) and make a very homogeneous group with only one variable site in ITS1 and one in ITS2. They represent a distinct clade, well separated from the hardwood-growing collections. Surprisingly, though, Asian and European F. hartigii (including a specimen growing in Europe on introduced Tsuga sp.) show several very constant mutations: 1b and 3b deletions in ITS1, 3b insertion in ITS2 and a few transitions/transversions. This represents only 3–4% sequence difference in BLAST search, but causes distinct clustering of American conifer collections, separately from European F. hartigii.
Taxonomy
Fomitiporia calkinsii (Murrill) Vlasák & Kout, comb. nov. — MycoBank MB518864
Basionym
Pyropolyporus calkinsii Murrill, The Polyporaceae of North America. II. The genus Pyropolyporus. B. Torrey Bot. Club 30:109–120, 1903.
Description
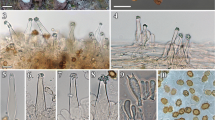
Basidiomes perennial, sessile, ungulate to applanate, up to 10 × 8 × 5 cm, woody hard; upper surface glabrous but somewhat grainy on touch, sulcate, making up to seven broad, seasonal zones, later becoming incrusted and slightly rimose, marginal zone yellowish brown, the older zones stepwise more and more dark reddish to blackish brown, sometimes with a silvery cover. Pore surface dark reddish- or grayish-brown, the pores circular, very regular, 6–8 per mm, with thick, entire dissepiments; young context yellowish brown, later reddish brown, zonate, up to 2 cm thick; tubes in many year layers, sometimes indistinctly stratified, brown, becoming whitish within, woody, up to 5 mm long.
Hyphal system dimitic in the context and hymenophoral trama, generative hyphae simple-septate, hyaline to pale yellowish, thin-walled, 1.5–3 μm wide, skeletal hyphae yellowish brown, thick-walled, nonseptate, 2.5–3.5 μm wide. Setae lacking. Cystidioles present in hymenium, hyaline, thin-walled, ventricose, ending in an elongated hyphoid apex, 13–16 μm long and 5–7 μm wide at the base, narrow apical part 1.5–2 μm wide. Basidia subglobose, 4-sterigmate, 10–12 × 8–10 μm. Basidiospores subglobose, hyaline, thick-walled, dextrinoid in Melzerś reagent, 5–6.5 (7) × 5–6 μm. On living oaks and hickory.
Holotypus
USA, Florida, on living live oak, Feb 1886, W.W. Calkins (NYBG742984).
Specimens examined
USA, TN, Great Smoky Mountains, on living Quercus rubra, Sep 2005, collected by J. Vlasak, PRM915979 and PRM915980; USA, NC, Great Smoky Mountains, on living Quercus sp. Apr 2004, coll. J. Vlasak Jr., PRM915978; USA, FL, Bulow Creek State Park, Carya sp., Dec 2002, coll. J. Vlasak Jr., JV0212/12-J; USA, TN, Great Smoky Mountains, Quercus falcata, Jun 1970, coll. M.A. Donk, TN35560.
Remarks
Fomitiporia calkinsii is very similar to European Fomitiporia robusta in both macro- and microscopic characteristics. Pileus surface is perhaps notably different. In F. robusta, it is usually green of algae except for the marginal zone; in rare cases of pilei without algae, the second or third zone from the margin is already dark blackish brown with a thick crust and the older zones more or less black. In F. calkinsii, the pileus surface is brown or rusty brown, indistinctly incrusted, in outer zones with lighter and brighter colors, in inner zones darker, but not just black (Figs. 1 and 2). Pores and spores are smaller in F. calkinsii and the contextual skeletal hyphae are distinctly narrower, as noted already by Lowe (1957).
Fomitiporia bakeri (Murrill) Vlasák & Kout, comb. nov. — MycoBank MB518865
Photograph in http://mykoweb.prf.jcu.cz/polypores.
Basionym
Pyropolyporus bakeri Murrill, Polyporaceae, Part 2. North American Flora 9:104, 1908.
Description
Basidiomes perennial, sessile, ungulate, rarely applanate, often triquetrous in section, up to 13 × 15 × 8 cm, not so hard as other pileate species of Fomitiporia; upper surface tomentose to glabrous, sulcate, later becoming incrusted and slightly rimose, marginal zone yellowish brown, the older zones stepwise more and more dark reddish to blackish brown, margin thick, rounded. Pore surface pale brown to golden brown, the pores circular or somewhat irregular, 5–6 per mm, with thin to thick dissepiments; context yellowish brown, shining, zonate, up to 5 cm thick; tubes brown, in distinct year layers, up to 10 mm long each year.
Hyphal system dimitic in the context and hymenophoral trama, generative hyphae simple-septate, hyaline to pale yellowish, thin-walled, 1.5–3 μm wide, skeletal hyphae yellowish brown, thick-walled, nonseptate, 3–6 μm wide. Setae lacking. Cystidioles present in hymenium, hyaline, thin-walled, ventricose, ending in an elongated hyphoid apex. Basidia subglobose, 4-sterigmate, 10–11 × 8–10 μm. Basidiospores subglobose, hyaline, thick-walled, dextrinoid in Melzer’s reagent, 6–8 × 5.5–6.5 μm.
Holotypus
USA, WI, St. Croix Falls, Betula lenta, 1897, C.F. Baker (NYBG742981).
Specimens examined
USA, PA, Norristown, Valley Forge, on living Betula lenta, Sep 2003, collected by J. Vlasak, PRM915948; USA, TN, Great Smoky Mountains, on living Betula allegheniensis, Apr 2004, coll. J. Vlasak Jr., JV0404/18-J.
Remarks
The basidiome is roughly similar to Fomitiporia calkinsii although often larger, not so hard, with larger and sometimes somewhat irregular pores that also have a more yellowish, brighter color. The context is often very thick, more yellowish brown, distinctly shining. Growth on birch (Betula sp.) is the most important field characteristic. Basidiocarps develop on dead standing stems unlike to F. calkinsii and F. robusta that grow mostly on living trees.
Discussion
In the second half of the 20th century, the pragmatic approach to mycological systematics, based on the assumption that different species must show clear microanatomical differences, enabled to reduce the number of described synonymous species several times and restored the basic order in polyporology. Unfortunately, the extensive study of herbarium specimens, often misshaped and untypical, collected in different stages of development and without notes about their ecology, had a consequence, sometimes, in dispraising some external morphological traits and important ecological characteristics in species delimitation.
Prominent American polyporologists, L.O. Overholts and J.L. Lowe, were particularly careful in describing new species, and American polypores without conspicuous differences in spore shape and/or size were usually identified as their European relatives. This is probably why they included all American pileate hymenochaetoid fungi, microscopically similar to Phellinus robustus, in this species. A similar approach was used by Gilbertson and Ryvarden (1987) who only separated species with strikingly different ecology: Phellinus texanus (Murrill) A. Ames. growing only in the southwest on non-oak, desert plants, and Phellinus hartigii (Allesch. & Schnabl) Pat., growing on conifers. In the latter combination, they again identified all American conifer-growing Phellinus robustus as the European species. Yet, P. hartigii is distinctly pileate in Europe and semipileate with sloping pileus or more often resupinate in the USA (Overholts 1953, p. 89). Murrill (1907) named this common American fungus Fomitiporia tsugina Murr., and Lowe (1957) also did not regard this American fungus as conspecific with European P. hartigii. Fischer and Binder (2004) in their ITS sequencing study of Phellinus s.l. found that American P. hartigii collections cluster separately of P. hartigii from Europe and Asia and we could only confirm this. The sequence difference is rather low (3–4% in the ITS region), but because of the very constant sequence of all American collections and different morphology, we believe that the American fungus should also be considered a good species even if closely related to F. hartigii. Some comments on Fomitiporia tsugina taxonomic status were also published by Decock et al. (2007).
According to Gilbertson and Ryvarden (1987), oak-growing Phellinus robustus-like fungi are distributed along the USA southern border from east to west. We believe that this distribution pattern describes the occurrence of Fomitiporia calkinsii and F. dryophila, another common and thermophilic, but semipileate, species from oaks (Decock et al. 2007). Yet, another oak-growing species probably occurs in this area, at least in the west. Overholts (1953) noted that specimens of Phelinus robustus from Pacific Coast are “less developed and usually nodulose-sessile”. We have only a small slice of this western species at hand, from a very large, thick and typically nodulose basidiocarp (according to collector's comments), growing in Santa Cruz, CA, on an oak stump, and its ribosomal RNA sequence is different from all other described species (Fomitiporia sp. in Table 1 and Fig. 1). Having no more material at present and no field knowledge of this species, we leave this problem open. Fischer and Binder (2004), who studied American Fomitiporia species collected in California using ribosomal RNA sequencing, described a new species F. polymorpha M. Fisch., collected on Salix and several other non-oak hosts, with effused-reflexed to bulbous basidiocarps that is nevertheless not related to our Fomitiporia sp. by its sequence. It may be identical with also bulbous and Salix-collected Pyropolyporus abramsianus (Murrill) Sacc. & Trotter that is also mentioned by Overholts (1953) and Decock et al. (2007) as Phellinus robustus-like species.
In summary, according to our results and literature data, hardwood-growing, pileate Fomitiporia species in the eastern USA include Fomitiporia bakeri having an exclusive preference for birch in the mid-west and east, Fomitiporia calkinsii on oak, beech and hickory, south of 35° parallel, and pseudopileate Fomitiporia dryophila on live oak in the very south. The occurrence of Fomitiporia robusta is very improbable, in our opinion, as we could not find any such fungus during years of exploring many ideal localities in the eastern USA. Fischer and Binder (2004) came to the same conclusion studying Fomitiporia species in California.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Dai YC, Cui BK, Decock C (2008) A new species of Fomitiporia (Hymenochaetaceae, Basidiomycota) from China based on morphological and molecular characters. Mycol Res 112:375–380
Decock C, Bitew A, Castillo G (2005) Fomitiporia tenuis and Fomitiporia aethiopica (Basidiomycota, Hymenochaetales): two undescribed species from the Ethiopian highlands: taxonomy and phylogeny. Mycologia 97:121–129
Decock C, Herrera Figueroa S, Robledo G, Castillo G (2007) Fomitiporia punctata (Basidiomycota, Hymenochaetales) and its presumed taxonomic synonyms in America: taxonomy and phylogeny of some species from tropical/subtropical areas. Mycologia 99:733–752
Fiasson JL, Niemelä T (1984) The Hymenochaetales: a revision of the European poroid taxa. Karstenia 24:14–28
Fischer M (1996) On the species complexes within Phellinus: Fomitiporia revisited. Mycol Res 100:1459–1467
Fischer M (2002) A new wood-decaying basidiomycete species associated with esca of grapevine: Fomitiporia mediterranea (Hymenochaetales). Mycol Prog 1:315–324
Fischer M, Binder M (2004) Species recognition, geographic distribution and host-pathogen relationships: a case study in a group of lignicolous Basidiomycetes, Phellinus s.l. Mycologia 96:799–811
Gilbertson RL, Ryvarden L (1987) North American polypores, Vol. 2. Fungiflora, Oslo, pp 435–885
Holmgren PK, Holmgren NH (1998) [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden′s Virtual Herbarium. – http://sweetgum.nybg.org/ih/
Kotlaba F (1984) Geographical distribution and ecology of polypores /Polyporales s.l./ in Czechoslovakia (in Czech). Academia, Praha, pp 1–194
Larsen MJ, Cobb-Poulle LA (1990) The genus Phellinus (Hymenochaetaceae). A survey of the world taxa. Synopsis Fung 3:1–206
Lowe JL (1957) Polyporaceae of North America. The genus Fomes. Syracuse New York State University College of Forestry, Technical Publication 80
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Murrill WA (1903) The Polyporaceae of North America. II. The genus Pyropolyporus. B Torrey Bot Club 30(2):109–120
Murrill WA (1907) Polyporaceae, part 1. North Am Flora 9(1):1–72
Murrill WA (1908) Polyporaceae, part 2. North Am Flora 9(2):104
Murrill WA (1914) Northern Polypores. New York, (published by the author)
Overholts LO (1920) Some mycological notes for 1919. Mycologia 12:135–142
Overholts LO (1953) Polyporaceae of the United States, Alaska and Canada. University of Michigan Press, Ann Arbor
Ryvarden L (1985) Type studies in the Polyporaceae 17. Species described by W.A. Murrill. Mycotaxon 23:169–198
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Vlasák J (2004) Collecting polypores in the USA (in Czech). Mykol Listy 90–91:15–19
Wagner T, Fischer M (2001) Natural groups and a revised system for the European poroid Hymenochaetales (Basidiomycota) supported by nLSU rDNA sequence data. Mycol Res 105:773–782
Wagner T, Fischer M (2002) Proceedings towards a natural classification of the worldwide taxa Phellinus s.l. and Inonotus s.l., and phylogenetic relationships of allied genera. Mycologia 94:998–1016
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols, a guide to methods and applications. Academic, New York, pp 315–322
Acknowledgements
We are grateful to Josef Vlasák Jr. for collecting many interesting polypore specimens and for critical reading of the manuscript. We also express our gratitude to Dr. R.H. Petersen from University of Tennessee, Knoxville, USA, for the loan of specimens and to Dr. Karel Petřík, BC ASCR, for the help with the phylogenetic analysis. This research was supported by AV0Z50510513 fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlasák, J., Kout, J. Pileate Fomitiporia species in the USA. New combinations Fomitiporia calkinsii and F. bakeri . Mycol Progress 10, 445–452 (2011). https://doi.org/10.1007/s11557-010-0715-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-010-0715-0