Abstract
Background
Recently, the PORT-C and LUNG-ART trials, which evaluated the role of postoperative radiation therapy (PORT), have significantly altered the treatment landscape for NSCLC pN2 patients who previously underwent surgery. In response, the Italian Association of Radiotherapy and Oncology Thoracic Oncology study group has initiated an observational multicenter trial to assess both acute and late toxicities of PORT in pN2 NSCLC patients treated with modern techniques.
Methods
Data on NSCLC patients submitted to PORT after radical surgery treated between 2015 and 2020 in six Italian Centers were collected. Heart, lung, and esophageal acute and late toxicities have been retrospectively analyzed and related to radiation therapy dosimetric parameters. Furthermore, loco-regional control, distant metastasis and overall survival have been analyzed.
Results
A total of 212 patients with a median age of 68 years from six different centers were included in this analysis (142 males and 70 females). Prior to undergoing PORT, 96 patients (45.8%) had a history of heart disease, 110 patients (51.9%) had hypertension, and 51 patients (24%) had COPD.
Acute toxicity was observed in 147 patients (69.3%), with lung toxicity occurring in 93 patients (G1 in 70 patients, G2 in 17 patients, and G3 in 4 patients), esophageal toxicity in 114 patients (G1 in 89 patients, G2 in 23 patients, and G3 in 1 patient), and cardiac toxicity in 4 patients (G1 in 2 patients and G3 in 2 patients). Late side effects were found in 60 patients (28.3%), predominantly involving the lungs (51 patients: 32 G1, 11 G2, and 1 G3) and the esophagus (11 patients: 8 G1 and 3 G2), with no reported late cardiac side effects.
Various clinical and dosimetric parameters were found to correlate with both acute and chronic toxicities. Over a median follow-up period of 54 months, 48 patients (22.6%) showed locoregional disease relapse, 106 patients (50%) developed distant metastases, and 66 patients (31.1%) died.
Conclusions
RAC-TAC retrospective multicentric study showed the low toxicity of PORT when advanced technology is used. At the same time, it’s noteworthy to underline that 50% of the patients develop distant recurrences in the follow up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The landscape of treatments for patients with pN2 non-small cell lung cancer (NSCLC) who have undergone surgery has been significantly transformed by recent developments in postoperative radiation therapy (PORT), as demonstrated by studies such as LUNG-ART and PORT-C [1, 2]. Clinical outcomes from both trials, particularly regarding overall survival (OS) (which did not reach the statistical significance in either study) and concerns related to radiotherapy (RT) toxicity, reduced the indication for PORT, especially among patients without risk factors [3].
Nonetheless, in our point of view, it is essential to scrutinize the results of these studies with a more balanced perspective. Notably, it is worth highlighting that the toxicity concerns observed in the Lung-ART trial were not substantiated by the findings in the PORT-C trial, which predominantly used advanced more sophisticated techniques (89.3% in PORT-C versus 11% in Lung-ART) such as volumetric modulated arc RT (VMAT) or intensity modulated RT (IMRT). Nevertheless, it remains critical to acknowledge that neither study demonstrated an advantage in OS and the substantial occurrence of deaths not related to cancer in the PORT arms of both trials underscores the necessity for a thorough assessment of the benefit/risk ratio associated with adjuvant approaches.
We believe that in the next future we will be able to correctly identify the pN2 patients that deserve adjuvant systemic therapies [4]. Among these strategies, RT should be performed in a proper way not to jeopardize the important advantage in mediastinal relapse (25% in PORT versus 46% in control group in the LUNG-ART trial).
In this context, the Italian Association of Radiotherapy and Oncology (AIRO) Thoracic Oncology Cancer study group has recently designed a multicenter retrospective observational study to assess both acute and late toxicities in NSCLC patients who received PORT using IMRT or VMAT techniques. The primary focus is on RT-related side effects. Secondary objectives include local control, distant control, overall survival, and dosimetry assessments of organs at risk (heart, lungs, and esophagus). This comprehensive approach, which includes toxicity assessment, dosimetry analysis, and clinical outcome evaluation, enhances our understanding of the treatment landscape and paves the way for further advancements in patient care.
Methods
Study design and population
The study adopted an observational retrospective design. Patients eligible for inclusion had histologically confirmed NSCLC. All patients underwent surgery with mediastinal exploration, which revealed mediastinal involvement (pN2) and subsequently received PORT using IMRT or VMAT techniques.
Exclusion criteria were: presence of documented metastases in a different lung lobe, pleural or pericardial effusion, history of prior thoracic RT, clinical progression during post-operative chemotherapy, and recent (within the last 6 months) severe cardiac or pulmonary diseases.
Data collection and database
To facilitate data collection, a dedicated database was established. Data collection spanned from January 1, 2015, to December 31, 2020, aiming to accumulate a sufficiently large dataset for comprehensive analysis. The data collected included a wide range of information, such as demographic details (gender, age, and recruitment date), medical history (comorbidities and smoking habits), ECOG status, tumor features (staging, histology, biomarkers), treatment characteristics (type of RT, concurrent/sequential chemotherapy and treatment start/end dates) and therapy response assessed using RECIST criteria. Radiation and dosimetry parameters for RT (technique, dose per fraction, total dose, duration) encompassing cardiac (V5, V30, Mean Dose, DMAX [0.1 cc]), pulmonary (Mean Dose, V20, V30), and esophageal (Mean Dose, V50, V55) dosimetry, toxicity assessments based on the CTCAE 4.0 scale (acute and late) were analyzed. Furthermore, clinical outcomes, including overall survival (OS), progression-free survival (PFS), local (LC) and symptom control were also evaluated. Sample size determination relied on consecutively treated patients at participating centers. OS and PFS were calculated from diagnosis until death or disease progression, using Kaplan–Meier method. Acute toxicity during and at the end of RT treatment was analyzed descriptively, presenting raw incidence rates and severity. Late toxicity, assessed at least 6 months post-radiotherapy completion, underwent similar descriptive analysis, reporting raw incidence rates and severity.
Statistical analysis
Data analysis involved a range of statistical techniques to examine the dataset. Descriptive statistics analyzed clinical and demographic variables, providing an overview of the study population. For the analysis of correlations, Pearson's correlation coefficient was used, allowing us to assess relationships between clinical factors, dosimetric parameters, and toxicity outcomes. Associations between categorical variables, such as comorbidities and toxicity, were explored using chi-squared or ANOVA where applicable. Logistic regression analysis was used for multivariate analysis. Statistical analyses used a two-sided approach and was considered as significant a p-value below 0.05. Data analysis was performed using IBM SPSS (IBM Corp, Armonk, NY, Version 23.0).
Results
Demographics
In this retrospective study, a total of 255 patients with a median age of 69 years (standard deviation 9.25, range 41–85 years) were included. One hundred sixty seven were males (65.5%) and 88 females (34.5%), among the participants, 42 (16.5%) were non-smokers, 166 (65.1%) former smokers and 44 (17.3%) current smokers. Clinical features of the entire cohort are summarized in Table 1.
Regarding the ECOG performance status, 143 patients (56%) had a score of 0, 101 (39.6%) had 1, 7 (2.7%) had 2, and 3 patients (1.2%) had a score of 3. Hypertension was present in 128 patients (50.2%), and the GOLD scale for chronic obstructive pulmonary disease (COPD) was distributed as follows: 137 (53.7%), 28 (11%), 25 (9.8%) and 2 (0.8%) patients had a score of 0, 1, 2 and 3, respectively.
The majority of patients were staged with 18 FDG PET/CT scan (228/255 patients, 88,7%), whereas brain MRI was used at baseline in only 30 patients (11,7%) and Endobronchial Ultrasound bronchoscopy for biopsy in 131 patients (51%).
Surgery and chemotherapy treatments
After completion of the clinical staging, 62 patients (24.3%) were staged as cN0, 26 (10.2%) as cN1, 137 (53.7%) as cN2, and a single patient (0.4%) as cN3. Surgical procedures included lobectomy in 183 patients (71.8%), segmentectomy in 9 patients (3.5%), pneumonectomy in 27 patients (10.6%) and sleeve resection in 15 patients (5.9%). After pathological review following surgery, two patients were staged as pN0 (0,8%) and three patients as pN1 (1,2%), whereas the median number of positive nodes was 3 (range 1–20), and the median number of nodes removed was 11 (range 1–75).
Regarding margin status, 175 patients (68.6%) had negative margins (R0), while 12 (4.7%) were classified as R1 (9/12) and R2 (3/12).
Adjuvant therapy consisted of sequential chemo-radiotherapy in most cases (125 of 255 patients, 49%), with 41 of 255 patients (16.1%) undergoing concomitant adjuvant chemo-radiotherapy. Twenty-one patients (8.2%) received neoadjuvant chemotherapy and finally 68 (26.6%) did not undergo chemotherapy. In the adjuvant setting, the median number of chemotherapy cycles administered was 3 (range 1–8); the most common regimen was represented by cisplatin doublets (165 patients, 64,7%). Adjuvant therapies are summarized in Table 2.
Radiotherapy treatment
All patients received RT using advanced techniques, with 15% undergoing Tomotherapy, 74.8% VMAT and the remaining ones IMRT. RT was usually completed without any interruption, with only 14.1% experiencing a treatment suspension. The median interruption duration was 7 days (range 2–40 days).
Median RT doses received by the critical structures were as follows:
-
Median doses to the heart for specific parameters were: V5 27%, V30 6%, mean and maximum dose were 7.83 Gy and 48 Gy;
-
For bilateral lungs were: mean dose 10.1 Gy, V20:16% and V30: 8%;
-
Median doses for the esophagus were 20 Gy as mean dose, 9.80% for V50, and no exposure for V55
Toxicity
Regarding acute toxicities, 179 patients (70.2%) experienced different adverse events summarized in Table 3.
Lung toxicities were observed in 106 patients being of grade 1 (G1) in 83 patients (46.4%), G2 in 19 (10.6%) and G3 in 4 patients (2.2%). Similarly, esophageal side effects occurred in 137 patients and were classified as G1, G2 and G3 in 109 (63.1%), 27 (15.6%) and one patient (0.6%), respectively. In terms of heart toxicity, cardiological events (pericarditis and atrial fibrillation) were observed only in 4 patients (2.2%), being G1 in 2 patients and G3 in 2 patients. Finally, 63 patients (27.9%) experienced asthenia, with severity categorized as G1 in 51 patients (22.6%), G2 in 11 (4.9%) and G3 in a single patient (0.4%). In 19 patients (7.5%) other types of acute toxicities were experienced, most of which were G1, including radiation dermatitis, nausea, and fever. It’s noteworthy to underline that the focus of this analysis was on toxicities specifically associated with radiotherapy, consequently, hematological toxicity hasn't been extensively addressed.
Moving to late toxicities, 71 patients (27.8%) developed lasting adverse effects that began at least six months after completing PORT. Chronic pulmonary toxicity was showed in 52 patients (20.4%), of whom 38 patients had G1 (14.9%), 13 patients G2 (5.1%) and only 1 patient G3 (0.4%). On the other hand, esophageal toxicity was noted in 12 patients (4.7%), being G1 in 9 patients (3.5%) and G2 in 3 patients (1.2%). Unfortunately, a single patient (0.4%) experienced severe (G3) late cardiac toxicity (heart failure). In addition, 18 patients (7.1%) exhibited other forms of chronic toxicity, predominantly G1.
Outcomes
In the latest analysis 78 patients (30.6%) were free from disease, 19 (7.5%) had local failures, 36 (14.1%) developed metastatic disease and 19 (7.5%) had both local and distant recurrences, considering a median follow-up of 49 months. Seventy-one (27.8%) even died for disease progression, while 13 (5.1%) passed away due to other causes (not tumor related). Finally, 19 patients (7.5%) were lost to follow-up. Median overall survival was 51 months, with a standard deviation of 4.54 months (95% CI 42–59 months).
Furthermore, 135 patients (52.9%) developed distant dissemination, showing a median metastasis-free survival of 20 months, with a standard deviation of 4.1 months (95% CI 11–28 months). The most prevalent sites of metastatic disease were brain, affecting 26 out of 135 patients (19.3%), followed by bone in 22/135 (16.3%), lung in 16/135 (11.9%) and liver involvement in 4 patients (3.0%). Twenty patients (14.8%) developed metastasis in other sites, while synchronous multifocal metastasis were found in 47/135 patients (34.8%).
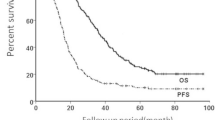
Finally, 52 patients (20.4%) experienced local recurrences, with a median PFS not reached, and a mean PFS of 71 months, with a standard deviation of 2.7 months (95% CI 66–77 months) (see Fig. 1).
Survival curves for the three outcomes. Panel A: Local recurrence survival, showing that among the patients studied, 52 individuals (20.4%) experienced local recurrences, with a median progression-free survival that was not reached. The mean progression-free survival in this group was 71 months, with a standard deviation of 2.7 months (95% CI 66–77 months). Panel B: Distant recurrence survival, indicating that 135 patients (52.9%) developed distant disease during the follow-up period. The median progression-free survival for this group was 20 months, with a standard deviation of 4.1 months (95% CI 11–28 months). Panel C: Overall survival, demonstrating that 84 patients (32.9%) passed away during the follow-up period. The calculated median overall survival for the entire cohort was 51 months, with a standard deviation of 4.54 months (95% CI 42–59 months)
Correlation with toxicity
All patient characteristics (including demographics, tumor-related and treatment-related parameters) were collected in a centralized digital database trying to find a correlation with acute or late side effects as well as with clinical outcomes. At univariate analysis we observed associations between acute lung toxicity and several factors, such as preexisting cardiac disease (p = 0.030), number of removed lymph nodes (p = 0.042), heart Dmax (p = 0.031) and lung V20 (p = 0.011), whereas at multivariate analysis only preexisting cardiac disease (p:0,028) and heart-Dmax (p:0,043) remained significant. Furthermore, esophageal acute toxicity seems to be associated with gender (most common in females, 64,8% versus 48,5% in males, p = 0.013), number of removed lymph nodes (p = 0.006) and heart Dmax (p = 0.016), whereas at multivariate analysis only sex remained significant (p:0,018).
Acute cardiac toxicity was significantly associated with mean lung radiation dose (p = 0.017) and heart V5 (p = 0.043), but only mean lung radiation dose was significant at multivariate analysis (p = 0.048). Acute asthenia was found to be correlated with age at both univariate (p = 0.008) and multivariate analysis (p:0,014). Regarding chronic toxicities, lung toxicity was correlated with ECOG performance status (p = 0.010), preexisting cardiac disease (p = 0.033), COPD (p = 0.010), and heart Dmax (p < 0.001), whereas at multivariate analysis only heart Dmax (p = 0.002) remained significant. In Fig. 2 parameters that resulted significant at multivariate analysis are summarized.
Correlation of significant factors with radiotherapy-related toxicities. The figure summarizes significant parameters identified through multivariate analysis in relation to radiotherapy-induced toxicities, with associated p values provided. Panel A: Comparison of maximum dose to the heart and acute lung toxicity (p = 0.043). Panel B: Relationship between heart comorbidities and acute lung toxicity (p = 0.028). Panel C: Gender-based analysis of esophageal toxicity (p = 0.018). Panel D: Mean lung dose and its correlation with acute heart toxicity (p = 0.048). Panel E: Correlation between age and acute asthenia (p = 0.014). Panel F: Relationship between acute and chronic toxicity and maximum dose to the heart (p = 0.002)
Discussion
In resected NSCLC pN2 the role of PORT has been a hot topic since 1998, when the PORT meta-analysis was published [5]. Although this analysis indicated a decrease in OS for patients receiving PORT, it has been criticized for utilizing outdated radiation therapy (RT) equipment and techniques, which can result in suboptimal dose distribution and increased radiation exposure to organs-at-risk, thereby worsening treatment-related mortality and limiting effectiveness [6, 7].
Due to the outdated nature of these data, its relevance to current practice was limited. However, there was still a lack of clear data to investigate the potential of PORT in this category of patients [8, 9]. In a monocentric retrospective analysis by Bruni, involving over 170 patients pN2 NSCLC, it was demonstrated that PORT might improve local control (p < 0.0009), with a 15% relapse rate in the PORT group compared to 32% in the non-PORT group [10], but without an impact in terms of distant metastasis and overall survival. The data on the use of PORT in conjunction with adjuvant chemotherapy are even more difficult to interpret, considering that the largest study was the ANITA secondary analysis [9].
Two recent phase III trials, LungART and PORT-C, found no benefits in terms of OS for PORT in stage III N2 NSCLC patients who had undergone surgery and adjuvant chemotherapy [1, 2]. The decision to use PORT, thus, involves considering several risk factors, such as resection margin, incomplete node dissection, extracapsular extension, multistation nodes, high ratio of positive nodes/excised nodes, impossibility to perform adjuvant chemotherapy or inadequate response to induction therapies, performance status and comorbidities [3, 11]. However, there is no unanimous consensus within the clinical community [3].
Future research should integrate clinical features and molecular biomarkers to identify the subsets of patients who may benefit or not from PORT [12, 13]. Upcoming studies should focus on enhancing the identification of high-risk patients for disease recurrence through the analysis of circulating tumor cells and the detection of postsurgical minimal residual disease.
Considering the above premises, it will be crucial to examine the impact of the radiation techniques to ensure the preservation of the advantages associated with enhanced local control. Indeed, in the last years the introduction of sophisticated techniques such as VMAT, IMRT or image guided radiotherapy in clinical practice has helped to improve the quality of radiation treatments in both the curative and palliative settings. The better knowledge and the longer experience in using these modern radiation therapy tools may increase the safety and the efficacy of the different treatments, particularly ameliorating the safety profile of RT [14]. Jairam conducted an analysis on the role of radiotherapy technique (IMRT vs 3D RT) in the PORT for NSCLC. They concluded that there was no correlation with toxicity in elderly patients, suggesting that the outcomes of PORT may be independent of the RT technique [15]. Nevertheless, our retrospective series contradicts these findings, as the toxicity outcomes were notably lower (toxicities ≥ G3: 2% for lung, 0,4% for heart) when compared to those documented in the LungART trial [1] (toxicities ≥ G3: 14% for lung, 5% for heart). Moreover, our results align closely with those of PORT-C [2] trial (toxicities ≥ G3: 0,7% for lung, 1,2% for heart), indicating a high degree of homogeneity, likely attributable to the use of more advanced RT technologies.
Regarding toxicity, a comprehensive study on different dosimetry parameters and dose constraints is essential to enhance the therapeutic ratio of modern RT. The safety and toxicity, particularly cardiopulmonary, remains a significant concern in relation to PORT, especially considering that some studies have not demonstrated a clear oncologic benefit. The excessive toxicity observed in patients receiving PORT in earlier trials, which led to non-cancer-related deaths, can be attributed to several factors such as suboptimal technique, large or excessive radiation volumes and the lack of CT-based planning. Several studies have investigated the role of modern radiation techniques with the mortality, suggesting that PORT does not impact the risk of death [16,17,18]. In this regard, a SEER analysis of PORT demonstrated that this technique was associated with an increased number of deaths from heart disease in patients between 1983 and 1988, but not in more recent cohorts [19]. The transition from conventional 2D to 3D radiation planning, and the subsequent advancements to 3D-CRT, IMRT, and VMAT, has significantly reduced radiation toxicity [20]. However, a dosimetric comparison of PORT plans using different techniques for pN2 NSCLC patients did not show absolute dosimetric advantages for all patients. Therefore, the choice of radiation therapy technique should be personalized, balancing target coverage with the protection of organs at risk.
Proton therapy offers a promising approach to reducing RT toxicity, with small series showing lower RT doses to heart and lungs [21, 22]. Boyce-Fappiano et al. found in a retrospective analysis proton beam PORT was associated with longer overall survival (OS) [22], due a better sparing of heart and lungs. Similarly, studies by Ma et al. [23] and Shepherd et al. [24] examined the correlation between dosimetry distribution to the heart and lungs and OS in patients treated with PORT. Ma et al. found that a higher dose to the heart and lungs was associated with a lower OS [23]. These findings align with the results from Shepherd et al., who reported a correlation between heart V8 and OS in PORT patients [24].
In this context, the results of our retrospective study provide valuable insights into the demographics and treatment characteristics, toxicity profiles, and outcomes of a cohort of 255 NSCLC patients treated with PORT with advanced RT techniques.
It’s also noteworthy to underline that the Impower010 trial has significantly impacted the landscape of resected stage III NSCLC by demonstrating the efficacy of adjuvant atezolizumab after chemotherapy, as evidenced by both disease free survival (DFS) [25] and OS (trend) [26]. Ongoing phase III clinical trials, such as BR.31 (durvalumab versus placebo) (NCT02273375), PEARLS/KEYNOTE-091 (pembrolizumab versus placebo) [27], ANVIL (nivolumab) [28], and ALCHEMIST [29], are investigating adjuvant immunotherapy in resected stage IB-IIIA NSCLC. Additionally, trials like Checkmate-816 [30], Keynote-671 [31], Checkmate-77t [32], Neotorch [33], and Aegean [34] are exploring neoadjuvant strategies combining chemotherapy and immunotherapy, showing promising results in obtaining complete pathological responses. This evolving landscape is expected to complicate the future management of resectable NSCLC in the near future.
Despite incorporating lower stages of NSCLC in these trials, the recurrence rate remains high, emphasizing the need for further investigation. Notably, in the IMpower010 trial, 29.8% of patients in the experimental arm experienced recurrence at 24 months, with disease relapse strongly correlated with OS in the immunotherapy arm [25]. Even though only 11% of patients in the experimental group and 16% in the observation group received subsequent salvage RT, details about the radiotherapy intent in this subgroup remain unclear [25].
Given this context, it is crucial to scrutinize the recurrence patterns in patients submitted to adjuvant combined systemic therapies and explore the different approaches within stratified risk classes. On the other hand, understanding whether immunotherapy may mitigate distant recurrence may also guide the consideration of local RT in select subsets of patients at high risk of local recurrence.
The demographic and clinical characteristics of the analyzed population reflect the heterogeneous nature of lung cancer patients and underline the importance of considering these factors in the whole treatment planning. It is noteworthy to underline that the decision regarding adjuvant chemotherapy was not uniform, with a notable subset of patients not receiving systemic therapy (24.7%). This variability may reflect individualized treatment decisions influenced by patient-specific factors and the evolving landscape of adjuvant therapies for lung cancer. Regarding RT, the low rate of temporary or definitive interruptions in our population confirmed the effective treatment planning and delivery with advanced techniques, minimizing disruptions that can affect treatment efficacy. Despite that, a significant proportion of patients experienced acute toxicities, with pulmonary and esophageal side effects being the most common, mostly mild to moderate. Notably, a small percentage of patients experienced acute cardiac toxicity, emphasizing the importance of cardiac sparing techniques during the radiotherapy planning. Chronic toxicities, though less frequent, highlight the actual need for long-term monitoring and management of treatment-related side effects.
The correlation analysis conducted in this study revealed several significant associations between clinical variables, dosimetry parameters and toxicity outcomes. Particularly, preexisting cardiac disease, the number of excised nodes and radiation dosimetry seem to play a significant role on influencing toxicity profiles. These findings also highlight the importance of personalizing each treatment plan considering patient-specific factors as well as minimizing the risk of treatment-related toxicities. In this scenario, the RT induced cardiac toxicity and coronary artery disease represent an important issue, as an important cause of mortality and morbidity in survivors p [35].
In conclusion, our study, as well as recent clinical trials, contributes to the evolving discussion on PORT inclusion criteria in resected stage III lung cancer. The interplay between historical data, contemporary trials, and our retrospective analysis offers a more comprehensive understanding of the complexities surrounding PORT in stage III resected NSCLC patients. While the debate continues, the emerging focus on factors such as diffuse use of advanced RT techniques, new dose constraints and novel therapeutic strategies may help to refine the selection of patients who stand to benefit most from PORT. Personalized decisions on treatment planning should always consider the broader context of each patient's unique characteristics with the ultimate goal of optimizing outcomes and enhancing the care of lung cancer patients. Further research and validation are still warranted to advance our knowledge and improve the management of this challenging disease.
References
Le Pechoux C, Pourel N, Barlesi F, Lerouge D, Antoni D, Lamezec B, Nestle U, Boisselier P, Dansin E, Paumier A, Peignaux K, Thillays F, Zalcman G, Madelaine J, Pichon E, Larrouy A, Lavole A, Argo-Leignel D, Derollez M, Faivre-Finn C, Hatton MQ, Riesterer O, Bouvier-Morel E, Dunant A, Edwards JG, Thomas PA, Mercier O, Bardet A (2022) Postoperative radiotherapy versus no postoperative radiotherapy in patients with completely resected non-small-cell lung cancer and proven mediastinal N2 involvement (Lung ART): an open-label, randomised, phase 3 trial. Lancet Oncol 23(1):104–114
Hui Z, Men Y, Hu C, Kang J, Sun X, Bi N, Zhou Z, Liang J, Lv J, Feng Q, Xiao Z, Chen D, Wang Y, Li J, Wang J, Gao S, Wang L, He J (2021) Effect of postoperative radiotherapy for patients With pIIIA-N2 non-small cell lung cancer after complete resection and adjuvant chemotherapy: the phase 3 PORT-C randomized clinical trial. JAMA Oncol 7(8):1178–1185
Süveg K, Le Pechoux C, Faivre-Finn C, Putora PM, De Ruysscher D, Widder J, Van Houtte P, Troost EGC, Slotman BJ, Ramella S, Pöttgen C, Peeters STH, Nestle U, McDonald F, Dziadziuszko R, Belderbos J, Ricardi U, Manapov F, Lievens Y, Geets X, Dieckmann K, Guckenberger M, Andratschke N, Glatzer M (2021) Role of postoperative radiotherapy in the management for resected NSCLC—decision criteria in clinical routine pre- and post-lungART. Clin Lung Cancer 22(6):579–586
Hendriks LEL, De Ruysscher DKM (2022) Postoperative radiotherapy in resected N2 non-small-cell lung cancer: Lung ART. Lancet Oncol 23(1):8–9
PORT Meta-analysis Trialists Group (1998) Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT meta-analysis trialists group. Lancet (Lond, Engl) 352(9124):257–263
Ahmad N, Attia G, El-Ghoneimy E, Radwan A, El-Badawy S (2009) Conventional (2D) versus conformal (3D) techniques in radiotherapy for malignant pediatric tumors: dosimetric perspectives. J Egypt Natl Canc Inst 21(4):309–314
Munro AJ (1998) What now for postoperative radiotherapy for lung cancer? Lancet (Lond, Engl) 352(9124):250–251
Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD (2006) Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clinic Oncol: Offici J Am Soc Clinic Oncol 24(19):2998–3006
Douillard JY, Rosell R, De Lena M, Riggi M, Hurteloup P, Mahe MA (2008) Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys 72(3):695–701
Scotti V, Meattini I, Saieva C, Agresti B, de Luca Cardillo C, Bastiani P, Livi L, Mangoni M, Di Cataldo V, Marrazzo L, Rampini A, Cipressi S, Bruni A, Santini P, Biti G (2010) Post-operative radiotherapy in N2 non-small cell lung cancer: a retrospective analysis of 175 patients. Radiother Oncol: J Eur Soc Ther Radiol Oncol 96(1):84–88
Levy A, Mercier O, Le Péchoux C (2022) Indications and parameters around postoperative radiation therapy for lung cancer. J Clinic Oncol: Off J Am Soc Clinic Oncol 40(6):556–566
Arcangeli S, Ramella S (2022) Postoperative radiotherapy (PORT) in NSCLC: the end of a love? It is never too good to trust what appears. Lung Cancer (Amst, Nether) 166:250–251
Zhu L, Xia B, Ma S (2022) Postoperative radiotherapy for patients with completely resected stage IIIA-N2 non-small cell lung cancer: opt-in or opt-out. Thorac Cancer 13(5):659–663
Giaj-Levra N, Borghetti P, Bruni A, Ciammella P, Cuccia F, Fozza A, Franceschini D, Scotti V, Vagge S, Alongi F (2020) Current radiotherapy techniques in NSCLC: challenges and potential solutions. Expert Rev Anticancer Ther 20(5):387–402
Jairam V, Pasha S, Soulos PR, Gross CP, Yu JB, Park HS, Decker RH (2021) Post-operative radiation therapy for non-small cell lung cancer: a comparison of radiation therapy techniques. Lung Cancer (Amst, Neth) 161:171–179
Machtay M, Lee JH, Shrager JB, Kaiser LR, Glatstein E (2001) Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J Clinic Oncol: Offici J Am Soc Clinic Oncol 19(19):3912–3917
Billiet C, Peeters S, Decaluwé H, Vansteenkiste J, Mebis J, Ruysscher D (2016) Postoperative radiotherapy for lung cancer: is it worth the controversy? Cancer Treat Rev 51:10–18
Wakelee HA, Stephenson P, Keller SM, Wagner H, Herskovic A, Komaki R, Marks RS, Perry MC, Livingston RB, Johnson DH (2005) Post-operative radiotherapy (PORT) or chemoradiotherapy (CPORT) following resection of stages II and IIIA non-small cell lung cancer (NSCLC) does not increase the expected risk of death from intercurrent disease (DID) in Eastern Cooperative Oncology Group (ECOG) trial E3590. Lung Cancer (Amst, Nether) 48(3):389–397
Lally BE, Detterbeck FC, Geiger AM, Thomas CR, Machtay M, Miller AA, Wilson LD, Oaks TE, Petty WJ, Robbins ME, Blackstock AW (2007) The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 110(4):911–917
Billiet C, Decaluwé H, Peeters S, Vansteenkiste J, Dooms C, Haustermans K, De Leyn P, De Ruysscher D (2014) Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol: J Eur Soc Ther Radiol Oncol 110(1):3–8
Remick JS, Schonewolf C, Gabriel P, Doucette A, Levin WP, Kucharczuk JC, Singhal S, Pechet TTV, Rengan R, SimoneBerman CBAT (2017) First clinical report of proton beam therapy for postoperative radiotherapy for non-small-cell lung cancer. Clin Lung Cancer 18(4):364–371
Boyce-Fappiano D, Nguyen QN, Chapman BV, Allen PK, Gjyshi O, Pezzi TA, De B, Gomez D, Lin SH, Chang JY, Liao Z, Lee P, Gandhi SJ (2021) Single institution experience of proton and photon-based postoperative radiation therapy for non-small-cell lung cancer. Clin Lung Cancer 22(5):e745–e755
Ma Z, Liu Y, Bao Y, Yuan M, Yang X, Men Y, Wang J, Deng L, Zhai Y, Bi N, Wang L, Hui Z (2023) Higher lung and heart doses decrease early and long-term survival, respectively, in patients with non-small cell lung cancer undergoing postoperative radiation. Adv Radiat Oncol 8(4):101213
Shepherd AF, Yu AF, Iocolano M, Leeman JE, Wild AT, Imber BS, Chaft JE, Offin M, Huang J, Isbell JM, Wu AJ, Gelblum DY, Shaverdian N, Simone CB, Gomez D, Yorke E, Jackson A, Rimner A (2021) Increasing heart dose reduces overall survival in patients undergoing postoperative radiation therapy for NSCLC. JTO clinic Res Rep 2(8):100209
Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H, Chen YM, Chella A, Sugawara S, Voong D, Wu F, Yi J, Deng Y, McCleland M, Bennett E, Gitlitz B, Wakelee H (2021) Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet (Lond, Engl) 398(10308):1344–1357
Felip E, Altorki N, Zhou C, Vallières E, Martínez-Martí A, Rittmeyer A, Chella A, Reck M, Goloborodko O, Huang M, Belleli R, McNally V, Srivastava MK, Bennett E, Gitlitz BJ, Wakelee HA (2023) Overall survival with adjuvant atezolizumab after chemotherapy in resected stage II-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase III trial. Ann Oncol: Offici J Eur Soc Med Oncol 34(10):907–919
O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, Esteban E, Isla D, Martinez-Marti A, Faehling M, Tsuboi M, Lee JS, Nakagawa K, Yang J, Samkari A, Keller SM, Mauer M, Jha N, Stahel R, Besse B, Peters S (2022) Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 23(10):1274–1286
Chaft JE, Dahlberg SE, Khullar OV, Edelman MJ, Simone CB, Heymach J, Rudin CM, Ramalingam SS (2018) EA5142 adjuvant nivolumab in resected lung cancers (ANVIL). J Clinic Oncol 36(15_suppl):8581–8581
Sands JM, Mandrekar SJ, Kozono D, Oxnard GR, Hillman SL, Wigle DA, Govindan R, Carlisle J, Gray J, Salama JK, Raez L, Ganti A, Foster N, Malik S, Bradley J, Kelly K, Ramalingam SS, Stinchcombe TE (2021) Integration of immunotherapy into adjuvant therapy for resected non-small-cell lung cancer: ALCHEMIST chemo-IO (ACCIO). Immunotherapy 13(9):727–734
Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson SJ, Kerr K, Wang C, Ciuleanu TE, Saylors GB, Tanaka F, Ito H, Chen KN, Liberman M, Vokes EE, Taube JM, Dorange C, Cai J, Fiore J, Jarkowski A, Balli D, Sausen M, Pandya D, Calvet CY, Girard N (2022) Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 386(21):1973–1985
Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Keller SM, Samkari A, Spicer JD (2023) Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med 389(6):491–503
Cascone T, Awad MM, Spicer JD, He J, Lu S, Sepesi B, Tanaka F, Taube JM, Cornelissen R, Havel L, Kuzdzal J, Petruzelka LB, Wu L, Pujol JL, Ito H, Coronado Erdmann C, Sathyanarayana P, Meadows-Shropshire S, Provencio Pulla M (2023) LBA1 CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. Ann Oncol 34:S1295
Lu S, Zhang W, Wu L, Wang W, Zhang P, Fang W, Xing W, Chen Q, Yang L, Mei J, Tan L, Sun X, Xu S, Hu X, Yu G, Yu D, Yang N, Chen Y, Shan J, Xing L, Tian H, Zhang X, Zhou M, Fang H, Wu G, Liu Y, Ye M, Cao L, Jiang J, Li X, Zhu L, Li D, Kang M, Zhong A, Chen K, Wu N, Sun Q, Ma H, Cai K, Wang C, Lin G, Zhu K, Zhang Y, Zhang X, Hu H, Zhang W, Chen J, Yang Z, Hang X, Hu J, Huang Y, Zhang Z, Zhang L, Zhang L, Liu L, Lin D, Zhang J, Chen G, Li Y, Zhu L, Wang W, Yu W, Cao D, Keegan P, Yao S (2024) Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA 331(3):201–211
Heymach JV, Mitsudomi T, Harpole D, Aperghis M, Jones S, Mann H, Fouad TM, Reck M (2022) Design and rationale for a phase III, double-blind, placebo-controlled study of neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab for the treatment of patients with resectable stages II and III non-small-cell lung cancer: the AEGEAN trial. Clin Lung Cancer 23(3):e247–e251
Banfill K, Giuliani M, Aznar M, Franks K, McWilliam A, Schmitt M, Sun F, Vozenin MC, Faivre Finn C (2021) Cardiac toxicity of thoracic radiotherapy: existing evidence and future directions. J Thorac Oncol: Offici Public Int Assoc Study Lung Cancer 16(2):216–227
Acknowledgements
The Authors thank the Scientific Committee and Board of the AIRO for the critical revision and final approval of the paper. The Authors also thank all the members of the Lung Cancer Study Group for their support in this project.
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by VN, AB, DF, BM, SV, PC, MS, AC, ED, GDM, AA, MM, MS, CG, DA, PB, and SC. The first draft of the manuscript was written by VN, AB, SV, PC, and MS and revised by MS, MS, CG, DA, PB, and SC. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no other relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Hospital Vanvitelli, Naples, under Protocol Number 0026796/I dated September 21, 2021 and then from all the other participating centers.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Explanation for number of Authors: This manuscript is the analysis of a large, multicenter study involving six different centers. The extensive nature of this research required a collaborative effort where each author made significant contributions to the study’s design, data collection, and analysis. The complexity and scope of this project necessitated the expertise and hard work of all 17 authors, ensuring the rigorous and comprehensive analysis presented in this manuscript. Each author’s unique input was essential for the successful completion of this study, making their inclusion as co-authors indispensable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nardone, V., Bruni, A., Franceschini, D. et al. Adjuvant modern radiotherapy in resected pN2 NSCLC patients: results from a multicentre retrospective analysis on acute and late toxicity on behalf of AIRO thoracic oncology study group: the RAC-TAC study. Radiol med (2024). https://doi.org/10.1007/s11547-024-01885-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11547-024-01885-w