Abstract
Purpose
The objective of this study was to investigate the role of myocardial perfusion imaging (MPI) stress tests using stress cardiac magnetic resonance (sCMR) and single-photon emission computed tomography myocardial perfusion imaging (SPECT-MPI) in non-cardiac surgery (NCS) pre-operatory management.
Materials and methods
This monocentric retrospective study enrolled patients with coronary artery disease or a minimum of two cardiovascular risk factors undergoing intermediate-to-high-risk non-cardiac surgeries. The primary composite endpoint comprised cardiac death, cardiogenic shock, acute coronary syndromes (ACS), and cardiogenic pulmonary edema occurring within 30 days after surgery, while the secondary endpoint was ACS.
Results
A total of 1590 patients were enrolled; among them, 669 underwent a MPI stress test strategy (sCMR: 287, SPECT-MPI: 382). The incidence of 30-day cardiac events was lower in the stress-tested group compared to the non-stress-tested group (1.2% vs. 3.4%; p 0.006). Adopting a stress test strategy showed a significant reduction in the risk of the composite endpoint (OR: 0.33, 95% CI: 0.15–0.76, p 0.009) and ACS (OR: 0.41, 95% CI: 0.17–0.98, p 0.046) at multivariable analysis, with similar cardiac events rate between stress CMR and SPECT (1.1% vs. 1.3%, p 0.756). Stress CMR showed a greater accuracy to predict coronary artery revascularizations (sCMR c-statistic: 0.95, ischemic cut-point: 5.5%; SPECT c-statistic: 0.85, ischemic cut-point: 7.5%).
Conclusion
Stress test strategy is related to a lower occurrence of cardiac events in high-risk patients scheduled for intermediate-to-high-risk non-cardiac surgeries. Both sCMR and SPECT-MPI comparably reduce the likelihood of cardiac complications, albeit sCMR offers greater accuracy in predicting coronary artery revascularization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perioperative cardiac complications among patients undergoing non-cardiac surgery (NCS) range from less than 1% to 5%, depending on the type of intervention, patient risk profile, and preoperative cardiac risk assessment strategy. In patients with known coronary artery disease (CAD) or at least two cardiovascular risk factors and poor functional capacity, an imaging stress test allows to detect inducible myocardial ischemia and should call for specific risk reduction strategies before intermediate-to-high-risk NCS [1]. However, data supporting cardiac imaging modalities in patients undergoing NCS are sparse and out of date [2,3,4,5]. In particular, there is little specific evidence on the use of stress cardiac magnetic resonance (sCMR) in this setting, even if this myocardial perfusion imaging (MPI) modality is widely used nowadays for myocardial ischemia detection in patients with known or suspected CAD[6, 7]. Moreover, modality-specific thresholds of inducible ischemia associated with an increased perioperative risk are poorly defined. As a result, there is no consensus on the best strategy for risk stratification in this setting. The aim of the present study is to assess the prognostic role of a myocardial perfusion imaging (MPI) stress test strategy based on sCMR or stress single-photon emission computed tomography myocardial perfusion imaging (SPECT-MPI) in terms of 30-day post-surgery cardiac events.
Methods
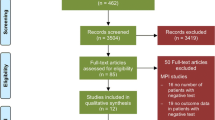
This is a single-center, retrospective cohort study conducted at a tertiary academic hospital. The study protocol was approved by the institutional ethics committee, and given the retrospective design of the study, the ethics committee waved the need of specific informed consent. Consecutive patients undergoing NCS between January 2015 and December 2021 were scrutinized and included according to prespecified inclusion and exclusion criteria (Fig. 1). Patients candidate to urgent, cardiac, and low-risk surgery were excluded, as well as those affected by severe valvular disease. Referral to CCTA after the preoperative cardiologic evaluation or direct referral to ICA was considered exclusion criteria.
Electronic medical records were retrospectively reviewed to collect clinical and outcomes data. Data of interest included symptoms at the time of first evaluation, laboratory test values, reports of ECG, echocardiogram, sCMR and SPECT-MPI, ICA, and cardiovascular outcomes at 30-day post-surgery follow-up. Moderate–severe chronic kidney disease was defined as a glomerular filtration rate inferior to 45 ml/min/1.73 m2 according to CKD-EPI formula. Functional capacity was expressed in metabolic equivalents (METS) and reported during cardiological evaluation by asking the patient about daily activities (MET-REPAIR or DASI questionnaires were alternatively used [8]). Different types of surgeries were classified according to ESC guidelines in intermediate and high risk; revised cardiac risk index (RCRI) was computed through analysis of electronic medical records and the scores were assessed as suggested by guidelines[1]. The American Society of Anesthesiologists (ASA) class was evaluated for each patient [9]. The referral for stress testing was guided by real life clinical practice, as the result of multidisciplinary integration of patient clinical presentation, risk stratification, and functional capacity (revised cardiac risk index score, METS). To address potential referral/selection bias, we performed a propensity score matching analysis.
Myocardial perfusion imaging stress test
Stress CMR was performed using adenosine (infusion rate 148 mcg/kg/min) or regadenoson (400 mcg i.v. bolus) with a rest stress protocol [10]. A Philips Achieva 1,5 T MRI system was used from 2015 to 2018; after this period, it was replaced by a Siemens Magnetom Aera 1,5 T MRI. Interpretation of CMR images and stress perfusion defects was made according to the recommendations of Society for Cardiovascular Magnetic Resonance [11]. Inducible perfusion defects were defined as an area of hypointensity signal that involves at least one myocardial segment with coronary distribution and persists for at least 3 phases after peak of contrast enhancement without evidence of LGE in the same position. A model of 17 segments was used to assess the number of ischemic segments and the percentage of ischemia (6% for each segment: 3% if less than 50% wall thickness and 6% in more than 50% wall thickness). CMR analysis was performed using Circle Cardiovascular Imaging software (cvi42, Calgary, Canada).
SPECT-MPI was conducted following a stress rest protocol; the choice of the type of stressor (physical, adenosine, regadenoson) was based on patients’ clinical characteristics. A Symbia Intevo™ Excel SPECT/CT Siemens System was used. All tests were performed using Technetium-99 m sestamibi. Acquisition protocol and images analysis followed the guidelines of the American Society of Nuclear Cardiology [12]. SPECT images were analyzed using standard 17-segment model. A grading scale from 0 to 4 was used to assess the severity of reduction in tracer uptake. Philips IntelliSpace Portal software was used for SPECT images postprocessing.
Definition of clinical outcome and follow-up.
The prespecified primary outcome was a composite endpoint of cardiovascular complications during the 30-day period after surgery, including cardiac death, cardiogenic shock, acute coronary syndromes (ACS), and cardiogenic pulmonary edema. Secondary endpoint was ACS (defined as myocardial infarction and unstable angina).
Statistical analysis
Descriptive statistics were obtained for all study variables. Continuous variables were presented as mean ± standard deviation (SD) or as median value and interquartile range (IQR), while categorical variables were presented as absolute number and percentage. Normality of data distribution was verified using the Shapiro–Wilk test. Comparisons between continuous variables were tested using the unpaired t-test or the Mann–Whitney test, as indicated. Relationships between categorical variables were tested using the Chi-square test or the Fisher’s exact test, as appropriate. Multivariable logistic regression analyses were performed to identify independent predictors of outcomes (primary composite endpoint and acute coronary syndromes) in the overall population and independent predictors of myocardial ischemia in the stress-tested group. Only variables that were significantly associated with the endpoint at univariable analysis (p < 0.05) were included in multiple multivariable logistic regression analyses. Results were expressed as odds ratio (OR) and confidence intervals (CI). Receiver operating characteristic (ROC) area under the curve (AUC), 95%CI, and the optimal cutoff points were calculated with Youden method to assess the predictive discriminatory power for coronary artery revascularization of percentage myocardial ischemia for both sCMR and SPECT-MPI. To examine the effect of imaging stress test in different subgroups, a test for interaction was performed; p for interaction was considered significant if < 0.05. Propensity score was calculated from a logistic regression model that included all baseline characteristics. Matching was done using a 1:1 nearest neighbor matching algorithm without replacement, with a caliper width of 0.2 of the standard deviation of the logit of the propensity score. To improve the discriminative capability of our models, we calculated the C-index for each model. The C-index, being numerically identical to the AUC of the ROC curve, provides an estimate of the model's discriminative power concerning the outcomes. We used the DeLong test to compare the AUCs of the ROC curves between different models. Statistical analysis was performed with Stata version 17.0 (StataCorp).
Results
Characteristics of the patient population
After the screening of 42743 surgical interventions, 1590 patients (42%) were finally included. Baseline clinical characteristics and outcomes of the study population, stratified according to the use of stress test modality, are shown in Table 1. Patients who underwent SPECT-MPI stress strategy showed higher ASA class and more often reported the assumption of cardioprotective therapies (i.e., anti-platelets and lipid-lowering drugs).
The median age of the patients was 71 years (65–77), and 38.8% (617) were female. Regarding cardiovascular risk factors, 81.1% (1290) of the patients had hypertension, 69.2% (1100) dyslipidemia, and 32.7% (520) diabetes mellitus. Moreover, 43.7% (695) of patients had a history of CAD; moderate-to-severe CKD had a prevalence of 16.3% (260).
Concerning symptoms, 42.8% (680) of patients suffered from dyspnea, 4.2% (67) from typical angina, and 26.8% (426) from atypical chest pain.
Electrocardiogram and echocardiogram were available in all patients: 94% (1495) were in sinus rhythm and 6% (95) in atrial fibrillation. Median left ventricular ejection fraction (LVEF) was 55% (51–55). There was a slightly greater incidence of grade II diastolic dysfunction in the stress-tested group (25.9% vs. 21.3%, p 0.035), whereas grade III dysfunction was more prevalent in the non-stress group (2.9% vs. 1.5%, p 0.013).
Type of surgery and surgical risk scores ( Fig. 2)
Type of surgeries, preoperative risk evaluation, and 30-day cardiac events. The most prevalent type of non-cardiac surgery was vascular (19.9%), followed by orthopedic (18.6%). About the half of surgeries were at high risk. Revised cardiac index was high (≥ 3) in 37.3% of patients, and ASA class III was present in 48.5% of cases. The study population was divided in two groups (no stress test vs. imaging stress test). Primary composite endpoint and secondary endpoint (ACS) were significantly lower in the imaging stress-tested group. ACS acute coronary syndrome, ASA American Society of Anesthesiologists, CMR cardiac magnetic resonance, GYNECOL gynecological surgery, ORT orthopedic surgery, SPECT-MPI single-photon emission computed tomography myocardial perfusion imaging, VASC vascular surgery
The most prevalent type of NCS was vascular (n = 316, 19.9%), followed by orthopedic (n = 296, 18.6%) and urological (n = 278, 17.5%). Almost half of surgeries were at high risk (50,1%). RCRI was calculated for each patient: 37.4% (595) had an index of 1, 39.6% (630) of 2, and 23% (366) had an index of more than 3. With regard to ASA class, 12.5% (199) of patients were in class I; 35.7% (567) in class II; 48.5% (772) in class III, and 3.2% (51) in ASA class IV.
Non-invasive imaging cardiac test
A total of 669 patients underwent a stress test before NCS. Results and outcomes of sCMR and SPECT-MPI are shown in Table 2; baseline clinical characteristics are available in supplemental Table 1. A sCMR was performed in 287 patients. Mean LVEF was 58.7% (± 10), and late gadolinium enhancement was present in 39.7% of cases. Inducible ischemia was detected in 52/287 patients (18.1%). The median value of ischemic segments was 3 (2–4), with a total percentage of stress myocardial ischemia of 8.6% (± 3.4).
A SPECT-MPI was performed in 382 patients and resulted positive for inducible ischemia in 49/382 cases (12.8%). The median value of reversible stress ischemic segments was 4 (2–4) with a total percentage of stress myocardial ischemia of 8.2% (± 4.4). Rest ischemia was detected in 31.2% of patients. Physical stress was performed in 57% of cases, while pharmacological in 43% of cases.
Indication to ICA after stress imaging was upon clinical judgment of the referring cardiologist.
Invasive coronary angiography
In the stress-tested group, a total of 105 patients (15.7%) underwent ICA before NCS and 82 (78% of the patients who underwent ICA) were revascularized. The rate of ICAs was not significantly different between sCMR and SPECT-MPI groups (18.8% vs. 13.3%, respectively, 0.054), despite a tendency toward higher rate of ICA in sCMR group with a similar rate of revascularizations (84.5% vs. 77.6%, respectively, p 0.448).
Myocardial ischemia predictors in patients undergoing non-cardiac surgery
Univariate and multivariate logistic regression analysis was performed to assess the association between clinical and echocardiographic parameters and the presence of myocardial ischemia in patients undergoing MPI stress test (Table 3 and supplemental Table 2). Independent predictors of myocardial ischemia were hypertension (OR 5.72, 95%CI 1.70–19.15, p 0.005), male sex (OR 1.74, 95% CI 1.09–2.79, p 0.020), moderate–severe CKD (OR 1.74, 95%CI 1.06–2.84, p 0.026), known CAD (OR 4.01, 95%CI 2.30–6.99, p < 0.001) and high-grade diastolic dysfunction (OR 1.85, 95%CI 1.30–2.64, p 0.001).
Myocardial ischemia and revascularization
AUC and the optimal cut-points were calculated both for sCMR and SPECT-MPI to identify the best ischemic thresholds for revascularization (Fig. 3). Stress CMR showed an AUC of 0.95 (SE: 0.98, SP: 0.92) when a threshold for myocardial ischemia of 5.5% was chosen. On the other side, the best cut-point for SPECT-MPI was a percentage of ischemia of 7.5% (AUC: 0.83, SE: 0.78, SP: 0.87).
ROC, AUC, and the optimal cut-points of sCMR and SPECT-MPI to predict coronary artery revascularization. AUC area under the curve, ROC receiver operating characteristics, sCMR stress cardiac magnetic resonance, SE sensitivity, SP specificity, SPECT-MPI single-photon emission computed tomography myocardial perfusion imaging
Cardiac events in non-cardiac surgery
The composite primary outcome occurred in 39 cases (2.4%) with a significantly greater incidence in the non-stress-tested group compared to the stress-tested group (respectively, 3.4% vs. 1.,2%, p 0.006; Table 1 and Fig. 2). Myocardial infarction was the most frequent cardiac complication (n = 20, 1.3% of cases), followed by unstable angina (n = 11, 0.6%), cardiac death (n = 3, 0.3%), cardiogenic pulmonary edema (n = 3, 0.3%), and cardiogenic shock (n = 2; 0.2%). ACS occurred more frequently in the non-stress-tested group (2.6% vs. 1.0%; p 0.027; Table 1 and Fig. 2).
Both primary outcome and ACS did not differ between sCMR and SPECT (primary outcome: 1.1% vs. 1.3%, p 0.756; ACS 0.7% vs. 1.3% p 0.441; Table 2).
After adjusting with multivariable logistic regression analysis, CAD (OR 2.33, 95%CI 1.11–4.88, p 0.025) was associated with an increased risk of the primary study endpoint, while higher METs grade (OR 0.26, 95%CI 0.12–0.55, p 0.001) and a stress test strategy (OR 0.33, 95%CI 0.15–0.76, p 0.009) were protective (Table 3 and supplemental Table 3). The independent protective effect of the MPI stress test strategy was consistent among all sub-categories, as shown by test for interaction (Fig. 4). Similarly, we performed a multivariable logistic regression analysis for the secondary endpoint ACS (Table 3). The protective effect of stress test strategy was confirmed also for ACS alone (OR 0.41, 95%CI 0.17–0.98, p 0.046).
Test for interaction of imaging stress test strategy among different subgroups. The independent protective effect of the MPI stress test strategy was consistent among all sub-categories. CAD Coronary artery disease, CKD chronic kidney disease, CI confidence intervals, LVEF left ventricular ejection fraction, METS metabolic equivalents, NYHA New York Heart Association, OR odds ratio
Propensity score analysis
Propensity score matching was utilized to create a balanced comparison, resulting in a cohort of 669 matched pairs. After matching, the baseline characteristics between the stress-tested and non-tested groups showed no significant differences, with the largest standardized mean difference being 0.08 for the presence of diabetes. The lower rate of postoperative cardiovascular events in the matched stress-tested group suggests a potential benefit of preoperative stress testing in reducing these events. The C-index values for different models were as follows: non-MPI (0.65), sCMR with revascularization (0.85), sCMR without revascularization (0.80), SPECT with revascularization (0.82), SPECT without revascularization (0.75). ROC analysis demonstrated AUC values ranging from 0.66 for non-MPI to 0.86 for sCMR with revascularization. The DeLong test indicated that sCMR with revascularization had significantly higher AUC compared to SPECT with revascularization (p = 0.03). Post-matching analysis indicated that the rate of 30-day postoperative cardiovascular events in the stress-tested group was significantly lower than the non-tested group (OR 0.34; 95% CI, 0.15–0.78; p = 0.009) and similarly resulted for the incidence of myocardial infarction (p = 0.046). When examining the outcomes of patients with positive stress test results, 18.1% in the sCMR group and 12.8% in the SPECT-MPI group had inducible ischemia, leading to ICA in 78% and a subsequent revascularization rate of 84.5% for the sCMR group and 77.6% for the SPECT-MPI group. The matched cohort analysis mitigates the effects of referral bias and provided a more accurate estimate of the impact of preoperative stress testing, overcoming the selection bias intrinsic to the retrospective design of the study. The lower rate of postoperative cardiovascular events in the matched stress-tested group also confirms a potential benefit of preoperative stress testing in reducing these events.
Discussion
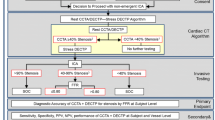
The aim of the present study was to assess the perioperative risk stratification value of a stress imaging test strategy with sCMR or SPECT-MPI in patients with ≥ 2 risk factors or known CAD undergoing intermediate-to-high-risk NCS. The main findings may be summarized as follows (Figs. 5 and 6):
-
A MPI stress test strategy, irrespective of the imaging modality used, was associated with a significantly reduced risk of perioperative cardiac complications;
-
Known CAD is an independent predictor of perioperative cardiac complications, while predicted METs and a MPI stress test strategy are independent protective factors;
-
The incidence of perioperative cardiac complications was comparable between patients undergoing sCMR and SPECT-MPI;
-
In the setting of perioperative risk stratification before NCS, the discriminative power of MPI ischemic threshold for coronary revascularization is excellent for sCMR (cut-point 5.5%) and good for SPECT-MPI (cut-point 7.5%).
Proposed algorithm to select patients who may benefit from a pre-operative stress test strategy. Both outcomes predictors and ischemic predictors must be considered during the preoperative evaluation before NCS. The suggested ischemic thresholds are 5.5% for sCMR and 7.5% for SPECT. The imaging stress test strategy is associated with a reduction in both MACE and ACS in the 30-day postoperative period. ACS acute coronary syndrome, CAD coronary artery disease, CI confidence intervals, CKD chronic kidney disease, DD diastolic dysfunction grade II and III, HTN hypertension, MACE major adverse cardiac events, METS metabolic equivalents, NCS non-cardiac surgery, OR odds ratio, sCMR stress cardiac magnetic resonance, SPECT single-photon emission computed tomography
Summary of study results. ACS: acute coronary syndrome. AUC: area under the curve. CAD: coronary artery disease. CI: confidence intervals. CMR: stress cardiac magnetic resonance. CKD: chronic kidney disease. DD: diastolic dysfunction grade II and III. HTN: hypertension. METS: metabolic equivalents. MPI: myocardial perfusion imaging. NCS: non-cardiac surgery. OR: odds ratio. SPECT: single-photon emission computed tomography
To further enhance the discriminative capability of different models with respect to the outcomes, we performed C-index analysis and compared the AUC values of different models using the DeLong test to evaluate whether the differences in discriminative ability were systematic or random. Results of ROC curves and AUC values demonstrated statistical differences in the discriminative capabilities of the models. The DeLong test confirmed that the variations in AUC values among the models were significant, indicating systematic differences in their discriminative abilities. This additional analysis underscores the discriminative power of sCMR and SPECT-MPI stress tests in predicting perioperative cardiac complications, thereby supporting their potential use in clinical practice for patients undergoing intermediate-to-high-risk NCS. Recently published guidelines [1] suggest the use of stress imaging test before high-risk elective NCS in patients with poor functional capacity and high likelihood of CAD or high cardiovascular risk, even if asymptomatic, to intercept patients with unfavorable trajectory after NCS. However, patients’ selection for stress imaging before NCS is often challenging in clinical practice, as the functional capacity and symptomatic state of surgical patients are difficult to evaluate due to the underlying diseases (i.e., cancer, orthopedic injuries, limited mobility). Moreover, current guidelines do not provide indications for specific imaging modalities, and existing evidence primarily focuses on stress echocardiography or SPECT-MPI [13,14,15,16,17,18]. Exercise stress test without imaging did not show promising results before non-cardiac surgery and guidelines do not recommend its use if an imaging test is available [1]. Resting transthoracic echocardiography is currently recommended to identify patients with severe valvular disease or cardiomyopathy, but its role for risk prediction before NCS is limited [19, 20]. Left ventricular ejection fraction is considered a borderline predictor of cardiac events, whereas diastolic dysfunction was identified as a significant predictor of events in several studies and one large meta-analysis [21]. In our study, all patients underwent an echocardiographic evaluation before surgery and, while none of the echocardiographic parameters were associated with the clinical endpoints, diastolic dysfunction (grade II and III) resulted as an independent predictor of myocardial ischemia, together with hypertension, known CAD, male sex and chronic kidney disease. Thus, our findings suggest considering stress imaging in patients with high-grade diastolic dysfunction to optimize risk stratification before surgery.
Most of published studies are focused on stress echocardiography and SPECT-MPI [13,14,15,16,17,18, 22]; there is general agreement in considering the absence of inducible ischemia as a marker of favorable clinical outcomes after NCS [23, 24]. However, a recent meta-analysis has shown that currently available evidence is not sufficient to demonstrate a benefit of a stress test strategy before NCS. Notably, only 5 of the 76 included studies in this meta-analysis compared imaging stress test vs. non-stress-tested groups, and none used sCMR [2]. In our study, adopting a stress imaging strategy with sCMR or MPI-SPECT, which targeted coronary revascularization, was independently associated with a significant risk reduction in cardiac events (OR 0.33; 95%CI 0.15–0.76). Of note, our study represents the first evidence for the use of sCMR in this scenario and demonstrates that sCMR has a greater accuracy than SPECT in terms of discriminative power of ischemic threshold for revascularization, together with known advantages of lower acquisition times and no radiation exposure related to the modality.
According to a meta-analysis including 79 studies and 1179 stable patients undergoing SPECT-MPI, less than 20% inducible myocardial ischemia does not significantly portend an increased risk of cardiac complications[25]. However, the impact of revascularization in this setting was not investigated and an ischemia threshold to treat patients undergoing NCS can only be hypothesized. In the general population, more than 10% of myocardial ischemia on nuclear imaging and at least 2 ischemic segments on sCMR are considered the reference thresholds for revascularization [26]. In our real-life retrospective study, referring cardiologists did not use a fixed cutoff value to refer patients to ICA, so some patients were scheduled for ICA with evidence of mild or moderate ischemia. The mean ischemic myocardial percentages were 8.6% and 8.2% for sCMR and SPECT, respectively, while the best accuracy thresholds were 5.5% for sCMR (AUC 0.95) and 7.5% for SPECT (AUC 0.83). Even if these cutoff values need a prognostic validation, they could be used to select patients who may benefit most from a pre-operative ICA, preventing useless delay of surgical intervention in case of false positive results. Our results may suggest a revision of current guidelines regarding revascularization treatment prior to NCS. A personalized approach can be favorable for these patients’ outcome, as the stress of surgery can exacerbate underlying cardiovascular conditions, requiring tailored ischemia thresholds and stress management. Currently, there is paucity of information regarding the role of ICA before NCS and the same recommendation of the non-surgical setting are used [1]. While there are many observational or non-randomized studies that suggest to treat moderate and severe ischemia to improve symptoms and quality of life in patients not presenting with ACS, recent results of large randomized trials (COURAGE and ISCHEMIA) showed no benefit of an early revascularization strategy in stable patients based on ischemia assessment in terms of myocardial infarction, cardiac death, and hospitalizations [27,28,29]. Moreover, in the setting of preoperative cardiological assessment before elective vascular surgery, CARP trial demonstrated no benefit of coronary revascularization in short- and long-term myocardial infarction mortality [30].
However, the pre-operative evaluation before NCS is a different scenario due to the planned surgical procedure itself, which exposes patients’ cardiovascular system to oxygen supply–demand imbalance, hemodynamic changes, as well as prothrombotic and pro-inflammatory states [23]. As reported in one large registry, major NCS is associated with a perioperative major adverse cardiovascular events incidence of about 3% [31]. A large prospective multicenter study showed an even higher incidence (13.1%) of myocardial infarction/injury with an extended 1-year follow-up [32]. In our study, including only intermediate-to-high-risk surgery, the overall incidence of 30-day cardiac events was 2.4%, and it dropped down to 1.2% in the MPI stress test strategy group. In conclusion, although the clinical impact of inducible ischemia in patients with known or suspected stable CAD has been largely questioned by recent trials, our results support the role of a MPI stress test, particularly sCMR, in the specific setting of pre-operative risk stratification for intermediate-to-high-risk NCS. Accordingly, we propose an algorithm to select patients who may benefit from a pre-operative stress test strategy (Fig. 5): along with outcomes predictors (CAD, low METS), ischemia predictors must be considered to further improve risk stratification before NCS (male sex, hypertension, CKD and diastolic dysfunction grade II and III). Stress CMR should be preferred, when available, over SPECT-MPI and an ischemia threshold of 5.5% is recommended as gatekeeper to ICA.
Study limitations
Our study should be interpreted considering some limitations. First, its single-center and observational retrospective nature determines intrinsic limitations in terms of generalizability and potential selection bias. However, we analyzed a wide population of moderate-to-high-risk patients candidates to different types of intermediate-to-high-risk surgery in a high-volume tertiary center. Moreover, the single-center fashion increased the reproducibility in terms of indications and results of stress imaging among different patients and propensity score analysis was performed to address potential referral/selection bias. Secondly, serum cardiac biomarkers were not systematically assessed and are not reported. The recommendation of a systematic assessment of troponin and BNP in patients undergoing NCS has been recently provided by ESC Guidelines, while our study focuses on a previous period. Third, the relatively small number of events in the MPI stress-tested group prevented us from further exploring any difference between sCMR and SPECT-MPI in terms of clinical events and might have led to possible type II error for the primary outcome. Notwithstanding, our study represents one of the largest single-center analyses exploring the role of an MPI stress test strategy in patients undergoing NCS and is the first study reporting data on the sCMR use in this setting. A final limitation regards the methodological discrepancies in terms of stressors (regadenoson vs. adenosine vs. physical effort). Nevertheless, our approach aligns with clinical guidelines and reflects a real-world clinical approach.
Conclusions
A stress imaging strategy is associated with a reduced incidence of cardiac events in high-risk patients candidate to intermediate-to-high-risk non-cardiac surgery. The probability of cardiac complications is similarly reduced by sCMR and SPECT-MPI, although sCMR is more accurate in predicting coronary artery revascularizations. Future randomized clinical trials are required to confirm and validate our findings.
Abbreviations
- NCS:
-
Non-cardiac surgery
- ACEi/ARBs:
-
Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers
- ACS:
-
Acute coronary syndromes
- ALP:
-
Alkaline phosphatase
- ASA:
-
American Society of Anesthesiologists
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the curve
- BNP:
-
B-type natriuretic peptide
- CABG:
-
Coronary artery bypass grafting
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary computed tomography angiography
- CKD:
-
Chronic kidney disease
- ECG:
-
Electrocardiogram
- ESC:
-
European Society of Cardiology
- ICA:
-
Invasive coronary angiography
- IQR:
-
Interquartile range
- LGE:
-
Late gadolinium enhancement
- LVEDV:
-
Left ventricular end-diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- METS:
-
Metabolic equivalents
- MPI:
-
Myocardial perfusion imaging
- NYHA:
-
New York Heart Association
- PCI:
-
Percutaneous coronary intervention
- RCRI:
-
Revised cardiac risk index
- ROC:
-
Receiver operating characteristic
- sCMR:
-
Stress cardiac magnetic resonance
- SD:
-
Standard deviation
- SPAP:
-
Systolic pulmonary arterial pressure
- SPECT-MPI:
-
Single-photon emission computed tomography myocardial perfusion imaging
- TAPSE:
-
Tricuspid annular plane systolic excursion
References
Halvorsen S, Mehilli J, Cassese S et al (2022) 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 43:3826–3924
Kalesan B, Nicewarner H, Intwala S et al (2019) Pre-operative stress testing in the evaluation of patients undergoing non-cardiac surgery: a systematic review and meta-analysis. PLoS ONE 14:1–22. https://doi.org/10.1371/journal.pone.0219145
Alkadhi H, Baettig E, Biondo A et al (2023) Risk stratification prior to non-cardiac surgery: the role of cardiac CT and MR imaging is underrecognized. Eur Radiol 33:8436–8438. https://doi.org/10.1007/s00330-023-09893-6
Di Cesare E, Cademartiri F, Carbone I et al (2013) Indicazioni cliniche per l’utilizzo della cardio RM. A cura del Gruppo di lavoro della Sezione di Cardio-Radiologia della SIRM. Radiol Medica 118:752–798. https://doi.org/10.1007/s11547-012-0899-2
Esposito A, Gallone G, Palmisano A et al (2020) The current landscape of imaging recommendations in cardiovascular clinical guidelines: toward an imaging-guided precision medicine. Radiol Medica 125:1013–1023. https://doi.org/10.1007/s11547-020-01286-9
Patel AR, Salerno M, Kwong RY et al (2021) Stress cardiac magnetic resonance myocardial perfusion imaging: JACC review topic of the week. J Am Coll Cardiol 78:1655–1668. https://doi.org/10.1016/J.JACC.2021.08.022/SUPPL_FILE/MMC1.DOCX
Catapano F, Moser LJ, Francone M et al (2024) Competence of radiologists in cardiac CT and MR imaging in Europe: insights from the ESCR Registry. Eur Radiol. https://doi.org/10.1007/s00330-024-10644-4
Jaeger C, Burkard T, Kamber F et al (2022) Quantification of metabolic equivalents (METs) by the MET-REPAIR questionnaire: a validation study in patients with a high cardiovascular burden. J Clin Anesth 76:110559. https://doi.org/10.1016/j.jclinane.2021.110559
Horvath B, Kloesel B, Todd MM et al (2021) The evolution, current value, and future of the american society of anesthesiologists physical status classification system. Anesthesiology 135:904–919. https://doi.org/10.1097/ALN.0000000000003947
Kramer CM, Barkhausen J, Bucciarelli-Ducci C et al (2020) Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 22:1–18. https://doi.org/10.1186/s12968-020-00607-1
Schulz-Menger J, Bluemke DA, Bremerich J et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update: society for cardiovascular magnetic resonance (SCMR): board of trustees task force on standardized post-processing. J Cardiovasc Magn Reson 22:1–22. https://doi.org/10.1186/s12968-020-00610-6
Dorbala S, Ananthasubramaniam K, Armstrong IS et al (2018) Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol 25:1784–1846. https://doi.org/10.1007/s12350-018-1283-y
Kontos MC, Brath LK, Akosah KO, Mohanty PK (1996) Cardiac complications in noncardiac surgery: relative value of resting two-dimensional echocardiography and dipyridamole thallium imaging. Am Heart J 132:559–566. https://doi.org/10.1016/S0002-8703(96)90238-0
Vanzetto G, Machecourt J, Blendea D et al (1996) Additive value of thallium single-photon emission computed tomography myocardial imaging for prediction of perioperative events in clinically selected high cardiac risk patients having abdominal aortic surgery. Am J Cardiol 77:143–148. https://doi.org/10.1016/S0002-9149(96)90585-8
Iskander S, Iskandrian AE (1998) Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol 32:57–62. https://doi.org/10.1016/S0735-1097(98)00177-6
Henein MY, O’Sullivan CA, Underwood R, Gibson D (1998) Assessment of cardiac risk before peripheral vascular surgery: a comparison of myocardial perfusion imaging and left ventricular long axis echocardiography at rest. Heart 79:125–132
Das MK, Pellikka PA, Mahoney DW et al (2000) Assessment of cardiac risk before nonvascular surgery. J Am Coll Cardiol 35:1647–1653. https://doi.org/10.1016/S0735-1097(00)00586-6
Hashimoto J, Nakahara T, Bai J et al (2007) Preoperative risk stratification with myocardial perfusion imaging in intermediate and low-risk non-cardiac surgery. Circ J 71:1395–1400. https://doi.org/10.1253/circj.71.1395
Chang HY, Chang WT, Liu YW (2019) Application of transthoracic echocardiography in patients receiving intermediate- or high-risk noncardiac surgery. PLoS ONE 14:e0215854. https://doi.org/10.1371/JOURNAL.PONE.0215854
Levitan EB, Graham LA, Valle JA et al (2016) Pre-operative echocardiography among patients with coronary artery disease in the United States Veterans Affairs healthcare system: a retrospective cohort study. BMC Cardiovasc Disord 16:173–173. https://doi.org/10.1186/S12872-016-0357-5
Fayad A, Ansari MT, Yang H et al (2016) Perioperative diastolic dysfunction in patients undergoing noncardiac surgery is an independent risk factor for cardiovascular events: a systematic review and meta-analysis. Anesthesiology 125:72–91. https://doi.org/10.1097/ALN.0000000000001132
Sabharwal NK, Lahiri A (2003) Role of myocardial perfusion imaging for risk stratification in suspected or known coronary artery disease. Heart 89:1291–1297. https://doi.org/10.1136/heart.89.11.1291
Cao D, Chandiramani R, Capodanno D et al (2021) Non-cardiac surgery in patients with coronary artery disease: risk evaluation and periprocedural management. Nat Rev Cardiol 18:37–57. https://doi.org/10.1038/s41569-020-0410-z
Patel AY, Eagle KA, Vaishnava P (2015) Cardiac risk of noncardiac surgery. J Am Coll Cardiol 66:2140–2148. https://doi.org/10.1016/j.jacc.2015.09.026
Etchells E, Meade M, Tomlinson G, Cook D (2002) Semiquantitative dipyridamole myocardial stress perfusion imaging for cardiac risk assessment before noncardiac vascular surgery: a metaanalysis. J Vasc Surg 36(3):534–540. https://doi.org/10.1067/mva.2002.126563
Knuuti J, Wijns W, Saraste A et al (2020) 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 41:407–477. https://doi.org/10.1093/eurheartj/ehz425
Boden WE, O’Rourke RA, Teo KK et al (2007) Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356:1503–1516. https://doi.org/10.1056/NEJMOA070829/SUPPL_FILE/NEJMOA070829SA1.PDF
Maron DJ, Hochman JS, Reynolds HR et al (2020) Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 382:1395–1407. https://doi.org/10.1056/NEJMOA1915922/SUPPL_FILE/NEJMOA1915922_DATA-SHARING.PDF
Shaw LJ, Berman DS, Maron DJ et al (2008) Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation 117:1283–1291. https://doi.org/10.1161/CIRCULATIONAHA.107.743963
Mcfalls EO, Ward HB, Moritz TE et al (2004) Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 351:2795–2804
Smilowitz NR, Gupta N, Ramakrishna H et al (2017) Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2:181–187. https://doi.org/10.1001/jamacardio.2016.4792
Puelacher C, Gualandro DM, Glarner N et al (2023) Long-term outcomes of perioperative myocardial infarction/injury after non-cardiac surgery. Eur Heart J 44:1690–1701. https://doi.org/10.1093/eurheartj/ehac798
Funding
The authors declare no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, guided by Fabio Fazzari. Material preparation, data collection, and analysis were performed by Costanza Lisi, Federica Catapano, Francesco Cannata, Stefano Figliozzi, and Federica Brilli. The first draft of the manuscript was written by Fabio Fazzari, with the help of Costanza Lisi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
The study protocol was approved by the institutional ethics committee, and given the retrospective design of the study, the ethics committee waved the need of specific informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fazzari, F., Lisi, C., Catapano, F. et al. Prognostic value of stress CMR and SPECT-MPI in patients undergoing intermediate-to-high-risk non-cardiac surgery. Radiol med (2024). https://doi.org/10.1007/s11547-024-01876-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11547-024-01876-x