Abstract
Objectives
Chronic kidney disease (CKD) is a disorder progressing to end-stage kidney failure. Early diagnosis and treatment are important for medical care. The aim of this prospective study was to define the strain index (SI) and resistivity index (RI) values in the same CKD group for each kidney separately at the same time, and also to compare the efficacy of SI and RI in the differentiation of normal population and CKD patients.
Materials and methods
Toshiba Aplio 500 USG device and 3.5–5 MHz convex probe were used for USG, CDUSG, and USG elastography examinations. The patients were referred to radiology clinique from nephrology and endocrinology cliniques after GFR calculation. Patients with renal cyst, tumor, or obstructive renal disease were excluded. Healthy volunteers according to laboratory and clinical examinations were selected from non-kidney disease patients.
Results
A total of 121 CKD (68 men, 53 women) and 40 healthy volunteers (19 men, 21 women) were participated. The mean SI and RI values of CKD were significantly higher than the normal healthy volunteers (p < 0.05). The SI and RI values of right and left kidney did not show any difference in CKD patients (p values were 0.381 for SI and 0.821 for RI). The sensitivity and the specificity of the SI were higher than RI.
Conclusion
The RI and SI values of kidneys in CKD patients were significantly higher than those of apparently normal kidneys. SI was more sensitive than RI in our study. Determining cut-off SI and RI values between normal and damaged renal parenchyma can help in the diagnosis and follow up of CKD patients.
Advances in knowledge
To the best of our knowledge, this is the first study comparing RI and SI in CKD patients, and SI is found to be more sensitive than RI for the evaluation of CKD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is an important and costly disorder that progresses to end-stage kidney failure. Early diagnosis and treatment are important for medical care. CKD is defined as kidney damage of three or more months duration caused by structural or functional abnormalities with or without a decreased glomerular filtration rate (GFR) by the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines. The diagnosis of CKD is based on the GFR < 60 mL/min/1.73 m2. CKD divides into five stages (stage 1 to stage 5) to express the severity according to GFR. Stage 1 indicates kidney damage with GFR ≥ 90 mL/min/1.73 m2, stage 2 indicates GFR 60–89 mL/min/1.73 m2 with evidence of kidney damage, stage 3 indicates GFR 30–59 mL/min/1.73 m2, stage 4 indicates GFR 15–29 mL/min/1.73 m2, and stage 5 (end-stage renal failure) indicates <15 mL/min/1.73 m2 [1]. Parenchymal corruption occurs during the CKD period [2].
Color Doppler ultrasonography (CDUSG) is a non-invasive real-time pulse-wave method that evaluates blood flow in vascular structures. The Doppler results indicate the flow parameters of related tissue and vessel. There are two different types are used in the clinic: color flow and spectral modes. Color flow and spectral modes give the operator pulsatility index (PI), resistivity index (RI), flow volume, and the other flow parameters about related vascular structure [3]. Presenting RI, CDUSG is used in daily practice in CKD patients [2, 4, 5]. In the measurement of intrarenal arterial resistance, RI is frequently used.
Elastography is a developing modality, in which working principle is based on tissue elasticity that requires dedicated probe and elastography software [6]. The principle of the elastography is to acquire data about the stiffness of the tissue to assist differential diagnosis. There are different kinds of USG elastography methods. These methods can be divided as dynamic and quasi-static according to the type of force, while shear-wave and strain according to method. We used the strain elastography which is divided into two types as qualitative real-time elastography and semi-quantitative strain elastography. Real-time elastography presents color scale according to the hardness of the related tissue, and the operator classifies the stiffness according to colors. Semi-quantitative strain elastography presents strain ratio or strain index using region of interests (ROI). Strain elastography is operator dependent due to the probe compressions and decompressions, while shear-wave does not need operator compressions by the aid of generating electro-mechanical waves [7, 8].
Organ damage affects the RI and SI values, which increase in CKD patients [2, 9–11]. Renal function is correlated with RI values [2]. There are various studies about RI and SI separately, but, to the best of our knowledge, there is no published study comparing RI and SI at the same in CKD patients. The efficacy of RI is well known in the literature; however, there is no evident knowledge about SI in the diagnosis of CKD [2]. We assume that besides RI, SI will also assist in the evaluation of CKD. Determining cut-off SI and RI values between normal and diseased renal parenchyma can help in the diagnosis and follow up of CKD patients.
The aim of this study was to define the SI and RI values in the same CKD group for each kidney separately at the same time, and also to compare the efficacy of SI and RI in the differentiation of normal population and CKD patients. To the best of our knowledge, this is the first study comparing RI and SI in CKD patients.
Material and methods
Informed consent form was obtained from all patients, and the study was performed in accordance with the ethical guidelines of the Helsinki Declaration and approved by the local ethics committee. No financial support was received for the present study.
In this prospective study, patients were referred to radiology clinique from nephrology and endocrinology cliniques after GFR calculation. Patients with renal cyst, tumor, or obstructive renal disease were excluded. Healthy volunteers according to laboratory and clinical examinations were selected from non-kidney disease patients. Demographic data (age and sex), creatinine in urine, and proteinuria (g/day) of the patients were recorded in nephrology and endocrinology cliniques, while RI and SI values of both right and left kidneys were recorded in radiology clinique. Demographic data and RI and SI values were recorded in the same week. There was no significant time interval between the radiologic measurements and blood and urine sampling.
The stages of the CKD were recorded, but the statistical analyses were not made by grouping according to CKD stages, because the aim of the study was not about the difference of RI and SI among stages.
Toshiba (Toshiba Medical Systems, Co, Ltd, Otawara, Japan) Aplio 500 US device and 3.5–5 MHz convex probe were used for USG, CDUSG, and USG elastography examinations.
The sonographic evaluations were made by a six-year-experienced radiologist who was completely blinded to the clinical information.
Color Doppler USG examination (CDUSG)
USG examinations were performed with the patient lying in supine, right decubitus, and left decubitus positions. Measurements were obtained from the optimal position even supine or decubitis. B-mode features, such as dimensions, parenchymal thickness, echogenicity, and collecting system, were evaluated. After angle correction, Spectral Doppler measurements of the interlobar arteries were obtained [12] (Fig. 1).
RI values were calculated as follows: RI = (peak systolic velocity-end diastolic velocity)/peak systolic velocity.
RI values were measured from the upper, mid, and lower poles of bilateral renal interlobar arteries, and the mean of the three measurements was used for statistical analysis for each kidney separately.
Sonoelastography examination
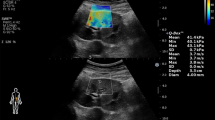
Elastography mode was used after grayscale B-mode and CDUSG. The elastography application of our device was strain elastography. Strain elastography is a semi-quantitative and semi-static method. Strain elastography calculates the strain ratio of interested lesion using adjacent soft tissue. The compression and decompression were applied to the adjacent tissue by the probe. Compression and decompression movements cause a sinusoidal wave that allows to follow the regularity and rhythmicity of the compression and decompressions (Figs. 2, 3). The monitor is divided into three windows. Right window is gray scale USG image, left window is color-coded USG elastography image, and the bottom window is sinusoidal wave. The measurement should be performed in decompression phase to avoid the external pressure effect. In all patients and healthy volunteers, ROI was adjusted to the parenchyma and reference ROI adjusted to the perirenal fat tissue to calculate the elasticity score. The size of ROI and reference ROI was 4 mm in diameter. We adjusted the ROI’s in the axial plane of the kidneys. The distance of the reference ROI to the USG transducer effects the strain ratio measurements; thus, we tried to adjust the reference ROI which was in the perirenal fat tissue to the closest position to sampling ROI in axial plane to obtain the most optimal measurement. The distance between reference ROI and parenchymal ROI was as close as we can. And also, we did not change the sampling procedure for all adjusting the reference ROI [13]. We took care about adjusting the reference ROI just in the perirenal fat tissue and avoided to include the renal capsule or the renal parenchyma. In atrophic kidneys, the ROI adjusted to the most appropriate and thick parenchymal area. The software calculates the elasticity score, known as SI, of the lesion based on the displacement using the ratio of the lesion and adjacent soft tissue.
Elastography USG image of the kidney with CKD. The monitor is divided into three windows. Right window is gray scale USG image, left window is color-coded USG elastography image, and the bottom window is sinusoidal wave of compression and decompression. The circles indicate the ROIs. The upper ROI is on the perirenal fat tissue and the lower ROI is on the parenchyma. The vertical white line on the sinusoidal wave indicates the point of measurement
Elastography USG image of the kidney with healthy patient. The monitor is divided into three windows. Right window is gray scale USG image, left window is color-coded USG elastography image, and the bottom window is sinusoidal wave of compression and decompression. The circles indicate the ROIs. The upper ROI is on the perirenal fat tissue and the lower ROI is on the parenchyma. The vertical white line on the sinusoidal wave indicates the point of measurement
SI values were calculated from upper, mid, and lower poles of bilateral renal parenchyma and perirenal fat tissue. The mean of three measurements was used for statistical analysis. Only one value was measured for each pole, and if the sinusoidal wave was irregular, the measurement was repeated. Calculations were made for each kidney separately.
Statistical analysis
IBM SPSS Statistics version 21 [IBM Corp. © Copyright IBM Corporation and other(s) 1989, 2012] was used to perform statistical analysis. Descriptive statistics were used for demographic data. One-Sample Kolmogorov-Smirnov test was performed to analyze the distribution of the data. Mann–Whitney U test was used to analyze the difference between the normal healthy individual volunteers and CKD groups for RI and SI values. Wilcoxon test was used to calculate the difference in RI and SI values of the right and left kidney. SI and RI cut-off values were calculated for each kidney and for the mean right and left kidney values [(right kidney + left kidney)/2]. ROC curve was made to calculate the cut-off value of SI and RI.
Continuous variables were expressed as arithmetical mean ± standard deviation; categorical variables were expressed as percentages (%). Level of significance was set at p ≤ 0.05.
Results
A total of 121 CKD (68 men, 53 women) and 40 healthy volunteers (19 men, 21 women) participated in this study. The mean age of CKD patients was 58.55 ± 12.33 years, and healthy volunteers were 52.60 ± 12.58 years. There were 28, 25, 37, 22, and 15 patients in Stage 1, 2, 3, 4, and 5, respectively.
The right kidney SI (RSI), the left kidney SI (LSI), the right kidney RI (RRI), the left kidney RI (LRI) values, and the mean kidney SI (SI), the mean kidney RI (SI) values of the CKD, and healthy volunteer are showed in Tables 1 and 2. The mean SI and RI values of CKD were significantly higher than the normal healthy volunteers (p < 0.05) (Tables 1, 2; Fig. 4).
The SI and RI values of right and left kidney did not show any significant difference in CKD patients. (Wilcoxon test p values were 0.381 for SI and 0.821 for RI). The SI and RI values of right and left kidney did not show any significant difference in healthy volunteers. (Wilcoxon test p values were 0.087 for SI and 0.964 for RI).
According to the ROC curve analysis, the cut-off values were 0.92, 0.92, 0.59, and 0.59 for right SI, left SI, right RI, and left RI, respectively. In addition, the cut-off values were 0.92 and 0.59 for SI and RI for mean kidney value [(right kidney + left kidney)/2] (Fig. 5). The sensitivity and specificity for SI was 71.90 and 90.00 %, respectively. The sensitivity and specificity for RI was 67.77 and 42.50 %, respectively. The sensitivity and the specificity of SI were higher than the RI (Table 3).
Discussion
The early diagnosis and follow-up of CKD is essential because of being costly and progressing to end-stage kidney failure [2]. Nowadays, it is known that RI is used for diagnosis and follow-up of CKD [2]. RI shows good correlation with renal function and histological damage scores in CKD patients. In recent studies, the availability of SI is researched in the diagnosis and follow-up of CKD, and showed also correlation with CKD [11, 14, 15].
In our study, the RI and SI values in both kidneys of CKD patients were higher than the normal population. And also, the SI and RI values of CKD patients did not show any significant difference between right and left kidney. We calculated the SI and RI cut-off values for each kidney, independently. According to our results, the cut-off value for SI was 0.92 and RI was 0.59 for each kidney. Thus, the mean SI and RI values of CKD were significantly higher than apparently healthy kidneys. Furthermore, the most important result according to our study was that the sensitivity and the specificity of SI were higher than RI.
RI is a value related with the blood flow, and shows increment with the CKD stages and renal damage [2]. RI is a good marker to follow up the progression of renal diseases [16]. RI showed almost the same sensitivity and the specificity as proteinuria in CKD patients [17]. In the study of Toshihiro et al., it was reported that the optimal RI value to discriminate chronic renal disease was 0.65. This value is similar to our finding 0.65 in CKD group [17]. Although, in most of the studies, it is reported that the cut of value for RI is 0.70, we found 0.65 [18]. The use of ACE inhibitors or other type of antihypertensive drugs may affect the RI value. The RI value of healthy group was 0.57 in our study, similar to Winther et al. (they reported 0.58) [19].
USG elastography presents the operator a value about the stiffness of the tissue. Recent studies showed that USG elastography has a place in the evaluation of CKD patients [11, 14, 15]. There are two basic different methods in USG elastography as shear-wave and strain. These two methods have advantages and disadvantages. Strain elastography can be used in ascites mediums, but shear-wave can not. Strain elastography is operator dependent, while shear-wave is not. But, recent studies showed that strain elastography method did not show inter- and intra-observer variability [20]. The application of elastography is based on compression and decompression to the related tissue by USG probe. The studies about various elastography methods in CKD patients demonstrated that elastography values were significantly higher than the normal healthy population, and useful in clinical usage. Guo et al. and Goya et al. reported that the shear-wave velocity of the CKD patients was higher than the normal healthy population in which ARFI method was used [14, 15, 21]. Some studies reported higher stiffness values in CKD patients than normal population in strain elastography and ARFI [11, 14, 15]. The SI values are not affected by the demographic features; thus, the changes indicate the disorders. In our study, the SI values of each kidney were not affected by age or gender, and were higher than those of apparently normal kidneys.
To the best of our knowledge, cardiovascular disorders do not affect the SI, unlike RI. Not only the parenchymal changes but also the vascular and hemodynamic disorders affect the RI. It is revealed that vascular lesions (arteriosclerosis, systemic vasculitis,…), antihypertensive drugs (ACE inhibitors, ARBs) age, and systolic blood pressure affect the RI value [4, 5, 22–24]. The tissue component and elasticity are the major factors affecting the SI. Chronic progressive disorders change the nature of the tissue. In CKD disease, chronic progression causes fibrosis and fibrosis causes increase in SI value [11, 12, 15].
The major limitations of our study are as follows: There are various etiologic factors of CKD. CKD has five stages according to severity. We recorded the etiology and the stages of CKD, but did not use these parameters for statistical analysis. Because our aim was to evaluate and compare the SI and RI values in CKD patients, the inter- and intra-observer consistency were not evaluated. Semi-quantitative elastography is dependent to operator; only one radiologist evaluated the patients. The quantitative elastography is superior than strain elastography, using strain elastography might be the other limitation. The other limitation was about anisotrophy and the tubular system. Grenier mentioned anisotrophy in the shear-wave elastography method. Anisotrophy is related with the direction of shear-waves and the tissue architecture. Degree of anisotropy, and the level of vascular and urinary pressure may have an impact on shear-wave velocity, and, therefore, on the elasticity values of the kidney tissue (more particularly the cortex). We used strain elastography in our study, not shear-wave; but, perhaps, anisotrophy influences the strain elastography as shear-wave. There should be more studies about anisotrophy and strain elastography [23].
In conclusion, the RI and SI values of kidneys in CKD patients were significantly higher than those of apparently normal kidneys. Determining cut-off SI and RI values between normal and damaged renal parenchyma which can help diagnosis and follow-up of CKD patients. In addition, SI can be used in daily usage like RI to follow up and evaluate the CKD patients. We can assume that SI is more sensitive than RI in the evaluation of CKD.
References
El Nahas AM, Bello AK (2005) Chronic kidney disease: the global challenge. Lancet 365(9456):331–340
Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T (2012) The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol 2012:139565
Jenderka KV, Delorme S (2015) Principles of Doppler sonography. Radiologe 55(7):593–609
Kimura N, Kimura H, Takahashi N, Hamada T, Maegawa H, Mori M, et al (2015) Renal resistive index correlates with peritubular capillary loss and arteriosclerosis in biopsy tissues from patients with chronic kidney disease. Clin Exp Nephrol 19(6):1114–1119
Carrol LE (2006) The stages of chronic kidney disease and the estimated glomerular filtration rate. J Lanc Gener Hosp 1(2):64–69
Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X (1991) Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 13(2):111–134
Onur MR, Göya C (2013) Ultrasound elastography: abdominal applications. Türkiye Klinikleri j Radiology-Special Topics 6(3):59–69
Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F et al (2013) EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: basic principles and technology. Ultraschall Med 34(2):169–184
Shimizu Y, Itoh T, Hougaku H, Nagai Y, Hashimoto H, Sakaguchi M et al (2001) Clinical usefulness of duplex ultrasonography for the assessment of renal arteriosclerosis in essential hypertensive patients. Hypertens Res 24(1):13–17
Florczak E, Januszewicz M, Januszewicz A, Prejbisz A, Kaczmarska M, Michalowska I et al (2009) Relationship between renal resistive index and early target organ damage in patients with never-treated essential hypertension. Blood Press 18(1–2):55–61
Menzilcioglu MS, Duymus M, Citil S, Avcu S, Gungor G, Sahin T et al (2015) Strain wave elastography for evaluation of renal parenchyma in chronic kidney disease. Br J Radiol 88:20140714
Ozkan F, Yavuz YC, Inci MF, Altunoluk B, Ozcan N, Yuksel M et al (2013) Interobserver variability of ultrasound elastography in transplant kidneys: correlations with clinical-Doppler parameters. Ultrasound Med Biol 39(1):4–9
Havre RF, Waage JR, Gilja OH, Odegaard S, Nesje LB (2011) Real-time elastography: strain ratio measurements are influenced by the position of the reference area. Ultraschall in der Medizin. [Epub ahead of print]
Guo LH, Xu HX, Fu HJ, Peng A, Zhang YF, Liu LN (2013) Acoustic radiation force impulse imaging for noninvasive evaluation of renal parenchyma elasticity: preliminary findings. PLoS One 8(7):e68925
Goya C, Kilinc F, Hamidi C, Yavuz A, Yildirim Y, Cetincakmak MG et al (2015) Acoustic radiation force impulse imaging for evaluation of renal parenchyma elasticity in diabetic nephropathy. AJR Am J Roentgenol 204(2):324–329
Krumme B (2006) Renal Doppler sonography–update in clinical nephrology. Nephron Clin Pract 103(2):c24–c28
Sugiura T, Wada A (2009) Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transpl 24(9):2780–2785
Viazzi F, Leoncini G, Derchi LE et al (2014) Ultrasound Doppler renal resistive index: a useful tool for the management of the hypertensive patient. J Hypertens 32(1):149–153
Winther SO, Thiesson HC, Poulsen LN, Chehri M, Agerskov H, Tepel M (2012) The renal arterial resistive index and stage of chronic kidney disease in patients with renal allograft. PLoS One 7(12):e51772
Havre RF, Elde E, Gilja OH, Odegaard S, Eide GE, Matre K et al (2008) Freehand real-time elastography: impact of scanning parameters on image quality and in vitro intra- and interobserver validations. Ultrasound Med Biol 34(10):1638–1650
Göya C, Hamidi C, Ece A, Okur MH, Taşdemir B, Çetinçakmak MG et al (2015) Acoustic radiation force impulse (ARFI) elastography for detection of renal damage in children. Pediatr Radiol 45:55–61
Mostbeck GH, Kain R, Mallek R, Derfler K, Walter R, Havelec L et al (1991) Duplex Doppler sonography in renal parenchymal disease. Histopathologic correlation. J Ultrasound Med 10(4):189–194
Bige N, Levy PP, Callard P, Faintuch JM, Chigot V, Jousselin V et al (2012) Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol 13:139
Ikee R, Kobayashi S, Hemmi N, Imakiire T, Kikuchi Y, Moriya H et al (2005) Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis 46(4):603–609
Acknowledgments
None of the authors involved in this study received financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Menzilcioglu, M.S., Duymus, M., Citil, S. et al. The comparison of resistivity index and strain index values in the ultrasonographic evaluation of chronic kidney disease. Radiol med 121, 681–687 (2016). https://doi.org/10.1007/s11547-016-0652-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0652-3