Abstract
Purpose
This study sought to investigate the effect of the hybrid iterative reconstruction (IR) algorithm (iDose, Philips Healthcare) on the improvement of image quality of computed tomography (CT) scans of the liver and determine the appropriate level of IR strength for clinical use.
Materials and methods
A total of 75 patients (41 men and 34 women; mean age, 59.5 years) with a primary abdominal malignancy who underwent two-phase liver CT scans for the work-up of their liver metastases, were included in this study. The CT images during the portal phase were reconstructed using either filtered back projection (FBP) or the hybrid IR algorithm with six different levels of IR strengths. The signal-to-noise ratio of the liver (SNRliver) and the contrast-to-noise ratio of the portal vein to muscle (CNRpv to m) were measured. For qualitative analysis, image noise, visibility of small intrahepatic vascular structures, beam-hardening artefact, lesion conspicuity, and overall image quality were graded by two radiologists.
Results
Quantitative analysis demonstrated that image noise was significantly reduced along with the increasing level of iDose and that the values of SNRliver and CNRpv to m were significantly better with iDose than those of FBP images. Qualitative assessment also showed significantly better results with iDose compared with FBP (p < 0.05) and the parameters for subjective image quality were highest with iDose level 4.
Conclusions
The hybrid IR technique is able to reduce image noise and to provide better image quality than FBP, and an intermediate strength of iDose (level 4) provided the highest quality images.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The explosive growth of the use of computed tomography (CT) can be attributed to its wide availability, speed, and diagnostic benefits [1, 2]. While this increase is associated with significant improvements in diagnostic performance, it has also caused an increase in radiation exposure and the risk of radiation-induced cancer [3]. Not only for patients who undergo multiple CT studies during the course of their follow-up, but also for paediatric patients who are sensitive to radiation hazards as well as the general population who undergo CT scans for routine checkups, radiation dose reduction is an important issue [4]. Owing to the radiation dose concerns associated with CT, several approaches, including automated tube current modulation, new image acquisition, and new reconstruction algorithms, have been used to reduce the radiation dose [5, 6]. Amongst these approaches, several iterative reconstruction (IR) algorithms of major vendors are widely used to achieve radiation dose reduction without image degradation [6].
Results of recent studies [5–7] have shown that new IR algorithms produce up to 66 % radiation exposure reduction without significant image degradation, compared with standard dose CT using filtered back projection (FBP). New iterative reconstructions assume that noise is not evenly distributed across the entire image [8]. With matrix algebra which is used to selectively identify and then subtract noise from the image, a less noisy image is acquired. However, an image-based IR method such as iterative reconstruction in image space (IRIS, Siemens Healthcare), resulting in profound changes in image appearance with higher levels of iterative weighing [9] and excessive image noise reduction, often results in “plastic”, “blotchy” or “pixilated” images which could adversely affect overall image quality or diagnostic accuracy [8]. This disadvantage could be attributed to the different frequency distribution of the noise when using the IR technique compared with the FBP technique. Indeed, this might be the primary reason why some radiologists are reluctant to take advantage of this technique in their clinical practice. More recently, following prior versions of the image-based IR technique, such as IRIS, several hybrid IR algorithms working in both image space and the raw data space, such as adaptive statistical iterative reconstruction (ASIR, GE Healthcare), iDose (Philips Healthcare), and sinogram-affirmed, iterative reconstruction (SAFIRE from Siemens Healthcare) were launched to solve image-quality-related problems and are believed to be more effective than image-based IR techniques [8, 10].
Therefore, the purpose of our study was to determine, through an evaluation of the objective and subjective image quality, whether the hybrid IR algorithm (iDose) can improve the image quality of liver CT by reducing image noise, and to assess which level of IR strength is acceptable for clinical use.
Materials and methods
Patient population
We obtained approval for this retrospective study from the institutional review board of our institution, and informed consent was waived. From December 2011 to February 2012, 75 consecutive patients (M:F = 41:34, mean age, 59.5 years; age range 35–83 years) with a known or suspected intra-abdominal malignancy underwent two-phase liver CT using a 64-channel, multidetector CT (MDCT) scanner (Philips Ingenuity, Philips Healthcare, Best, The Netherlands). Amongst these 75 patients, 30 had intrahepatic lesions, including metastases (n = 16), cyst (n = 12), and haemangioma (n = 2). Amongst them, ten patients with metastases underwent liver biopsy or surgery, and hepatic metastasis was confirmed pathologically. In addition, in the other six patients, the hepatic lesions noted on the liver CT were clinically diagnosed as metastases either based on their characteristic magnetic resonance (MR) findings for metastases, including irregular or ill-defined borders which displayed high signal intensity on high b value diffusion-weighted imaging (DWI) and the lower apparent diffusion coefficient (ADC) value of the lesion compared with that of the adjacent liver parenchyma or interval growth in the longest axial diameter of at least 20 % on the next follow-up CT. The cysts or haemangiomas in 14 patients were diagnosed based on characteristic imaging features on liver CT or MR imaging and absence of interval growth on follow-up CT scan.
CT technique
For all patients, two-phase liver CT consisting of precontrast and portal phase images was performed. All patients underwent two-phase liver CT using a 64-channel, multidetector CT (Philips Ingenuity) scanner. The CT parameters were as follows: rotation time, 0.5 s; beam collimation, 64 × 0.6 mm; reconstruction section thickness, 3 mm; beam pitch, 0.891; field of view, 32 ~ 38 cm; and matrix size, 512 × 512 pixels. Iodinated contrast medium (iopromide, Ultravist 370; Bayer-Schering, Berlin, Germany) at a dose of 1.5 mL/kg (=555 mgI/kg) body weight, was injected for 30 s using a power injector (Stellant Dual; Medrad, Indianola, PA, USA) and was then followed by injection of 30–40 mL of normal saline. Portal phase imaging was performed 70 s after attaining 150 HU attenuation of the descending aorta, as measured using the bolus-tracking technique. The tube potential was fixed to 120 kV and the automatic tube current modulation technique (Z-axis Dose modulation; Philips Healthcare) was used with a reference current of 180 mAs. The mean CTDIvol of the portal phase CT scans was 6.85 ± 1.33 mGy (mean ± standard deviation; range 4.91–13.21) and the mean dose-length product (DLP) was 375 ± 91 mGy·cm (mean ± standard deviation; range 227–799). Using the conversion coefficient of k = 0.015, the mean effective dose was 5.62 mSv.
Image reconstruction
Images were reconstructed for each patient using the same parameters, i.e. 3-mm section thickness and a 2-mm interval, during the portal phase and using a standard FBP algorithm with a standard, soft-tissue kernel and an iDose algorithm with a level from 1 to 6. The seven different reconstruction images, i.e. FBP, iDose level 1 (iDose-1) through iDose level 6 (iDose-6) were generated from the same raw data for each patient and were then sent to and archived in our picture archiving and communications system (Maroview 5.4, Infinitt, Seoul, Korea).
iDose is provided with an additional parameter, i.e. the iDose level (scale 1–7), which is used to define the strength of the IR technique for reducing image quantum mottle noise. Increasing levels of iDose indicate a greater strength of noise removal, i.e. iDose-1 = 11 %, 2 = 16 %, 3 = 23 %, 4 = 29 %, 5 = 37 %, 6 = 45 %, and 7 = 55 %, relative to the corresponding FBP reconstruction [11]. For example, if the original scan at 200 mAs is associated with the contrast-to-noise ratio of 200 mAs (CNR), then using an iDose reconstruction with a level of 4 for a scan performed with 100 mAs would compensate for the increase in noise in the same homogeneous region of interest (ROI). The level can be defined independent of the radiation dose for every acquisition acquired and this allows targeting of the iDose application depending on the clinical goal, i.e. dose reduction, image quality improvement or a combination of both.
Qualitative analysis
Two radiologists independently assessed the image noise, visibility of small intrahepatic vascular structures, beam-hardening artefact, lesion conspicuity, and the overall image quality as determined on portal phase CT images. The reading was performed at a workstation (Advantage Windows 6200, Hewlett-Packard, CA, USA) with a spatial resolution of 1600 × 1200 (Totoku, Japan) using our picture archiving and communications system (Maroview 5.4, Infinitt, Seoul, Korea). The readers were blinded to the CT parameters and reconstruction methods, and the order of image sets was totally randomised so as to minimise recall bias. To improve interobserver agreement, the criteria for image grading were established by consensus between the two radiologists beginning the image reading. If there were multiple lesions, the readers were asked to grade the smallest lesion with a diameter of at least 1 cm.
Subjective image noise level was assessed using a five-point scale (1 = unacceptable image noise, 2 = above average noise, 3 = average image noise, 4 = less than average noise, and 5 = minimal image noise), and visibility of small structures such as small portal vein branch vessels, was graded using a five-point scale (1 = unacceptable visualisation, 2 = suboptimal visibility, 3 = acceptable visibility, 4 = above average visibility, and 5 = excellent visualisation). Subjective visual lesion conspicuity was assessed using a five-point scale (1 = margins definitely an artefact mimicking a lesion, 2 = probably an artefact mimicking a lesion, 3 = subtle lesion, 4 = well-seen lesion with poorly visualised margins, and 5 = well-seen lesion with well-visualised margins). Beam-hardening artefacts were defined as streak artefacts and were assessed using a three-point scale (1 = present and affecting image interpretation, 2 = present but not affecting interpretation, 3 = absent). In addition, overall image quality including artificial sensation was assessed using a five-point scale (1 = unacceptable diagnostic image quality with severe blotchy, or pixilated appearance impairing lesion evaluation, 2 = sub-diagnostic image quality with obvious blotchy, or pixilated artefacts impairing lesion evaluation, 3 = average diagnostic image quality with subtle blotchy or pixilated artefacts, 4 = better image quality than average with subtle pixilated appearance, and 5 = better image quality than average with better edge delineation and no pixilated appearance). The image quality attributes assessed in our study have been described in the European Guidelines on Quality Criteria for Computerized Tomography document [12] and have been used in numerous previous studies published in the radiology literature [13].
Quantitative analysis
Quantitative measurements were performed at a commercially available workstation (Advantage Windows 4.2; GE Healthcare) by experienced radiology research personnel who was blinded to the image review results. The seven image sets were displayed side by side with a preset soft-tissue window (window width, 350 HU; window level, 40 HU). We measured the image noise and attenuation values of the liver, portal vein, and paraspinal muscle, as also described in previous reports [14, 15]. Objective image noise was measured for 525 image sets (seven image sets each from 75 patients) as the standard deviation of the pixel values from a circular or ovoid ROI (10–50 mm2) placed in a homogenous region of subcutaneous fat of the anterior abdominal wall. To ensure consistency, all measurements were performed three times at the level of the main portal vein, after which the mean values were calculated.
Mean CT attenuation values (in Hounsfield units) of the portal vein, liver parenchyma, and paraspinal muscle were obtained on the portal phase of CT scanning. The attenuation of the portal vein was measured from a single ROI (10–50 mm2) placed at the portal vein confluence level. The liver attenuation was recorded as the mean of the measurements of four ROIs (50–100 mm2) in the medial and lateral segments of the left hepatic lobe and in the anterior and posterior segments of the right hepatic lobe [14]. Areas of focal changes in the liver parenchyma, visible blood vessels, bile ducts, and prominent artefacts were carefully avoided in the ROI measurements. The attenuation of the paraspinal muscles was recorded as the mean attenuation of two ROIs (10–50 mm2), while taking care to avoid macroscopic fat infiltration in the right and left paraspinal muscle at the level of the right portal vein. For all measurements, the size, shape, and position of the ROIs were kept constant by applying the copy-and-paste function at the workstation.
The CNR values relative to muscle (m) for the portal vein (pv) were calculated using the following equation [16]: CNRpv to m = (ROIpv − ROIm)/SDn, where ROIpv is the mean attenuation of the portal vein, ROIm is the mean attenuation of the paraspinal muscle, and SDn is the mean image noise. SNRliver values were calculated by dividing the mean attenuation of the liver by the mean image noise.
Statistical analysis
All statistical analyses were performed using commercially available software (MedCalc, version 12.3.0.0, 2012; MedCalc, Mariakerke, Belgium) and data are graphically presented as mean ± 95 % confidence interval (CI). The Kruskal–Wallis test was used to determine the results of the qualitative analysis data. When there were statistically significant differences, post hoc analysis was performed using the Student–Newman–Keuls test (Mann–Whitney). Interobserver agreement was measured using the kappa test. The scale for the k coefficients for interobserver agreement was as follows: <0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and 0.81–1.00, almost perfect [17]. A p value of <0.05 was considered significant. The one-way ANOVA test was used to compare statistically significant inter-group differences in the quantitative measurement data.
Results
Qualitative analysis
The image noise and beam-hardening artefact decreased significantly as the iDose level increased (p < 0.05). However, the scores for subjective image quality, such as the visibility of small intrahepatic vascular structures, lesion conspicuity, and overall image quality were highest with iDose level 4 (Fig. 1). Both observers complained of the artificial, plastic look of the images at iDose level 5 or 6 (Fig. 2). Interobserver agreement for the qualitative analysis was moderate to almost perfect (Table 1).
Bar graphs showing qualitative analysis results. With regard to subjective image noise (a) and beam-hardening artefact (c), the higher iDose level used, the lower image noise and beam-hardening artefacts noted (higher score). However, visibility of small intrahepatic vascular structures (b), lesion conspicuity (d), and overall image quality (e) are highest with iDose level 4
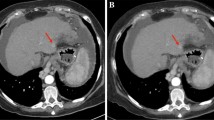
Axial contrast-enhanced multidetector CT images in a 60-year-old man with a history of subtotal gastrectomy due to gastric cancer. Images obtained during the portal phase with a FBP, b iDose level 2, c iDose level 4, d iDose level 6 shows gradually reduced image noise with increasing iDose level. However, due to excessive noise reduction in iDose level 6 image, both observers complained of “plastic” appearance of the image
Quantitative analysis
An increased iDose level was associated with a linear noise reduction, no change in CT attenuation, and a linear improvement in SNR and CNR (Table 2). Compared with FBP, the mean noise reduction was 13, 18, 23, 28, 34, and 41 % for reconstructions with iDose levels 1 through 6, respectively. When compared directly with FBP, the noise decreased significantly at all of the iDose levels (p < 0.05). There was also no significant difference in the mean CT attenuation of liver, portal vein or back muscle in the image sets. There was a significant increase in the SNR and CNR with an increased iDose level. Compared with FBP, there was a mean increase in liver SNR of 12, 17, 23, 29, 36, and 43 % for reconstructions using iDose levels 1 through 6, respectively. The SNR of the portal vein, compared with FBP, showed a mean increase of 12, 17, 23, 28, 35, and 43 % for reconstructions using iDose levels 1 through 6. The CNR of the portal vein also showed a mean increase of 11, 16, 22, 28, 35, and 42 % for reconstructions using iDose levels 1 through 6 when compared with FBP. When compared directly with FBP, all the iDose levels were associated with a significant reduction in SNR and CNR (p < 0.05).
Discussion
In our study, both quantitative and qualitative analyses demonstrated that the iDose technique with different levels of IR strengths showed a significant noise reduction and provided improved SNRliver and CNRpv to the m as well as better image quality compared with FBP images. Our study results were in good agreement with those of previous studies regarding various IR techniques such as ASIR (GE Healthcare) [6, 14, 18], or with an IRIS (Siemens Healthcare) [19–21] or adaptive iterative dose reduction (AIDR, Toshiba Healthcare) [22], both of which demonstrated that IR has the potential to provide improved image quality at lower radiation doses than FBP. Furthermore, in the qualitative analysis, intermediate strength IR (level 4) was more preferred than FBP or higher strength IR (level 5 or 6), despite the fact that quantitative analysis showed better results of image noise, SNRliver, and CNRpv to the m as the strength of the IR increased. Based on our study results on noise reduction, we believe that the use of iDose with intermediate IR strength can be used to reduce the radiation dose of routinely used abdominal CT while maintaining image quality.
In our study, when we evaluated the appropriate level of IR strength, i.e. the iDose level, iDose effectively reduce image noise, while the images did not have an artificial, blotchy, pixilated or plastic appearance, up to the intermediate strength of IR. In addition, although the CT vendor, Philips, claimed that iDose is able to preserve the image texture and its natural appearance by minimising the shift of noise power spectrum (NPS), even at the maximum noise removal to reproduce the noise texture of classical FBP images that radiologists expect to achieve [23]. iDose uses a combination of processing in the projection and image domains, thus providing clinical advantages such as the ability to effectively remove photon starvation-related artefacts, e.g. streaks. In addition, with iDose, radiation dose reduction is achievable while maintaining the spatial resolution. Prior studies have evaluated the measurement of spatial resolution with phantom experiments using modulation transfer function (MTF) and concluded that there is no decline in spatial resolution while noise level and radiation dose decreased [10, 11]. In addition, through the use of dynamic frequency noise removal techniques in such IR techniques, it is possible to effectively remove noise across all frequencies. Consequently, the noise texture of images reconstructed with iDose and FBP did not differ, although the noise itself was significantly reduced by applying the iDose algorithm [24]. However, both reviewers of our study preferred intermediate strength reconstruction (level 4) because some of the images reconstructed with iDose-5 or iDose-6 appeared artificial. We believe that this kind of mismatch between quantitative analysis and qualitative analysis could be related to some differences in the noise power spectrum (NPS) at higher IR strengths of iDose compared to FBP [24]. Another explanation could be that radiologists who review the image sets were not comfortable with the texture of the images reconstructed with iDose. In our study, when iDose level 4 was used for image reconstruction, image noise (iDose, 8.8) was reduced by 28.5 % compared with FBP-reconstructed image (Noise, 12.3). Theoretically, as radiation dose decreased by 1/χ, the image noise increases by the square root of χ. For example, 50 % (1/2) less radiation exposure was achieved by increasing the noise index (NI) by a factor of \( \sqrt 2 \) [25]. According to a previous study, it has been reported that when using iDose in the clinical routine, the clinician has the opportunity to reduce the radiation dose up to 80 % while preserving the image quality or maintain the radiation dose to improve the spatial resolution up to 68 % [11]. For example, for paediatric imaging where radiation dose reduction is paramount, iDose allows a significantly lower radiation dose while maintaining diagnostic image quality. In other situations, where image quality, spatial resolution, is of a higher priority than dose reduction, such as in the assessment of coronary stent patency, iDose allows significantly improved spatial resolution. However, based on our study results on noise reduction, we believe that the use of iDose at intermediate level of dose reduction could reduce the radiation dose up to 40 % of routinely used abdominal CT dose while maintaining image quality. Currently, at our hospital, based on these study results, we were able to reduce the radiation dose up to 30 % for liver CT scanning with iDose level 4 reconstruction compared with the conventional scanning method using FBP.
Currently, several types of IR techniques are being developed by many vendors and are being used at many medical centres as a tool to reduce the radiation dose or to improve image quality. In our study, we used different levels of iterative strengths of the hybrid IR algorithm. The iDose algorithm can be classified as a hybrid IR technique, and, theoretically, it can be more successful than image-based iterative techniques, such as IRIS, as it uses projection-based data as well as image-based data. Recent studies reporting on SAFIRE and AIDR 3D, which are also classified as hybrid IR technique since they iterate in both the raw data space and the image space, have shown that radiation dose reduction up to 50–52 % with mean objective image noise reduction of 31 % without altering diagnostic information is possible [7, 26]. SAFIRE is provided with five reconstruction strength levels and the noise modelling is supported by raw data: in each iteration, the noise content is estimated in each voxel by analysing the contribution of raw data to this voxel, after which noise is removed. The vendor recommends a medium reconstruction strength level of three and states that up to 60 % dose reduction can be achieved [26]. AIDR 3D images are created by blending the final iterative images with the initial image. It is based on a CT model and a statistical noise model. The initial high noise image undergoes a number of iterations leading to an image with reduced noise. The vendor declares that a dose reduction of 75 % or more is possible based on the assumption that noise in CT images is inversely proportional to the square root of the applied dose [7]. More recently, a model-based iterative reconstruction (MBIR) technique, marketed under the commercial name of VEO, has been developed. Unlike ASIR, VEO is a fully iterative method working in the raw data domain which models not only the data statistics but also the geometry of the scanner itself by considering the voxel volumes of the scanned object, the focal spot size, the active area size of the detector, etc. However, although VEO seems to mark a major breakthrough in CT reconstruction, it requires a high computational power leading to a reconstruction time as long as 30 min ~1 h, and is therefore not yet suitable for all clinical situations [22].
Our study has some limitations. First, our study design was retrospective and nonrandomised and was comprised of a small number of patients seen at a single medical centre. Multicentre prospective clinical trials will be needed to validate our data. Second, we did not investigate the performance of iDose in terms of image quality improvement depending on the patients’ body habitus and using the body mass index (BMI). Further studies will be needed to determine whether hybrid IR is also effective in obese patients in whom CT image quality is typically worse. Third, as lesion conspicuity was assessed using hepatic metastases seen on the portal phase, we did not evaluate arterial-phase image quality on the hybrid IR algorithm. Therefore, the lesion conspicuity of hypervascular tumours, such as hepatocellular carcinomas, should be evaluated in future studies. Lastly, as there was no comparison between our hybrid IR and other dose-saving techniques, it was not evident whether potential dose savings using hybrid IR would be associated with a greater clinical benefit than other techniques.
In conclusion, the hybrid IR technique, including noise and anatomical spaces, yields a higher SNR and CNR than FBP without losing its natural image appearance when applied with intermediate strength of reconstruction.
References
Brenner DJ, Hall EJ (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med 357:2277–2284
(2007) CT market summary report. IMV Medical Information Division, Des Plaines
Berrington de Gonzalez A, Mahesh M, Kim KP et al (2009) Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med 169:2071–2077. doi:10.1001/archinternmed.2009.440
Nakayama Y, Awai K, Funama Y et al (2005) Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology 237:945–951
Sagara Y, Hara AK, Pavlicek W et al (2010) Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am J Roentgenol 195:713–719. doi:10.2214/AJR.09.2989
Hara AK, Paden RG, Silva AC et al (2009) Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol 193:764–771. doi:10.2214/AJR.09.2397
Gervaise A, Osemont B, Lecocq S, Noel A, Micard E, Felblinger J, Blum A (2012) CT image quality improvement using adaptive iterative dose reduction with wide-volume acquisition on 320-detector CT. Eur Radiol 22(2):295–301. doi:10.1007/s00330-011-2271-7
Utsunomiya D, Weigold WG, Weissman G, Taylor AJ (2012) Effect of hybrid iterative reconstruction technique on quantitative and qualitative image analysis at 256-slice prospective gating cardiac CT. Eur Radiol 22:1287–1294. doi:10.1007/s00330-011-2361-6
Leipsic J, Labounty TM, Heilbron B et al (2010) Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 195:649–654. doi:10.2214/AJR.10.4285
Noel PB, Fingerle AA, Renger B et al (2011) Initial performance characterization of a clinical noise-suppressing reconstruction algorithm for MDCT. AJR Am J Roentgenol 197:1404–1409. doi:10.2214/AJR.11.6907
Scibelli A (2011) iDose4 iterative reconstruction technique. Philips Healthcare Website. http://clinical.netforum.healthcare.philips.com/global/Explore/White-Papers/CT/iDose4-iterative-reconstruction-technique
European Commission (2000) EUR 16262 EN European guidelines on quality criteria for computed tomography. European Commission, Luxembourg. http://www.drs.dk/guidelines/ct/quality/
Singh S, Kalra MK, Moore MA et al (2009) Dose reduction and compliance with pediatric CT protocols adapted to patient size, clinical indication, and number of prior studies. Radiology 252:200–208
Marin D, Nelson RC, Schindera ST et al (2010) Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm—initial clinical experience. Radiology 254:145–153. doi:10.1148/radiol.09090094
Awai K, Takada K, Onishi H, Hori S (2002) Aortic and hepatic enhancement and tumor-to-liver contrast: analysis of the effect of different concentrations of contrast material at multi-detector row helical CT. Radiology 224(3):757–763
Spielmann AL, Nelson RC, Lowry CR et al (2002) Liver: single breath-hold dynamic subtraction CT with multi-detector row helical technology feasibility study. Radiology 222:278–283
Schindera S, Nelson R, Yoshizumi T et al (2009) Effect of automatic tube current modulation on radiation dose and image quality for low tube voltage multidetector row CT angiography phantom study. Acad Radiol 16:997–1002
Silva AC, Lawder HJ, Hara A et al (2010) Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am J Roentgenol 194:191–199. doi:10.2214/AJR.09.2953
Ghetti C, Ortenzia O, Serreli G (2012) CT iterative reconstruction in image space: a phantom study. Phys Med 28:161–165. doi:10.1016/j.ejmp.2011.03.003
Pontana F, Duhamel A, Pagniez J et al (2011) Chest computed tomography using iterative reconstruction vs filtered back projection (part 2): image quality of low-dose CT examinations in 80 patients. Eur Radiol 21:636–643. doi:10.1007/s00330-010-1991-4
Pontana F, Pagniez J, Flohr T, Faivre JB, Duhamel A, Remy J, Remy-Jardin M (2011) Chest computed tomography using iterative reconstruction vs filtered back projection (part 1): evaluation of image noise reduction in 32 patients. Eur Radiol 21(3):627–635. doi:10.1007/s00330-010-1990-5
Mieville FA, Gudinchet F, Brunelle F et al (2013) Iterative reconstruction methods in two different MDCT scanners: physical metrics and 4-alternative forced-choice detectability experiments—a phantom approach. Phys Med 29:99–110. doi:10.1016/j.ejmp.2011.12.004
Fleischmann D, Boas FE (2011) Computed tomography—old ideas and new technology. Eur Radiol 21:510–517. doi:10.1007/s00330-011-2056-z
Funama Y, Taguchi K, Utsunomiya D et al (2011) Combination of a low-tube-voltage technique with hybrid iterative reconstruction (iDose) algorithm at coronary computed tomographic angiography. J Comput Assist Tomogr 35:480–485
Boedeker KL, McNitt-Gray MF (2007) Application of the noise power spectrum in modern diagnostic MDCT: part II. Noise power spectra and signal to noise. Phys Med Biol 52:4047–4061. doi:10.1088/0031-9155/52/14/003
Grant KRR (2012) SAFIRE: sinogram affirmed iterative reconstruction. siemens medical solutions whitepaper. http://usa.healthcare.siemens.com/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/siemens_hwem-hwem_ssxa_websites-context-root/wcm/idc/groups/public/@us/@imaging/@ct/documents/download/mdaw/ndq2/~edisp/safire-00308312.pdf
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, J.S., Lee, J.M., Sohn, J.Y. et al. Hybrid iterative reconstruction technique for liver CT scans for image noise reduction and image quality improvement: evaluation of the optimal iterative reconstruction strengths. Radiol med 120, 259–267 (2015). https://doi.org/10.1007/s11547-014-0441-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-014-0441-9