Abstract
Purpose
Few studies have correlated computed tomography enterography (CTE) findings with Crohn’s disease (CD) clinical and biochemical activity. The aim of this study was to evaluate correlations between CTE findings with CD activity.
Materials and methods
The CTE datasets from 62 patients were retrospectively reviewed for different parameters: bowel wall thickening and hyperenhancement, mesenteric alterations, abdominal free fluid and complications related to the disease (fistulas, strictures, abscesses). Activity was assessed using the Crohn’s Disease Activity Index (CDAI) and some biochemical markers (C-reactive protein, erythrocyte sedimentation rate, alpha 2-globulins, fibrinogen, platelets, haemoglobin). Correlations between CTE parameters, clinical activity score and laboratory parameters were assessed by logistic regression.
Results
CDAI was significantly correlated with increased fat density (p = 0.03) and intestinal strictures (p = 0.04). Platelet counts were elevated in patients with enlarged mesenteric lymph nodes (p = 0.009) and the comb sign (p = 0.05). Serum alpha 2-globulins were higher in the presence of the comb sign (p = 0.03).
Conclusion
The CTE finding of perienteric inflammation (increased fat density) and vascular engorgement of the vasa recta in CD patients suggest that the disease is clinically active and that these patients may require more aggressive treatment than patients without these findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessment of inflammatory activity in Crohn’s disease (CD) has become crucial to drive therapeutic choices and monitor their effect [1]. In the past, this evaluation was based on clinical indexes, mainly the Crohn’s Disease Activity Index (CDAI), integrated by laboratory parameters including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and orosomucoids, and endoscopic findings [2, 3]. Indeed, endoscopy can assess only mucosal inflammation, which, in a transmural disease like CD, might not be enough [4].
Recently, a few papers have suggested the role of radiological techniques such as computed tomography enteroclysis or enterography (CTE) and magnetic resonance enteroclysis or enterography (MRE) not only in detecting extramural complications but also in the evaluation of disease activity [5–9].
The CTE finding of either enteric signs such as mural stratification and hyperenhancement or perienteric signs like mesenteric hypertrophy or engorged vasa recta, the so-called “comb sign”, has been found to correlate either with endoscopic or histological inflammation or with acute-phase proteins, with sometimes discrepant results [6, 7, 10–12]. The importance of recognising the different patterns of disease, especially early in the disease course, may help in tailoring specific therapies, predicting future complications, and possibly offering better timing of surgical interventions [13]. A good classification of the patient is of primary importance, and sometimes a clinical suspicion is not confirmed on more invasive investigation [14].
In this context, CTE today appears as a valid technique for the detection of disease complications, and thus a valid alternative for disease staging. It seems to be a very powerful technique which can be performed quickly in most radiological departments, even though some concerns have been raised regarding radiation exposure [15, 16]. In this respect, correlating CD clinical activity with the CTE findings could help to select patients to be referred for CTE, reducing unnecessary radiation exposure [9].
However, the relationships between the CTE findings of bowel inflammatory activity, and clinical and serum biochemical markers of inflammation have not yet been thoroughly investigated. The aim of this study was to correlate the CTE findings with CD clinical activity, assessed by CDAI, and biochemical activity, assessed by a comprehensive panel of markers including ESR, CRP, alpha 2-globulins, fibrinogen, platelets and haemoglobin.
Materials and methods
Patients
We retrospectively reviewed all the clinical records of CD patients followed up in our inflammatory bowel disease clinic (a tertiary referral centre for gastroenterological disease), between September 2008 and September 2011. Patients were included if they had a definite diagnosis of small bowel CD based on clinical, endoscopic and histological findings [17] and had been subjected to CTE, ordered as part of routine clinical workup by experienced gastroenterologists for suspicion of strictures or abscesses. Exclusion criteria, routinely applied at our department for this type of studies, were age younger than 18 years, renal insufficiency (serum creatinine >2 mg/dL), pregnancy, intestinal obstruction and documented allergic reaction to iodinated contrast material. The study population consisted of 62 patients (33 male; median age 40 years; range 20–66 years; median duration of disease 52 months; range 1–468 months) with CD. At the time of CTE examination, 61.3 % of patients were treated with steroids and mesalazine, 12.9 % were on conventional immunomodulators (azathioprine or methotrexate), 14.5 % were receiving anti-TNF agents (infliximab or adalimumab) and 12.9 % were on aminosalicylates alone. The demographic and clinical characteristics of patients enrolled in the study are reported in Table 1. Clinical activity was assessed using the CDAI. Biochemical activity was evaluated with clinical data and biochemical data, obtained within 2 weeks before CTE (ESR, CRP, complete blood count, serum alpha 2-globulins and fibrinogen). In 40 patients CDAI was higher than 150, while in the remaining 22 CDAI was <150, so the disease was considered in remission. All patients enrolled were asked to sign an informed consent form for the processing of their personal data in accordance with the law, and each patient had also read an informative report about the tutelage of the personal data, in accordance with the same law. Statistical analyses were done after removing personal data.
CTE technique
Prior to the examination, all patients underwent an intestinal preparation according to the following plan: a diet free of fruit and vegetables for at least 3 days before the examination; a semiliquid diet and 2 L of water and macrogol 4,000 solution (Selg-Esse 1000, PROMEFARM, Milan, Italy) the day before the examination. This was necessary to eliminate residual faeces, avoid reflux into the small bowel and ensure a faster transit of the negative intraluminal contrast agent. The patients obtained adequate small bowel distension with oral administration of 2 L of iso-osmotic polyethylene glycol (PEG) solution within 60 min prior to scanning. PEG was chosen for its no osmotic effects and for its pleasant, sweet taste, which makes it more acceptable to patients. To minimise potential artefacts due to peristaltic bowel movement, to obtain homogeneous small bowel distension and to reduce abdominal discomfort, all patients underwent intravenous (IV) administration of an anticholinergic compound, 20 mg of N-butyl-joscine bromide (Buscopan, Boehringer Ingelheim, Reggello, Florence, Italy) 10 min before the CT scan [18]. All patients were studied with a 64-slice MDCT scanner (Brilliance 64, Philips Medical System, Cleveland, Ohio, USA) using the following scan parameters: collimation, 64 × 0.625 mm; gantry rotation time, 420 ms; slice thickness, 1.5 mm; slice increment, 0.7 mm, 140 kV, 250 mAs. We used the CT scanner’s automatic dose reduction method for all the examinations. The dose reduction software allowed modulation of the mAs parameter during the scan using a maximum of 250 mAs with a dose–length product mean of 730 mGy*cm with a range of 678–923 mGy*cm [19–21]. The examinations were performed 50 s after the IV administration of 1.5 mL/kg of iodinated nonionic contrast medium (iodixanol, Visipaque 320, GE Healthcare S.r.l. Milan, Italy) at a mean flow rate of 3 mL/s [18]. The CTE scan was performed with the patient in the supine position, and the scanning volume was acquired from the diaphragm to the perineum during a single breath-hold.
Visual image evaluation
Acquired data were analysed on an advanced computer workstation where, besides the axial images, multiplanar, maximum intensity projection and volume rendering reconstructions were visualised. CT images were analysed in random order and were reviewed by two experienced gastrointestinal radiologists, blinded to all endoscopic and clinical data to ensure objective interpretation of image findings. To evaluate intraobserver variability, images were blindly re-analysed by the same observer after 2 weeks. To evaluate interobserver variability, all the images were presented in random order to both observers, who were unaware of each other’s results. In each patient we assessed site and number of abnormal bowel segments, bowel wall thickening (BWT), mural hyperenhancement, mural stratification and mesenteric alterations (Table 2). BWT was defined as pathological if ≥3 mm on an adequately distended loop [22]. Mural hyperenhancement was defined as segmental enhancement in all or part (in the case of mural stratification) of the small bowel wall, greater than in the adjacent small bowel loops. Each of these bowel wall characteristics was rated as present or absent. Mesenteric alterations included fibro-fatty proliferation (known as “creeping fat” of the mesentery), increased fat density, hypervascularity (consisting of the presence of the “comb sign”) and lymph nodes. Fibro-fatty proliferation refers to fatty deposition along the mesenteric border of bowel segments affected by CD; it was defined as a focally increased and inhomogeneous fluid attenuation in the mesenteric fat [+20/+60 Hounsfield units (HU)], compared with the appearance of subcutaneous fat or perienteric fat adjacent to noninflamed bowel loops [23]. Increased fat density refers to fluid density in the fat surrounding thickened or abnormally enhancing bowel, resulting from inflammatory infiltration of the perienteric adipose tissue. The comb sign refers to hypervascularity of the involved mesentery because of the presence of dilated and tortuous vasa recta that penetrate the bowel wall perpendicular to the bowel lumen, mimicking the appearance of a comb [24]. Mesenteric lymph nodes located near the affected intestinal segments were considered pathological if their transverse diameter was >15 mm [25]. Each of these mesenteric effects were graded along a three-point scale as not present (0), definitely present to a mild-moderate (1) or a severe (2) degree.
Finally, the presence of abdominal free fluid and other complications related to the disease (fistulas, strictures, abscesses) were assessed. All the CTE features are summarised on Table 2.
Statistical analysis
All continuous variables were described as median and range, while categorical variables were expressed as frequency and percentage. The patients were grouped according to the clinical activity of disease: inactive (CDAI <150); mild activity (CDAI 150–220); moderate activity (CDAI 220–450). To explore univariate associations in the distribution of categorical data, the Chi-squared test or Fisher’s exact test was used, as appropriate. Differences in mean continuous variables between groups of patients were analysed by the t test. A p value <0.05 was considered statistically significant. Multiple linear regression analysis was performed to identify independent predictors of CTE findings as continuous dependent variables in all groups of patients. Variables found to be associated with the dependent variable at univariate analyses were included in multivariate regression models. Multiple logistic regression models were used to assess the relationship of CTE findings to demographic, clinical and laboratory characteristics of CD patients. Regression analyses were performed using PROC LOGISTIC, PROC REG, and subroutines in Statistical Analysis Software (SAS Institute, Inc., Cary, NC).
Results
According to the Montreal classification [26], CD patients presented the following disease location: L1, 32.3 %; L2, 6.5 %; L3, 58.0 % and L4, 3.2 %. 21 % had nonstricturing nonpenetrating disease, 38 % stricturing, 9 % penetrating, 16 % both stricturing and penetrating disease and ten patients (16 %) had perianal involvement. In 40 patients, CDAI was higher than 150, while in the remaining 22 CDAI was <150, so the disease was considered in remission.
Twenty patients (32.2 %) showed intestinal strictures (Fig. 1); 19 of them also showed bowel wall hyperenhancement and comb sign, and were therefore classified as inflammatory stenosis (Figs. 2, 3). Other signs were fistulas in 22 (35.5 %, Fig. 4) and abscesses in 12 (19.3 %, Fig. 5). All the CTE findings are summarised in Table 2, while the association between CTE findings according to CD clinical activity is summarised in Table 3. Univariate analysis showed that the presence of increased fat density (p = 0.008) and intestinal strictures (p = 0.03) were significantly associated with CDAI score above 150 (Table 3). Furthermore, the presence of abdominal free fluids was marginally related to clinical disease activity (p = 0.054). Based on the multivariate linear logistic regression model, the increased fat density [p = 0.03, odds ratio (OR) 2.5, 95 % confidence interval (CI) 1.057–5.923] and intestinal strictures (p = 0.04, OR 3.0, 95 % CI 1.042–8.613) were significantly and independently associated with an elevated CDAI score (Figs. 2, 6). Univariate analysis showed that the presence of enlarged mesenteric lymph nodes (p = 0.009) and comb sign (p = 0.05) were significantly associated with a high platelet count (Table 4). Furthermore, the serum alpha 2-globulins were the only serum biomarker significantly and independently associated with the presence of the comb sign (p = 0.03, OR 13.9, 95 % CI 1.158–168.491).
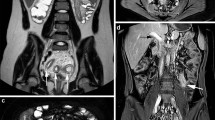
Female patient with mild Crohn’s disease activity (CDAI 320). Axial and coronal contrast-enhanced images of computed tomography enterography show increased fat density and the comb sign with nodal involvement (arrow), and mural hyperenhancement of the small bowel wall with an inflammatory pseudopolyp (arrowhead)
The correlation between the two observers in the visualisation of Crohn’s disease bowel manifestations was found to be 98.4 %, with only one case of disagreement. Intraobserver variability did not show a significant difference.
Discussion
In referral centres, CTE has outnumbered barium examinations to assist in Crohn’s disease diagnosis [23, 27]. CTE has been reported to influence disease management in 28 % of cases, mainly due to detection of complications and assessment of disease activity [27]. Moreover, radiological findings are mostly relevant for assessing the “damage score” as well as mucosal and histological healing [28]. CTE provides both anatomical and practical advantages: depiction of the entire bowel wall, multiplanar imaging and no obscuration of small bowel loops due to superimposition, and better evaluation of both luminal and extramural manifestations [29]; in addition, it is not an operator-dependent technique [30]. From the patient’s perspective, CTE has the advantage of being less invasive because it does not require intubation or uncomfortable palpation, it only requires getting on and off the table once, and it is generally faster. CTE adds useful information to the clinical assessment providing definition of extraluminal involvement and ensuring stricture detection and characterisation.
Our study shows that a CTE finding of increased fat density has a good correlation with the CDAI score. Although the CDAI has been criticised because of its subjectivity and interobserver variability, it remains the most widely used index for evaluating outcome in clinical trials on Crohn’s disease [31]. Increase in fat density is a highly specific marker of active CD, reflecting the presence of inflammatory infiltrate within the perienteric fat due to mesenteric adipocytes which are supposed to play a major role in the CD inflammatory cascade [32]. Compared to fibro-fatty proliferation, the increased fat density is an earlier change and is also more specific than bowel wall thickening, which could give false-positive findings in the presence of underdistended bowel loops.
Conflicting data have appeared in the medical literature concerning the relationship between radiological signs of inflammation (approached by either CTE or MRE) and disease activity in CD. Maccioni et al. [33] reported a significant correlation between MR signs of intestinal inflammation (oedema in the perienteric fat, wall thickening, contrast enhancement) with a biological activity score which included white blood cells, orosomucoid levels and CRP in 20 CD patients. These results were not confirmed by other authors [34–36]. Few data exist about the relationship between signs of perienteric inflammation, such as those explored in our study, with clinical or biochemical indices of activity. Lee et al. [37] first described the association of prominent perienteric or pericolic vasculature and active CD. Colombel et al. [4] found a significant relationship between radiological findings of perienteric inflammation expressed by increased fat density and CRP. Minordi et al. [8] evaluated both mural signs (parietal thickness, target sign or alternating rings of low and high density in the bowel wall) and extraenteric inflammation. There was a positive correlation between the target sign and fibro-fatty proliferation with the CDAI and among wall thickness, the comb sign and perienteric stranding with CRP. When we explored the relationship between the biomarkers and the CTE findings, we found a correlation of alpha 2-globulins and platelet count with vascular extraluminal findings (comb sign). Alpha 2-globulins are a fraction of serum proteins containing orosomucoids, in particular alpha 1-acid glycoprotein, which, though less commonly used in clinical practice, had been shown to have a better correlation with CDAI than CRP [38]. The relationship with the comb sign, which is an expression of more advanced and extensive CD, could suggest the presence of prolonged active disease. These results may provide new insight into the meaning and clinical implications of elevated expression of these markers in Crohn’s patients. Many studies have connected vascular inflammation to the pathogenesis of inflammatory bowel disease [39, 40]. CTE may offer a noninvasive way to detect vascular abnormalities in Crohn’s disease that was not previously available. There are some discrepancies between our results and those of previous studies, which could be explained by differences in sample size, patient recruitment and referral and heterogeneity of technical approach. Minordi et al. [8] used both enterography and enteroclysis in their study, whereas Colombel et al. [4] enrolled patients undergoing colonoscopy and the primary endpoint of the study was to correlate CTE with endoscopy and serum biomarkers and not with clinical activity. Our case series has the advantage of being totally homogenous since all patients are referred to the radiology department by a single gastroenterology team dedicated to the care of IBD patients and the technical approach was uniform. Our study confirms the role of CTE as a useful tool not only in the diagnostic workup of suspected CD but also in the evaluation of disease activity, which we believe is an integrated process which involves clinical symptoms and signs, serum acute-phase reactants and endoscopic findings.
To our knowledge, few studies in the literature have correlated CDAI, biochemical activity and CTE signs with attention focused also on nodal involvement and abdominal free fluid. The major limitation of the study pertains to the CT technique and is the use of ionising radiation that we tried to limit by using dose reduction software. In conclusion, CTE might help to better address and monitor response to therapy, for example, to define the so-called “window of opportunity” for biologics, and this aspect deserves further studies since it has not been evaluated in the present study or in the literature. However, to prove the value of CTE in the clinical assessment of Crohn’s disease, we need prospective studies that can determine whether the informations gained from CTE actually change clinical decision-making and clinical outcomes.
References
Biancone L, De Nigris F, Del Vecchio Blanco G et al (2002) Monitoring the activity of Crohn’s disease. Aliment Pharmacol Ther 16:29–33
Sandborn WJ, Feagan BG, Hanauer S et al (2002) A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology 122:512–530
Sostegni R, Daperno M, Scaglione N et al (2003) Review article: Crohn’s disease: monitoring disease activity. Aliment Pharmacol Ther 17:11–17
Colombel JF, Solem CA, Sandborn WJ et al (2006) Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut 55:1561–1567
Choi D, Jin Lee S, Ah Cho Y et al (2003) Bowel wall thickening in patients with Crohn’s disease: CT patterns and correlation with inflammatory activity. Clin Radiol 58:68–74
Del Campo L, Arribas I, Valbuena M et al (2001) Spiral CT findings in active and remission phases in patients with Crohn disease. J Comput Assist Tomogr 25:792–797
Gourtsoyiannis N, Papanikolaou N, Grammatikakis J et al (2004) Assessment of Crohn’s disease activity in the small bowel with MR and conventional enteroclysis: preliminary results. Eur Radiol 14:1017–1024
Minordi LM, Vecchioli A, Guidi L et al (2009) CT findings and clinical activity in Crohn’s disease. Clin Imaging 33:123–129
Desmond AN, O’Regan K, Malik N et al (2012) Selection of symptomatic patients with Crohn’s disease for abdominopelvic computed tomography: role of serum C-reactive protein. Clin Gastroenterol Hepatol 10:886–892
Bodily KD, Fletcher JG, Solem C et al (2006) Crohn disease: mural attenuation and thickness at contrast-enhanced CT enterography-correlation with endoscopic and histologic findings of inflammation. Radiology 238:505–516
Booya F, Fletcher JG, Huprich JE et al (2006) Active Crohn disease: CT findings and interobserver agreement for enteric phase CT enterography. Radiology 241:787–795
Meyers MA, McGuire PV (1995) Spiral CT demonstration of hypervascularity in Crohn disease: "vascular jejunization of the ileum" or the "comb sign". Abdom Imaging 20:327–332
Al-Hawary MM, Kaza RK, Platt JF (2013) CT enterography: concepts and advances in Crohn’s disease imaging. Radiol Clin N Am 51:1–16
Higgins PD, Caoili E, Zimmermann M et al (2007) Computed tomographic enterography adds information to clinical management in small bowel Crohn’s disease. Inflamm Bowel Dis 13:262–268
Lee SS, Kim AY, Yang SK et al (2009) Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology 251:751–761
Kroeker KI, Lam S, Birchall I, Fedorak RN (2011) Patients with IBD are exposed to high levels of ionizing radiation through CT scan diagnostic imaging: a five-year study. J Clin Gastroenterol 45(1):34–39
Hanauer SB, Sandborn WJ (2007) European evidence-based consensus on the diagnosis and management of Crohn’s disease. Gut 56:161–163
Lo Re G, Galia M, Bartolotta TV et al (2007) Forty-slice TCMD enteroclysis: evaluation after oral administration of isotonic solution in Crohn’s disease. Radiol Med 112:787–797
McCollough CH, Bruesewitz MR, Kofler JM Jr (2006) CT dose reduction and dose management tools: overview of available options. Radiographics 26:503–512
Huda W, Mettler FA (2011) Volume CT dose index and dose–length product displayed during CT: what good are they? Radiology 258:236–242
Al-Hawary MM, Zimmermann EM (2010) Choosing the right cross-sectional imaging technique: trading image quality for radiation risk. Inflamm Bowel Dis 17:1089–1091
Horsthuis K, Bipat S, Bennink RJ (2008) Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: metaanalysis of prospective studies. Radiology 247:64–79
Goldberg HI, Gore RM, Margulis AR et al (1983) Computed tomography in the evaluation of Crohn disease. AJR Am J Roentgenol 140:277–282
Madureira AJ (2004) The comb sign. Radiology 230:783–784
Lucey BC, Stuhlfaut JW, Soto JA (2005) Mesenteric lymph nodes: detection and significance on MDCT. Radiographics 25:351–365
Silverberg MS, Satsangi J, Ahmad T et al (2005) Toward and integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 19:5–36
Fishman EK, Wolf EJ, Jones B et al (1987) CT evaluation of Crohn’s disease: effect on patient management. AJR Am J Roentgenol 148:537–540
Van Assche G (2009) Mucosal healing as a treatment goal in Crohn’s disease. J Gastroenterol Hepatol (NY) 5:558–559
Hara AK, Alam S, Heigh RI et al (2008) Using CT enterography to monitor Crohn’s disease activity: a preliminary study. AJR Am J Roentgenol 190:1512–1516
Pariente B, Peyrin-Biroulet L, Cohen L et al (2011) Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. AJR Am J Roentgenol 197:42–49
Van Assche G, Dignass A, Panes J et al (2010) The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis 4:7–27
Desreumaux P, Ernst O, Geboes K et al (1999) Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 117:73–81
Maccioni F, Viscido A, Broglia L et al (2000) Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging 25:219–228
Neurath MF, Vehling D, Schunk K et al (2002) Noninvasive assessment of Crohn’s disease activity: a comparison of 18F-fluorodeoxyglucose positron emission tomography, hydromagnetic resonance imaging, and granulocyte scintigraphy with labeled antibodies. Am J Gastroenterol 97:1978–1985
Schunk K, Kern A, Oberholzer K et al (2000) Hydro-MRI in Crohn’s disease: appraisal of disease activity. Invest Radiol 35:431–437
Solem CA, Loftus EV Jr, Tremaine WJ et al (2005) Correlation of C-reactive protein with clinical, endoscopic, histologic and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 11:707–712
Lee SS, Ha HK, Yang SK et al (2002) CT of prominent pericolic or perienteric vasculature in patients with Crohn’s disease: correlation with clinical disease activity and findings on barium studies. AJR Am J Roentgenol 179:1029–1036
Brignola C, Campieri M, Bazzocchi G et al (1986) A laboratory index for predicting relapse in asymptomatic patients with Crohn’s disease. Gastroenterology 91:1490–1494
Hatoum OA, Binion DG (2005) The vasculature and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflamm Bowel Dis 11:304–313
Hatoum OA, Binion DG, Otterson MF et al (2003) Acquired microvascular dysfunction in inflammatory bowel disease: loss of nitric oxide-mediated vasodilation. Gastroenterology 125:58–69
Conflict of interest
Giuseppe Lo Re, Maria Cappello, Chiara Tudisca, Massimo Galia, Claudia Randazzo, Antonio Craxì, Calogero Cammà, Andrea Giovagnoni, Massimo Midiri declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo Re, G., Cappello, M., Tudisca, C. et al. CT enterography as a powerful tool for the evaluation of inflammatory activity in Crohn’s disease: relationship of CT findings with CDAI and acute-phase reactants. Radiol med 119, 658–666 (2014). https://doi.org/10.1007/s11547-013-0377-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-013-0377-5