Abstract
Objective
The authors sought to evaluate the diagnostic accuracy of high-resolution computed tomography (HRCT) in the detection of pulmonary veno-occlusive disease (PVOD) in patients with pre-capillary pulmonary arterial hypertension (PAH) of unknown aetiology, and to identify the role of CT in diagnosis and therapy.
Materials and methods
The CT scans of 96 patients were retrospectively reviewed and assessed for specific HRCT findings: ground-glass opacities, septal lines and mediastinal lymph nodal enlargement (short diameter ≥1 cm). According to the HRCT findings, patients were divided into PVOD-suspicious and not PVOD-suspicious. Subsequently, a clinical-instrumental evaluation was performed, and the response to therapy and histopathological reports were evaluated.
Results
Radiological evaluation based on HRCT findings revealed 29 patients as PVOD-suspicious and 67 as not PVOD-suspicious. The final diagnosis was PVOD in 22 patients and idiopathic PAH in 74 patients. The CT scan showed 95.5 % sensitivity, 89 % specificity, 72.5 % positive predictive value, and 98.5 % negative predictive value, with a diagnostic accuracy of 90.5 % in identifying patients with PVOD.
Conclusions
Chest CT can be considered a screening test in the assessment of patients with PAH of unknown aetiology, and the radiologist can help the clinician to identify patients with CT findings that make PVOD highly probable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With less than 200 cases described in the literature, pulmonary veno-occlusive disease (PVOD) is a rare form of pulmonary arterial hypertension (PAH) [1] which is associated with a worse prognosis compared to idiopathic PAH (IPAH) [2, 3]. Although the aetiology has not yet been identified, several risk factors are thought to be involved including infection, chemotherapy toxicity [4], radiation, autoimmunity and genetic predisposition [5–8].
Signs and symptoms are not specific and mimic those of other disorders; they include dyspnoea, fatigue, chest pain, haemoptysis, cyanosis, pulmonary crackles on auscultation and clubbing on physical examination. Haemosiderin-laden macrophages in the bronchoalveolar lavage (BAL) fluid, low diffusion of carbon monoxide (DLCO) in pulmonary function tests as well as low partial pressure of oxygen in arterial blood (PaO2) may also be found.
The key histopathological hallmark is a widespread fibrous intimal proliferation, typically “patchy” in distribution that predominantly involves the pulmonary venules and small veins, with a gradual reduction of the vascular lumen until complete occlusion [9]; the central recanalisation of the obstructed venous vessel is frequently observed [10]. Other histopathological alterations involve the interlobular septa, lung parenchyma and pulmonary arteries. The interlobular septa are typically oedematous and contain dilated lymphatic vessels. In the lung parenchyma, patchy areas of alveolar capillary dilatation (so-called loop-like appearance) are present upstream from the narrowed and occluded veins; in these areas there is often interstitial fibrosis, haemorrhage, and intra-alveolar haemosiderin-laden macrophages, probably due to chronic passive congestion. With regard to the pulmonary arterial circulation, the muscular pulmonary arteries show medial hypertrophy, while the arterioles become muscularised secondary to post-capillary obstruction of venous drainage [11, 12].
PVOD remains a clinical entity difficult to classify; it shares some features with pre-capillary forms of PAH, but there are histopathological, clinical and therapeutic differences. For this reason, together with pulmonary capillary haemangiomatosis (PCH), PVOD has been included in a distinct category but not completely separated from IPAH, according to the latest guidelines of the European Society of Cardiology and European Respiratory Society (Table 1) [13].
As PVOD and PCH are clinically, radiologically and therapeutically indistinguishable, the differential diagnosis between these two conditions is based on histological examination, when available: the most distinctive histological feature of PCH is the proliferation of capillary channels within the alveolar walls, with secondary arterial alterations, typically without alterations in venules and interlobular septa [12].
Although the definitive diagnosis of PVOD is based on histology, affected individuals are often too debilitated to undergo a lung biopsy, which is an invasive procedure associated with a significant risk of mortality [14]; thus, diagnostic confirmation is usually obtained through autopsy in the event of death, or through examination of the explanted lung in the case of lung transplantation. However, in some cases, histological examination fails to provide sufficient information to discriminate between PVOD and PCH, such as when the biopsy material is insufficient and does not contain septa or when the loop-like capillary dilation and the proliferative lesions of the capillaries are indistinguishable.
For these reasons, the diagnosis is routinely established by algorithms of various investigative tests and procedures (clinical suspicion, physical examination, and radiological findings) which provide a diagnosis of high probability of PVOD [15]. Table 2 summarises the instrumental findings suggestive of PVOD.
In PVOD, the X-ray examination only shows Kerley B lines and peripheral interstitial thickening in addition to the classic signs of PAH (ectasia of the pulmonary arteries/pulmonary trunk and cardiomegaly with a prevalence of right sections).
Computed tomography (CT), especially the high-resolution study of the lung parenchyma (HRCT), is nowadays considered an integral part of the diagnostic workup of PVOD [16], owing to the detection of smooth thickening of the interlobular septa, ground-glass opacities, increased size of the mediastinal lymph nodes, pleural and pericardial effusions [17, 18] (even though the importance of pleuro-pericardial effusion is still controversial) [19].
Because of the lack of response of IPAH to drug therapy which consists in vasodilators (prostanoids, antagonists for the endothelin receptor, inhibitors of phosphodiesterase type 5) and which might even worsen the prognosis due to the onset of severe, at times fatal, pulmonary oedema, the only curative therapy for PVOD is represented by cardiopulmonary or lung transplantation [20, 21]. Therefore, a correct and timely diagnosis becomes crucial to start an adequate medical treatment.
The aim of our study was to evaluate the diagnostic accuracy of HRCT for POVD in a large group of patients with pre-capillary PAH of unknown aetiology, and assess the influence of HRCT findings in determining the diagnosis and subsequent treatment.
Materials and methods
We retrospectively evaluated the CT scans of 131 patients with pre-capillary PAH of unknown aetiology performed between 2005 and 2012, as part of the diagnostic algorithm “Pulmonary Hypertension” of our institute. Patients with CT signs of chronic pulmonary emphysema, interstitial fibrosis and left ventricular failure were excluded from the study. We finally considered a total of 96 patients (71 % women and 29 % men), aged between 19 and 86 years, with a mean age of 51 years.
CT protocol
All the examinations were performed with multidetector CT (SOMATOM Cardiac 16, Siemens, Forchheim, Germany), with the patent in the supine position and during one deep inspiratory breath-hold; they included HRCT scans for the evaluation of the lung parenchyma and a CT angiography study of the pulmonary arteries.
Standard parameters were as follows: slice thickness, 0.75 mm; reconstruction increment, 0.5 mm; 120 kV; 180 mAs; during postprocessing, 2-mm-thick sections with a reconstruction increment of 1 mm both with window for lung parenchyma and with window for the mediastinum.
The CT angiography study was performed with the bolus-tracking method (delay of 5 s from the detection of an enhancement threshold of 200 HU within a region of interest placed in the pulmonary artery), with the intravenous injection of 80 ml of nonionic iodinated contrast medium (Iomeron 400, Bracco SpA, Milan, Italy), followed by a bolus of normal saline (40 ml), infused with an automatic injector at a flow of 4 ml/s.
Image analysis
The CT images were analysed by four thoracic radiologists who evaluated the HRCT findings specific for POVD:
-
Ground-glass opacities, characterised by increased parenchymal density with preservation of bronchial and vascular structures.
-
Smooth thickening of the interlobular septa, characterised by thin linear or polygonal opacities which define pulmonary lobules.
-
Enlargement of mediastinal lymph nodes (short diameter ≥1 cm).
Findings consistent with but not specific for POVD were evaluated as well, such as dilatation of the pulmonary arteries (diameter of the common trunk >30 mm), pleural and pericardial effusion, dilatation of right heart chambers, thromboembolic phenomena and cardiac/lung malformations.
Diagnostic process
The radiological assessment divided patients into two groups based on the presence of at least two of the three abovementioned specific HRCT signs: POVD-suspicious (group 1) and not POVD-suspicious (group 2). We subsequently evaluated the results of other noninvasive clinical and instrumental investigations, such as PaO2, DLCO and the 6-minute walking test (6MWT), based on which the therapy was started. Finally, a post-therapy assessment was performed based on the development of pulmonary oedema secondary to treatment with vasodilators, and on the histological results, if available.
Statistical analysis
The significance of each HRCT finding for the diagnosis of POVD was calculated with the Fisher exact test. Sensitivity, specificity, positive predictive value, negative predictive value, diagnostic accuracy of CT and likelihood ratio were calculated using the software Openepi (www.openepi.com, copyright ‘The Open Source Initiative’).
Results
On the basis of the presence of at least two HRCT findings specific to POVD, 29 patients (8 men and 21 women) were considered POVD-suspicious (30 %, group 1), and 67 not POVD-suspicious (70 %, group 2). All patients had both a dilatation of the pulmonary arteries and right heart chambers, consistent with PAH; in no case was thromboembolism or cardiopulmonary malformations documented.
As a result of the clinical-instrumental analysis, among the 29 patients of group 1, 15 were finally classified as IPAH and therefore received treatment with vasodilators; eight patients did not present any post-therapy complications, while seven patients developed pulmonary oedema leading to a subsequent reclassification of their diagnosis from IPAH to POVD. In group 2, all 67 patients were classified as IPAH, with only one patient developing a severe pulmonary oedema after treatment and being reclassified as POVD (Fig. 1).
Diagnostic efficacy of high-resolution computed tomography (HRCT). According to the HRCT findings, patients were subdivided into pulmonary veno-occlusive disease (PVOD)-suspicious and not PVOD-suspicious. After an overall clinical evaluation and a post-therapy revaluation, a final diagnosis of PVOD was made in 22 patients and one of idiopathic pulmonary arterial hypertension (IPAH) in 74 patients
The histological diagnosis was available for 11 subjects (autopsy in one case and histological examination of the explanted lung after transplantation in 10 cases): POVD was confirmed in five, while IPAH was demonstrated in six subjects (Fig. 1). From the association of radiological, clinical-instrumental and post-treatment evaluation as well as of histological examination (when available), the final diagnosis was POVD in 22 cases and IPAH in the remaining 74.
Based on the presence of at least two specific HRCT signs, the CT evaluation showed a sensitivity of 95.5 % (95 % CI 78.2–99.2), a specificity of 89 % (95 % CI 80.1–94.4), a positive predictive value of 72.5 % (95 % CI 54.3–85.3) and negative predictive value of 98.5 % (95 % CI 92–99.7), with a diagnostic accuracy of 90.5 % (95 % CI 83.1–94.9), positive likelihood ratio of 8.83 and a negative likelihood ratio of 0.05.
Among the HRCT signs, septal thickening and ground-glass opacities demonstrated greater levels of significance (p < 0.0001) than the enlargement of mediastinal lymph nodes (p = 0.01). The presence of pleural and pericardial effusion was not statistically linked to POVD (p = 0.056 and p = 0.3).
Although the histological confirmation of POVD was available for only a small number of patients, all five of them (100 %) showed smooth thickening of the interlobular septa associated with ground-glass opacities and enlargement of the mediastinal lymph nodes (Fig. 2); in two cases pleural and pericardial effusion was also detected. In none of the six patients with a histological diagnosis of IPAH, were septal thickening or enlarged lymph nodes documented, while ground-glass opacities were observed in three cases.
Discussion and conclusions
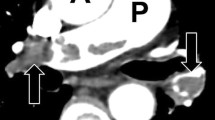
In the diagnostic classification of patients with evidence of PAH of unknown aetiology and pre-capillary pulmonary hypertension at right heart catheterisation, the early identification of those with POVD and the differential diagnosis with IPAH are crucial. In fact, if these patients are treated with combined therapies of vasodilators, which are the gold-standard in IPAH, they may develop a severe, at times fatal, pulmonary oedema [22, 23] (Fig. 3).
The findings of noninvasive instrumental investigations most suggestive of POVD are DLCO <55 % than predicted (64.3 % sensitivity, 89 % specificity) and PaO2 < 70 mmHg (with a significant difference between patients with POVD and with IPAH, 61.3 ± 17.3 versus 75.4 ± 13.8 mmHg, respectively) [24].
CT plays an important role in the diagnosis since it allows the identification of some specific signs highly suggestive of POVD. In our study, the thickening of the interlobular septa and ground-glass opacities showed the highest level of significance for POVD (p < 0.0001), in agreement with data available in the literature [17, 18], followed by mediastinal lymphadenomegalies (p = 0.01). By contrast, the presence of pleural and pericardial effusion, even though quite frequent in these patients, is not sufficiently specific for the differential diagnosis with other forms of pre-capillary PAH.
The association of at least two specific HRCT signs among septal thickening, ground glass and enlarged lymph nodes, showed a sensitivity of 95.5 % and a specificity of 89 %, with a positive predictive of 72.5 %. Moreover, thanks to the high negative predictive value of CT (98.5 %), the absence of specific HRCT signs allows the exclusion of POVD.
From the comparison between the HRCT findings and the histological examination, we found that all patients with a histological diagnosis of POVD showed smooth thickening of the interlobular septa, ground-glass opacities and enlarged lymph nodes. None of the six patients with a histological diagnosis of IPAH presented septal thickening or enlarged lymph nodes, and three of them had ground-glass only; this is further evidence of the importance of the association of the three HRCT signs for the diagnosis of POVD.
Our study has some limitations, including the small number of histological examinations and the lack of data from BAL, which is not routinely performed. In our opinion, BAL should be integrated with other radiological and clinical investigations because it is a repeatable and easy examination which allows the identification of haemosiderin-laden macrophages (frequently observed in POVD and rarely in IPAH) [25–27].
To conclude, chest CT should be considered in the screening of patients with PAH of unknown aetiology; the radiologist could cooperate with the clinician in the recognition of individuals with a suspected POVD, whose treatment is different from that of IPAH due to the risk of a fatal pulmonary oedema. According to the international guidelines, these patients should be followed in highly specialised centres and closely monitored for the early identification of possible complications due to the administration of vasodilator therapy.
References
Holcomb BW Jr, Loyd JE, Ely Ew et al (2000) Pulmonary veno-occlusive disease: a case series and new observations. Chest 118:1671–1679
Takeda Y, Yamamoto K, Tomimoto S et al (2011) Pulmonary venous occlusion and death in pulmonary arterial hypertension: survival analyses using radiographic surrogates. BMC Pulm Med 11:47. doi:10.1186/1471-2466-11-47
Mandel J, Mark EJ, Hales CA (2000) Pulmonary venoocclusive disease. Am J Respir Crit Care Med 162:1964–1973
Valmary S, Dorfmüller P, Montani D (2010) Human gamma-herpesviruses EBV and HHV-8 are not detected in the lungs of patients with severe pulmonary arterial hypertension. Chest 139:1310–1316
Swift GL, Gibbs A, Campbell IA et al (1993) Pulmonary veno-occlusive disease and Hodgkin’s lymphoma. Eur Respir J 6:596–598
Ibrahim NB, Burnley H, Gaber KA et al (2005) Segmental pulmonary veno-occlusive disease secondary to lung cancer. J Clin Pathol 58:434–436
Knight BK, Rose AG (1985) Pulmonary veno-occlusive disease after chemotherapy. Thorax 40:874–875
Ozsoyoglu A, Swartz J, Farver CF et al (2006) High-resolution computed tomographic imaging and pathologic features of pulmonary veno-occlusive disease: a review of three patients. Curr Probl Diagn Radiol 35:219–223
Eltorky MA, Headley AS, Winer-Muram H et al (1994) Pulmonary capillary hemangiomatosis: a clinicopathologic review. Ann Thorac Surg 57:772–776
Carrington CB, Liebow AA (1970) Pulmonary venoocclusive disease. Hum Pathol 1:322–324
Wagenvoort CA, Wagenvoort N, Takahashi T (1985) Pulmonary veno-occlusive disease: involvement of pulmonary arteries and review of the literature. Hum Pathol 16:1033–1041
Frazier AA, Franks TJ, Mohammed TL et al (2007) From the archives of the AFIP: pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. Radiographics 27:867–882
Galiè N, Hoeper MM, Humbert M et al (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30:2493–2537
Nawaz S, Dobersen MJ, Blount SG Jr et al (1990) Florid pulmonary veno-occlusive disease. Chest 98:1037–1039
Pietra GG, Capron F, Stewart S et al (2004) Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43:25–32
Grunig E, Barner A, Bell M et al (2011) Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with updated commentary of the Cologne consensus conference 2011. Int J Cardiol 154S:S3–S12
Montani D, Kemp K, Dorfmuller P et al (2009) Idiopathic pulmonary arterial hypertension and pulmonary veno-occlusive disease: similarities and difference. Semin Respir Crit Care Med 30:411–412
Resten A, Maitre S, Humbert M et al (2004) Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. AJR Am J Roentgenol 183:65–70
Resten A, Maître S, Capron F et al (2003) Pulmonary hypertension: CT findings in pulmonary veno-occlusive disease. J Radiol 84:1739–1745
Humbert M, Maître S, Capron F et al (1998) Pulmonary edema complicating continuous intravenous prostacyclin in pulmonary capillary hemangiomatosis. Am J Respir Crit Care Med 157:1681–1685
Masters K, Bennett S (2013) Pulmonary veno-occlusive disease: an uncommon cause of pulmonary hypertension. BMJ Case Rep. doi:10.1136/bcr-2012-007752
Montani D, Jaıs X, Price LC et al (2009) Cautious epoprostenol therapy is a safe bridge to lung transplantation in pulmonary veno-occlusive disease. Eur Respir J 34:1348–1356
O’Callaghan DS, Dorfmuller P, Jaïs X (2011) Pulmonary veno-occlusive disease: the bête noire of pulmonary hypertension in connective tissue diseases? Presse Med 40:65–78
Montani D, Achouh L, Dorfmuller P et al (2008) Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine 87:220–233
Montani D, Price LC, Dorfmuller P et al (2009) Pulmonary veno-occlusive disease. Eur Respir J 33:189–200
Montani D, O’Callaghan D, Savale L et al (2010) Pulmonary veno-occlusive disease: recent progress and current challenges. Respir Med 104:23–32
Rabiller A, Jaïs X, Hamid A (2006) Occult alveolar haemorrhage in pulmonary veno-occlusive disease. Eur Respir J 27:108–113
Conflict of interest
Giangaspare Mineo, Domenico Attinà, Martina Mughetti, Caterina Balacchi, Fiorella De Luca, Fabio Niro, Federica Ciccarese, Luigi Lovato, Vincenzo Russo, Francesco Buia, Cecilia Modolon, Alessandra Manes, Massimiliano Palazzini, Nazareno Galiè, Maurizio Zompatori declare no conflict of interest with the publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mineo, G., Attinà, D., Mughetti, M. et al. Pulmonary veno-occlusive disease: the role of CT. Radiol med 119, 667–673 (2014). https://doi.org/10.1007/s11547-013-0363-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-013-0363-y