Abstract
Background
Immuno-oncology combinations have achieved survival benefits in patients with metastatic renal cell carcinoma (mRCC).
Objective
The ARON-1 study (NCT05287464) was designed to globally collect real-world data on the use of immuno-combinations as first-line therapy for mRCC patients.
Patients and Methods
Patients aged ≥ 18 years with a cytologically and/or histologically confirmed diagnosis of mRCC treated with first-line immuno-combination therapies were retrospectively included from 47 International Institutions from 16 countries. Patients were assessed for overall survival (OS), progression-free survival (PFS), and overall clinical benefit (OCB).
Results
A total of 729 patients were included; tumor histology was clear-cell RCC in 86% of cases; 313 patients received dual immuno-oncology (IO + IO) therapy while 416 were treated with IO-tyrosine kinase inhibitor (IO + TKI) combinations. In the overall study population, the median OS and PFS were 36.5 and 15.0 months, respectively. The median OS was longer with IO+TKI compared with IO+IO therapy in the 616 patients with intermediate/poor International mRCC Database Consortium (IMDC) risk criteria (55.7 vs 29.7 months; p = 0.045). OCB was 84% for IO+TKI and 72% for IO + IO combination (p < 0.001).
Conclusions
Our study may suggest that immuno-oncology combinations are effective as first-line therapy in the mRCC real-world context, showing outcome differences between IO + IO and IO + TKI combinations in mRCC subpopulations.
Clinical Trial Registration
NCT05287464.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We showed real-world data on the use of immuno-combinations in patients with metastatic renal cell carcinoma. |
Our data seem to suggest a better outcome for patients treated with immunotherapy plus anti-angiogenic agents compared with dual immunotherapies. |
1 Introduction
Renal cell carcinoma (RCC) is one of the most frequent urinary tract tumors worldwide, and its incidence has been predicted to increase in the coming years [1, 2]. About 30% of patients present with local or distant recurrence after nephrectomy for localized disease [3]. Systemic treatment of metastatic RCC (mRCC) has been completely revolutionized by the development of immunotherapy-based combinations, which have improved the outcome and quality of life of mRCC patients [4,5,6,7,8,9,10,11,12,13].
Two distinct type of immuno-oncology (IO) combinations have been developed. The first one, defined as IO+IO, involves the use of two different immune checkpoint inhibitors, anti-programmed death (PD)-1 nivolumab and anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) ipilimumab. The second combination, defined as IO + tyrosine kinase inhibitors (TKIs), involves the use of agents directed against-PD1 (nivolumab, pembrolizumab) or its ligand PD-L1 (avelumab, atezolizumab) combined with anti-vascular endothelial growth factor (VEGF) monoclonal antibody (bevacizumab) or VEGF receptors (VEGFR)-TKIs (axitinib, lenvatinib, cabozantinib) [4,5,6,7,8,9,10,11,12,13].
Although the rate of patients experiencing progression as best response (defined as primary refractory) to immune-based combinations is significantly lower than with anti-VEGFR TKIs [14,15,16], the necessity of identifying potential factors influencing their prognosis still represents a hot topic for uro-oncologists.
Currently, the choice of the best combination is mainly based on patients’ clinical and histological characteristics and, even more, on clinicians’ experience. In this scenario, real-world data may offer a crucial contribution to guide the decision-making process in patients with mRCC [17,18,19]. The ARON project has been designed to create a global network to allow uro-oncologists to share and discuss their experiences on the use of immunotherapy and other emerging drugs for patients with genitourinary tumors. Specifically, the ARON-1 study (ClinicalTrials.gov identifier NCT05287464) was designed to globally collect real-world data on the use of immuno-oncologycombinations as first-line therapy for mRCC.
2 Patients and Methods
2.1 Study Population
The ARON-1 study (NCT05287464) retrospectively collected data from patients aged ≥ 18 years with a cytologically and/or histologically confirmed diagnosis of mRCC treated with first-line immuno-combination therapies.
The ARON-1 study collected data of patients treated from January 1, 2016 to July1, 2022 in 47 International Institutions from 16 countries. Clinical data and laboratory parameters from patients’ paper and electronic charts were collected. The study population included adults with clear-cell RCC (ccRCC) or non-clear-cell RCC (nccRCC). Data on histology, nephrectomy status, International mRCC Database Consortium (IMDC) criteria, sites of metastases, type of immuno-combination, and response to therapy were retrospectively collected. Patients without enough data on tumor assessment or response to therapy were excluded from our study.
Follow-up was usually carried out by means of physical examination and laboratory tests every 4–6 weeks, while imaging was performed following standard local procedures every 8–12 weeks.
2.2 Study Endpoints
Disease status was evaluated using standard RECIST 1.1 criteria [20]. Overall survival (OS) was calculated from the start of first-line immuno-oncology combination until death. Progression-free survival (PFS) was defined as the time from the start of treatment to progression or death from any cause, whichever occurred first. Patients without tumor progression or death or lost to follow-up at the time of the analysis were censored at the last follow-up visit. Data on tumor response (complete [CR] or partial responses [PR], stable [SD] or progressive disease [PD]) were collected and analyzed.
2.3 Statistical Analysis
The Kaplan-Meier method with Rothman’s 95% confidence intervals (CI) was used to estimate the survival curves of both OS and PFS. Comparisons were performed using the log-rank test. Univariate and multivariate analyses were carried out with Cox proportional hazard models. A survival receiver operating characteristic (ROC) analysis was adopted to identify potential cut-offs that better stratify patients into risk groups. The chi-square test was used to compare categorical endpoints. Differences were considered statistically significant when the p value was <0.05, and all p values were two-sided. The statistical analysis was performed by MedCalc version 19.6.4 (MedCalc Software, Broekstraat 52, 9030 Mariakerke, Belgium).
The research was carried out in accordance with approval from the ethics committee of the Marche Region (2021-492) and was performed in accordance with the Declaration of Helsinki.
3 Results
3.1 Study Population
Seven hundred and twenty-nine patients were included in our analysis. The median follow-up time was 18.1 months (95% CI 14.4−67.8); 540 patients (74%) were male. The median age was 63 years (range 25−88). Tumor histology was predominantly ccRCC (625, 86%); among the 104 nccRCC patients, histology showed a papillary type I or II RCC in 28 cases and chromophobe RCC in 11 cases (Table 1); sarcomatoid differentiation was observed in 117 patients (16%).
Lung (70%), lymph nodes (51%), and bone (34%) were the most common sites of metastasis. Basing on IMDC criteria, 113 patients (16%) were at favorable risk, 425 (58%) at intermediate risk, and 191 (26%) at poor risk. Patients’ characteristics are summarized in Table 1. No significant differences were found in terms of baseline clinico-pathological features between patients receiving IO + IO and those treated with IO + TKI, except for a higher proportion of patients with lung metastases treated with nivolumab and ipilimumab and for a different IMDC group stratification, related to the fact that the IO + IO combination was approved only for intermediate- and poor-risk RCC patients (Table 1).
Nivolumab and ipilimumab comprised first-line therapy in 313 patients (43%), while 416 patients (57%) received IO + TKI combinations; by the time of analysis, 101 (32%) and 60 patients (14%), respectively, treated with IO + IO or IO + TKI had died at the time of analysis.
3.2 Survival Analysis
In the overall study population, the median OS was 36.5 months (95% CI 24.8−60.8). One hundred and sixty-one patients (22%) were dead at the time of analysis. Male patients showed longer median OS than females, although the difference was not statistically significant (55.8 vs 28.4; p = 0.104, Fig. 1). Furthermore, no statistically significant differences were observed between patients aged ≥ 70 years and < 70 years (28.1 months, 95% CI 25.9−60.8, vs 41.0 months, 95% CI 32.7−55.7; p = 0.117, Fig. 1).
In patients with good-, intermediate- and poor-risk criteria, the median OS was not reached (NR, 95% CI NR−NR), 55.7 months (95% CI 28.4−60.8), and 19.2 months (95% CI 12.5−32.7), respectively (p < 0.001, Fig. 1). Previous nephrectomy was associated with median longer OS (55.7 months, 95% CI 41.0−60.8, vs 18.4 months, 95% CI 16.2−28.1; p < 0.001, Fig. 1).
Patients with ccRCC showed longer median OS compared with those with nccRCC histology (41.0 months, 95% CI 29.7−60.8, vs 18.0 months, 95% CI 12.6−36.5; p = 0.005, Fig. 1). Of note, the presence of sarcomatoid differentiation was correlated with shorter median OS (26.4 months, 95% CI 20.0−41.0, vs 36.5 months, 95% CI 28.4−60.8; p = 0.014, Fig. 1).
The best cut-off for the number of metastatic sites was > 3, calculated by ROC curve. In our study population, 141 patients (19%) presented with more than three metastatic sites and had a significantly shorter median OS (22.1 months, 95% CI 16.8−41.0) compared with patients with three or fewer metastatic sites (55.7 months, 95% CI 30.7−60.8; p < 0.001; Fig. 2). Brain metastases were associated with the worst median OS (18.4 months, 95% CI 13.2−41.0 vs 36.5 months, 95% CI 29.7−60.8; p = 0.024, Fig. 2), followed by bone (26.0 months, 95% CI 20.0−29.7 vs 60.8 months, 95% CI 36.5−NR; p < 0001, Fig. 2) and liver metastases (28.4 months, 95% CI 19.1−60.8 vs 41.0 months, 95% CI 29.7−60.8; p = 0.024, Fig. 2).
In the overall study population, median PFS was 15.0 months (95% CI 12.2−17.1). No statistically significant differences in terms of PFS were found between men and women (14.6 months, 95% CI 12.0−17.0 vs 15.9 months, 95% CI 9.9−44.5; p = 0.979) and between patients aged ≥ 70 years and < 70 years (15.5 months, 95% CI 10.4−21.5, vs 15.0 months, 95% CI 12.2−18.8; p = 0.742).
Patients with good, intermediate, and poor risk criteria showed a median PFS of 28.4, 15.0, and 11.0 months, respectively (p < 0.001, Fig. S1 in the electronic supplementary material [ESM]). Longer median PFS was observed in patients who underwent previous nephrectomy (20.1 months, 95% CI 15.9−25.1, vs 9.0 months, 95% CI 6.8−11.3; p < 0.001, Fig. S1, see ESM), while no significant difference was found between ccRCC and nccRCC patients (15.2 months, 95% CI 12.2−18.7, vs 13.0 months, 95% CI 6.9−25.1; p = 0.168). Sarcomatoid differentiation was correlated with shorter PFS (6.7 months, 95% CI 5.5−15.8, vs 15.5 months, 95% CI 12.9−21.6, p < 0.001, Fig. S1, see ESM), as well as the presence of more than three metastatic sites (6.9 months, 95% CI 4.8−11.3, vs 16.4 months, 95% CI 14.1−21.5; p < 0.001, Fig. S1, see ESM). Bone metastases were associated with worst PFS (10.4 months, 95% CI 8.0−12.9, vs 20.1 months, 95% CI 15.2−28.4; p < 0.001, Fig. S1, see ESM), while no statistically significant differences were found with the presence of liver (11.3 months, 95% CI 7.5−21.7, vs 15.8 months, 95% CI 12.9−18.8; p = 0.129) or brain metastases (10.4 months, 95% CI 5.5−13.0, vs 15.8 months, 95% CI 12.9−18.8; p = 0.088).
3.3 Role of Prognostic Factors
In the univariate analysis, IMDC criteria, previous nephrectomy, tumor histology, sarcomatoid differentiation, number of metastatic sites greater than three, bone, liver and brain metastases were significant predictors of OS (Table 2). At multivariate analysis, IMDC criteria, previous nephrectomy, tumor histology, sarcomatoid differentiation, and bone metastases proved to be significantly associated with OS (Table 2).
As for PFS, previous nephrectomy, sarcomatoid differentiation, number of metastatic sites greater than three, and bone metastases were significantly associated with OS in both univariate and multivariate analyses, while IMDC criteria did not prove to be significantly correlated with PFS in the multivariate analysis (Table 2).
3.4 Comparison of Overall Survival: IO + IO vs IO + TKI
At the time of data cut-off, nivolumab plus ipilimumab was ongoing in 158 of the 313 patients. The median follow-up time for this combination was 18.8 months (95% CI 15.5−88.8). At the time of data cut-off, treatment with IO + TKI was ongoing in 307 of the 416 patients, with a median follow-up time of 17.6 months (95% CI 16.9−64.3). Second- and third-line treatments stratified by first-line immuno-combination are reported in Table S1 (see ESM).
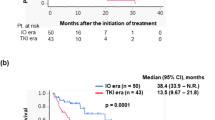
In the 616 patients with intermediate/poor IMDC risk criteria, the use of an IO + TKI combination yielded a longer median OS, as compared with the IO+IO doublet (55.7 months, 95% CI 27.3−60.8, vs 29.7 months, 95% CI 25.9−41.0; p = 0.045, Fig. 3).
We further stratified IMDC intermediate-/poor-risk patients by clinico-pathological features. Stratified by sex, no significant differences were observed in male patients treated with IO + TKI versus IO + IO (male: 55.7 months, 95% CI 22.1−60.8, vs NR, 95% CI NR−NR; p = 0.364; females: NR, 95% CI NR−NR, vs 25.0 months, 95% CI 16.0−41.0; p = 0.089). The two combinations showed similar median OS in intermediate-/poor-risk patients aged > 70 years (IO+TKI: 28.1 months, 95% CI 18.4−60.8, vs IO+IO: 26.4 months, 95% CI 22.2−28.4; p = 0.859).
A trend toward a longer median OS was observed in intermediate-/poor-risk patients who underwent nephrectomy treated with IO + TKI, although the difference was not statistically significant (55.7 months, 95% CI 32.7−60.8, vs 41.0 months, 95% CI 29.7−41.0; p = 0.682).
Stratified by tumor histology, the IO+TKI combination registered a not statistically significant longer median OS in both ccRCC (55.7 months, 95% CI 27.3−60.8, vs 30.2 months, 95% CI 26.0−41.0; p = 0.162) and nccRCC intermediate-/poor-risk patients (18.0 months, 95% CI 12.6−18.0, vs 15.2 months, 95% CI 7.6−16.5, p = 0.107).
Among the 114 mRCC cases with sarcomatoid differentiation, 54 received an IO+TKI combination, reporting a statistically nonsignificant prolongation of the median OS compared with the 60 patients treated with IO+IO therapy (NR, 95% CI NR−NR, vs 25.0 months, 95% CI 8.9−41.0; p = 0.190).
Finally, based on site of metastases, the difference in favor of IO + TKI combinations was statistically significant in intermediate-/poor-risk patients with lung (60.8 months, 95% CI 27.3−60.8, vs 28.3 months, 95% CI 20.0−41.0; p = 0.028, Fig. 3) and liver metastases (55.7 months, 95% CI 22.1−60.8, vs 25.9 months, 95% CI 10.0−30.2; p = 0.033, Fig. 3), while it was not significant in patients with bone (27.3 months, 95% CI 19.1−55.7, vs 22.2 months, 95% CI 15.4−28.3; p = 0.159) or brain metastases (22.1 months, 95% CI 18.0−22.1, vs 13.2 months, 95% CI 6.0−41.0; p = 0.221).
3.5 Comparison of Progression-Free Survival: IO + IO Versus IO + TKI in Intermediate-/Poor-Risk Patients
The median PFS was longer in patients with intermediate-/poor-risk IMDC criteria treated with IO+TKI compared with an IO + IO combination (15.9 months, 95% CI 11.0−20.6 vs 11.1 months, 95% CI 7.2−14.6; p = 0.011, Fig. S2, see ESM).
The median PFS was longer in females treated with IO+TKI vs IO+IO combination (44.5 months, 95% CI 9.6−44.5, vs 5.9 months, 95% CI 4.4−15.8; p = 0.004, Fig. S2, see ESM). No significant differences were observed in males (13.0 months, 95% CI 10.4−20.1, vs 12.2 months, 95% CI 8.3−15.2; p = 0.208), patients aged > 70 years (23.2 months, 95% CI 8.5−27.6, vs 11.3 months, 95% CI 6.3−16.4; p = 0.097), and those with previous nephrectomy (16.6 months, 95% CI 11.4−27.6 vs 15.8 months, 95% CI 10.4−23.9; p = 0.612), clear-cell histology (14.7 months, 95% CI 10.4−20.1 vs 12.0 months, 95% CI 7.8−15.2; p = 0.078), or sarcomatoid differentiation (6.6 months, 95% CI 4.0−18.8 vs 6.7 months, 95% CI 4.2−17.1; p = 0.723). On the other hand, nccRCC patients showed longer median PFS with an IO + TKI combination (NR, 95% CI NR−NR vs 6.9 months, 95% CI 4.0−15.2; p = 0.018, Fig. S2, see ESM).
By stratifying patients according to metastatic sites, the use of an IO + TKI combination registered a statistically significant longer median PFS compared with IO+IO only in intermediate-/poor-risk patients with liver metastases (16.6 months, 95% CI 10.8−27.6 vs 5.8 months, 95% CI 3.6−11.5; p = 0.004, Fig. S2, see ESM), while no significant differences were found in patients with lung (11.4 months, 95% CI 9.6−18.8 vs 10.4 months, 95% CI 6.5−15.0; p = 0.120), bone (11.0 months, 95% CI 8.0−16.6 vs 6.8 months, 95% CI 4.8−11.3; p = 0.077), or brain metastases (10.4 months, 95% CI 3.6−13.0 vs 5.5 months, 95% CI 2.1−10.4; p = 0.306).
3.6 Comparison of Response to First-Line Therapy: IO + IO vs IO + TKI
In the overall study population, the percentages of patients experiencing a CR, PR, SD, and PD were 6%, 43%, 30%, and 21%, respectively. In patients treated with an IO + IO combination, the response rates were CR = 11%, PR = 32%, SD = 29%, and PD = 28% (Table S1, see ESM). Otherwise, IO + TKI combinations yielded CR = 3%, PR = 51%, SD = 30% and PD = 16% (Table S1, see ESM). The difference between the type of responses obtained by these two combinations were statistically significant (p < 0.001, Table S1, see ESM).
4 Discussion
The selection of the ideal candidate to receive IO + IO or IO + TKI combinations is challenging due to the lack of direct comparisons between these different approaches. In this situation, the use of real-world data is integral to understanding the utilization patterns and outcomes of new treatments among cancer patients treated in both academic and community settings and provides fundamental data on the outcome of patients ineligible for clinical trials [21, 22], who constitute a not negligible proportion in daily clinical practice; and indeed, the use of rigorous real-world evidence has been advocated for across different malignancies [23].
The ARON-1 study has been designed to investigate the presence of factors influencing the prognosis of mRCC treated with immuno-oncology combinations and to retrospectively compare the efficacy of the different combinations available across the globe. Our data showed that the main prognostic factors validated for mRCC patients treated with targeted monotherapy can also be applied to patients treated with immuno-combinations (i.e., IMDC, liver-bone-brain metastases, nephrectomy, sarcomatoid differentiation, number of metastatic sites, clear-cell vs non-clear-cell histology).
If some of these factors are well known, some more insight is needed on the putative favorable prognostic role of a previous cytoreductive nephrectomy. For years, this role has been a cornerstone in the overall management of mRCC, being supported not only by old, randomized data from the age of cytokine-based immunotherapy, but also by huge retrospective series [24, 25] and by at least one meta-analysis [26]. Our results further increase the amount of data still suggesting a positive role for cytoreductive nephrectomy, leading to a key question: in terms of evidence making, which is more important, a single, randomized, controlled, phase III clinical trial (itself not free from criticism) or a bulk amount of retrospective evidence from real-world practice? In the absence of a clear-cut answer, the clinical judgment for each given patient should replace any dogmatic attitude, as already claimed by Motzer and Russo in the editorial comment to the CARMENA publication [27].
As far as the indirect comparison between the two strategies goes, the median OS was longer with IO + TKI compared with IO + IO therapy in patients with intermediate-/poor-risk features. Furthermore, OCB was + 11% higher with the IO + TKI combination. These data were consistent with those recently published in a meta-analysis on first-line immuno-combinations [28].
Another interesting (and to a certain extent worrisome) finding emerging from our study is the extremely low percentage of patients who did receive a second- or third-line treatment. Although the relatively short follow-up may account, at least in part, for this finding, it is clear that the use of combinations ultimately limits our choice for further treatment lines.
Of course, our study presents several limitations, including its retrospective nature. At first, our follow-up of 18 months and the 22% of deaths may represent a bias for OS assessment. Secondly, we did not perform a centralized review of radiological imaging, and we had no available data on the concomitant use of medications that could influence the efficacy of first-line therapy. As a consequence of all the above, our findings should be interpreted with caution and are possibly in need of a larger prospective validation.
Nevertheless, our data clearly suggest for patients for whom dimensional reduction of disease burden is needed (e.g., spinal cord compression, pain), one of the available IO-TKI combinations might be the best choice, considering the lower percentage of primary refractory patients compared with IO-IO combinations in the present study; moreover, these data are consistent with the data from randomized trials [6,7,8,9,10,11].
In 2019, Dudani et al. [29] published a first retrospective comparison between 75 patients treated with IO + IO and 113 with IO + TKIs from the IMDC dataset, with a median follow-up of 11.7 months. In our study, reporting a longer follow-up and a larger study population, the efficacy of IO + IO and IO + TKI combinations varies across different clinico-pathological subgroups.
5 Conclusions
Our study may suggest that immuno-oncology combinations are effective as first-line therapy in the mRCC real-world context, showing outcome differences between IO + IO and IO + TKI combinations in mRCC subpopulations. Prospective clinical trials directly comparing distinct IO + IO and IO + TKI combinations are thus sorely needed.
References
Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009.
Santoni M, Piva F, Porta C, Bracarda S, Heng DY, Matrana MR, et al. Artificial neural networks as a way to predict future kidney cancer incidence in the United States. Clin Genitourin Cancer. 2021;19(2):e84–91.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24.
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J ClinOncol. 2010;28(6):1061–8.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15.
Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–15.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41.
Rizzo A, Mollica V, Dall’Olio FG, Ricci AD, Maggio I, Marchetti A, et al. Quality of life assessment in renal cell carcinoma Phase II and III clinical trials published between 2010 and 2020: a systematic review. Future Oncol. 2021;17(20):2671–81.
Rathi N, Maughan BL, Agarwal N, Swami U. The tango of immunotherapy and targeted therapy in metastatic renal cell carcinoma. Transl Cancer Res. 2019;8(8):E1–6.
Heng DY, Mackenzie MJ, Vaishampayan UN, Bjarnason GA, Knox JJ, Tan MH, et al. Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol. 2012;23(6):1549–55.
Santoni M, Massari F, Bracarda S, Grande E, Matrana MR, Rizzo M, et al. Cabozantinib in patients with advanced renal cell carcinoma primary refractory to first-line immunocombinations or tyrosine kinase inhibitors. Eur Urol Focus. 2022;S2405–4569(22):00049–59.
Teishima J, Murata D, Inoue S, Hayashi T, Mita K, Hasegawa Y, et al. Prediction of early progression of metastatic renal cell carcinoma treated with first-line tyrosine kinase inhibitor. Curr Urol. 2021;15(4):187–92.
Gan CL, Dudani S, Wells JC, Donskov F, Pal SK, Dizman N, et al. Cabozantinib real-world effectiveness in the first-through fourth-line settings for the treatment of metastatic renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium. Cancer Med. 2021;10(4):1212–21.
Ravi P, Mantia C, Su C, Sorenson K, Elhag D, Rathi N, Bakouny Z, Agarwal N, Zakharia Y, Costello BA, McKay RR, Narayan V, Alva A, McGregor BA, Gao X, McDermott DF, Choueiri TK. Evaluation of the safety and efficacy of immunotherapy rechallenge in patients with renal cell carcinoma. JAMA Oncol. 2020;6(10):1606–10.
Santoni M, Massari F, Myint ZW, Iacovelli R, Pichler M, Basso U, et al. Clinico-pathological features influencing the prognostic role of body mass index in patients with advanced renal cell carcinoma treated by immuno-oncology combinations (ARON-1). Clin Genitourin Cancer. 2023;S1558–7673(23):00065–74.
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7.
Nemoto Y, Ishihara H, Nakamura K, Tachibana H, Fukuda H, Yoshida K, et al. Efficacy and safety of immunotherapy-based combinations as first-line therapy for metastatic renal cell carcinoma in patients who do not meet trial eligibility criteria. Target Oncol. 2022;17(4):475–82.
Ishihara H, Nemoto Y, Nakamura K, Tachibana H, Ikeda T, Fukuda H, et al. Comparison of outcomes between therapeutic combinations based on immune checkpoint inhibitors or tyrosine kinase inhibitor monotherapy for first-line therapy of patients with advanced renal cell carcinoma outside of clinical trials: a real-world retrospective multi-institutional study. Target Oncol. 2023;18(2):209–20.
Yang DD, Nguyen PL. The increasing importance of rigorous real-world evidence. JNCI Cancer Spectr. 2022;6(4):pkac051.
Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–6.
Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the international metastatic renal cell carcinoma database consortium. Eur Urol. 2014;66:704–10.
Massari F, Di Nunno V, Gatto L, et al. Should CARMENA really change our attitude towards cytoreductive nephrectomy in metastatic renal cell carcinoma? A systematic review and meta-analysis evaluating cytoreductive nephrectomy in the era of targeted therapy. Target Oncol. 2018;13:705–14.
Motzer RJ, Russo P. Cytoreductive nephrectomy—patient selection is key. N Engl J Med. 2018;379:481–2.
Massari F, Rizzo A, Mollica V, Rosellini M, Marchetti A, Ardizzoni A, Santoni M. Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer. 2021;154:120–7.
Dudani S, Graham J, Wells JC, Bakouny Z, Pal SK, Dizman N, et al. First-line immuno-oncology combination therapies in metastatic renal-cell carcinoma: results from the international metastatic renal-cell carcinoma database consortium. Eur Urol. 2019;76(6):861–7.
Acknowledgements
None to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Matteo Santoni has received research support and honoraria from Janssen, Bristol Myers Squibb, Ipsen, MSD, Astellas, and Bayer, all unrelated to the present paper. R. Kanesvaran has received fees for speaker bureau and advisory board activities from the following companies: Pfizer, MSD, BMS, Eisai, Ipsen, Johnson and Johnson, Merck, Amgen, Astellas, and Bayer. Enrique Grande has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Adacap, AMGEN, Angelini, Astellas, Astra Zeneca, Bayer, Blueprint, Bristol Myers Squibb, Caris Life Sciences, Celgene, Clovis-Oncology, Eisai, Eusa Pharma, Genetracer, Guardant Health, HRA-Pharma, IPSEN, ITM-Radiopharma, Janssen, Lexicon, Lilly, Merck KGaA, MSD, Nanostring Technologies, Natera, Novartis, ONCODNA (Biosequence), Palex, Pharmamar, Pierre Fabre, Pfizer, Roche, Sanofi-Genzyme, Servier, Taiho, and Thermo Fisher Scientific. EG has received research grants from Pfizer, Astra Zeneca, Astellas, and Lexicon Pharmaceuticals. Tomas Buchler has received research support and honoraria from Roche, Bristol Myers Squibb, Ipsen, Exelixis, Eisai, Merck Sharp Dohme, Merck, Eli Lilly, and AstraZeneca, all unrelated to the present paper. Aristotelis Bamias has received honoraria/advisory/research support by Pfizer, BMS, AZ, MSD, Roche, Janssen, Ipsen, Bayer, and Merck. Fernando Sabino Marques Monteiro has received research support from Janssen and Merck Sharp Dome and honoraria from Janssen, Ipsen, Bristol Myers Squibb, and Merck Sharp Dome. Camillo Porta has received honoraria from Angelini Pharma, AstraZeneca, BMS, Eisai, General Electric, Ipsen, and MSD and acted as a Protocol Steering Committee Member for BMS, Eisai, and MSD. The other authors declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Funding
No external funding was used in the preparation of this manuscript.
Ethics approval, Consent to Participate
This retrospective research was carried out in accordance with the approval from the ethics committee of the Marche Region (2021-492) and was performed in accordance with the Declaration of Helsinki.
Availability of data
All data generated or analyzed during this study are included in this published article (and its supplementary information files). The datasets generated during and/or analyzed during the current study are not publicly available in accordance with all the centers participating to the ARON project but are available from the corresponding author on reasonable request.
Author contributions
Conceptualization: Matteo Santoni, Camillo Porta, Gaetano Aurilio. Data curation: Matteo Santoni, Francesco Massari, Fernando Sabino M. Monteiro, Zin W. Myint, Umberto Basso, Jindrich Kopecky, Jakub Kucharz, Mimma Rizzo1 Luca Galli, Thomas Büttner, Ugo De Giorgi, Ondřej Fiala, Paolo Andrea Zucali, Giuseppe Fornarini, Maria T Bourlon, Sarah Scagliarini, Javier Molina-Cerrillo, Marc R Matrana, Renate Pichler, Carlo Cattrini, Emmanuel Seront, Alvaro Pinto, Rossana Berardi, Anca Zgura, Giulia Mammone, Jawaher Ansari, Francesco Atzori, Rita Chiari, Orazio Caffo, Giuseppe Procopio, Maria Bassanelli, Sara Merler, Carlo Messina, Zsófia Küronya, Alessandra Mosca, Dipen Bhuva, Nuno Vau, Lorena Incorvaia, Sara Elena Rebuzzi, Giandomenico Roviello. Formal analysis: Matteo Santoni. Investigation: Francesco Massari. Methodology: Alessandro Rizzo, Ignacio Ortego Zabalza, Roberto Iacovelli, Martin Pichler. Project administration: Giulia Sorgentoni. Supervision: Enrique Grande, Aristotelis Bamias, Fabio Calabrò. Roles/writing—original draft: Matteo Santoni, Veronica Mollica, Sebastiano Buti. Writing—review & editing: Tomas Büchler, Ravindran Kanesvaran, Camillo Porta, Nicola Battelli, Rodolfo Montironi.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santoni, M., Massari, F., Myint, Z.W. et al. Global Real-World Outcomes of Patients Receiving Immuno-Oncology Combinations for Advanced Renal Cell Carcinoma: The ARON-1 Study. Targ Oncol 18, 559–570 (2023). https://doi.org/10.1007/s11523-023-00978-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00978-2